
Cost-effectiveness analysis of rhTPO and rhIL-11 in the treatment of chemotherapy-induced thrombocytopenia in hematological tumors based on real-world data
Introduction
Chemotherapy-induced thrombocytopenia (CIT) results from the inhibitory effect of antitumor drugs on bone marrow megakaryocytes, leading to a platelet count in peripheral blood lower than 100×109/L (1). CIT, one of the most common chemotherapy-related hematological toxicities, can increase the risk of bleeding, prolong hospitalization, increase medical costs, reduce chemotherapy effectiveness, impact patients’ quality of life, affect prognosis, and in severe cases, lead to death (1,2). The incidence of CIT is reported to be related to the type of chemotherapeutic drug, combination therapy, and tumor (3-5). Primary and secondary prevention for high-risk patients can help reduce the incidence and severity of CIT (1).
The treatment of CIT mainly includes platelet transfusion and the administration of platelet-promoting growth factors (1,6). Platelet transfusion is the fastest and most effective method for the treatment of severe CIT and can effectively reduce the risk of major hemorrhage and mortality. However, the clinical use of platelets is limited due to the possibility of transfusion-related viral and bacterial infections, allogeneic immune responses, and a tight blood supply. Recent studies have shown that the use of platelet growth factors can effectively increase platelets, reduce the risk of bleeding, reduce the need for platelet transfusion, and ensure that chemotherapy is carried out on schedule and in sufficient quantities (6). Recombinant human thrombopoietin (rhTPO) and recombinant human interleukin-11 (rhIL-11) have been approved by the Food and Drug Administration (FDA) of China for CIT treatment. After long-term clinical application, both have been shown to reduce the extent of platelet count decline after chemotherapy for solid tumors such as lung, breast, and ovarian cancers, shorten the duration of thrombocytopenia, and reduce the number of platelet transfusions (6-8).
In recent years, there has been gradual recognition of the therapeutic effectiveness of rhTPO and rhIL-11 in hematological tumors. However, since rhTPO in the treatment of thrombocytopenia in patients with hematological tumors is off-label use, most of the existing research conclusions come from real-world data. In fact, more and more attention has been paid to real world study at home and abroad (9,10). In 2016, the United States passed the 21st Century Cures Act, which authorized research using real-world evidence for expanded drug indications (11). In December 2018, FDA announced the Real World Evidence Protocol Framework, which provided a relatively clear roadmap for achieving the Real World Evidence’s goal of supporting drug approval decisions (12). Unfortunately, the sample size of the existing studies on the treatment of CIT by rhTPO and rhIL-11 in patients with hematological tumors is generally small, and there is a large bias between different studies. In addition, the treatment costs of the two drugs vary several times, so the comprehensive evaluation of the two drugs is not consistent (1,13-15). A meta-analysis showed that compared with rhIL-11, rhTPO could increase the maximum value of platelet recovery, shorten the duration of platelet ≤50×109/L, shorten the time to platelet recovery to ≥75×109/L, and reduce the incidence of adverse reactions (15). However, no significant difference was found between the two in improving the minimum value of platelets after chemotherapy and the time for patients to recover to platelets ≥100×109/L. In addition, from an economic perspective, the unit price and the price per treatment course of rhTPO are both higher than that of rhIL-11. The more cost-effective regimen of the two for the treatment of CIT requires further investigation. In fact, relatively few studies are available on the pharmacoeconomics of rhTPO and rhIL-11 in the treatment of CIT at home and abroad, and the conclusions are inconsistent (16-18). Chen et al. (16) showed that rhIL-11 and rhTPO were similar in the treatment of thrombocytopenia caused by gemcitabine-based combination chemotherapy in patients with lung cancer. Measured by the least-cost method, rhIL-11 has certain advantages in terms of economics. Yang et al. (17) used cost-effectiveness analysis (CEA), combined with literature reports and expert consultation, to carry out an economic evaluation of rhIL-11 and rhTPO in the treatment of CIT. The results showed that rhTPO was more cost-effective in treating CIT than rhIL-11.
In summary, there are certain differences in clinical efficacy, safety, and economics between rhIL-11 and rhTPO in the treatment of different diseases. In this study, we retrospectively analyzed clinical data for CIT in patients with hematological malignancies at our hospital from the past 7 years. Propensity score matching (PSM) was used to eliminate confounding factors. At the same time, the efficacy and economy of rhIL-11 and rhTPO in the treatment of CIT in the real world were evaluated from the perspective of the health care system. This study aimed to provide a basis for the rational selection of CIT treatment drugs for patients with hematological tumors. We present the following article in accordance with the CHEERS reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-880/rc).
Methods
Data sources
We retrospectively collected the data of hematologic oncology patients treated for CIT with rhTPO (TPIAO, 3SBIOINC., SFDA Approval No. S20050048) or rhIL-11 (Jijufen, Hangzhou Jiuyuan Gene Engineering Co., Ltd., SFDA Approval No. S20063110; Juhe granules, Qilu Pharmaceutical Co., Ltd., SFDA Approval No. S20030016) at our hospital between January 2014 and December 2020. The patients’ basic information, disease diagnosis, medications, laboratory test results, disease course records, and treatment costs were collected. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As a retrospective study, the patients did not provide informed consent. The study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Soochow University (Ethics Approval No. 258, 2022).
The inclusion criteria were: (I) aged 18–65 years, (II) a pathological diagnosis of hematological tumors, and (III) a reduction in platelets caused by chemotherapy (platelet <100×109/L).
The exclusion criteria were: (I) combined radiotherapy; (II) other nonchemotherapeutic factors that may have caused thrombocytopenia; (III) no follow-up blood test report; (IV) no continuous drug use; (V) cross user of rhIL-11 and rhTPO in the same cycle; (VI) the use of other platelet-elevating drugs; (VII) severe heart, liver, or kidney failure, especially with a history of organic heart disease; (VIII) a history of active bleeding and thrombosis; and (IX) incomplete records with key information missing.
PSM was used to eliminate confounding factors. PSM was a method to match individuals in the treatment group and the control group based on propensity score. The difference in outcome could be expressed by calculating the average treatment effect of the two groups, which can effectively reduce the confounding effect. Therefore, non-randomized data could be used to estimate the relationship between treatment factors and outcomes, making real-world data studies close to the most realistic randomized controlled trial (RCT) studies (18). The observational variables included gender, age, body mass index (BMI), liver function, renal function, disease classification, chemotherapy regimen, CIT grade, target drug application time, length of hospital stay and so on, were included as covariates in the logistic regression model, the confounding bias of nonrandomized controlled subjects was balanced by 1:1 PSM, and a certain number of cases were obtained for analysis (19,20).
Treatment regimens
The rhTPO group received a daily intravenous injection of 1.5×104 U, and the rhIL-11 group received a daily intravenous injection of 1.5 mg.
According to the World Health Organization (WHO), thrombocytopenia after chemotherapy is classified as grade I to grade IV, with grade I corresponding to 100×109/L > platelet ≥75×109/L, grade II to 75×109/L > platelet ≥50×109/L, grade III to 50×109/L > platelet ≥25×109/L, and grade IV to platelet <25×109/L.
The patient population was divided into a grade I–II thrombocytopenia group and grade III–IV thrombocytopenia group.
Efficacy evaluation criteria
Routine blood tests were performed for all enrolled patients with hematologic diseases. Platelet data of the two groups were collected before implementation of the platelet-elevating regimen and on day 7 and 14 after implementation of the platelet-elevating regimen. The platelet compliance rate of patients with different CIT grades on day 7 and 14 after implementation of the platelet-elevating regimen were evaluated as an indicator of efficacy. In addition, bleeding in the two groups of patients was evaluated within 14 days of platelet-elevating treatment.
Platelet compliance was defined as platelet ≥100×109/L or an increase of 50×109/L compared to the original level. The platelet compliance rate was the number of patients with platelet compliance divided by the total number of patients.
Economic analysis
Cost calculation
This study conducted an economic evaluation of rhTPO and rhIL-11 in the treatment of CIT in patients with hematological tumors from the perspective of the health care system. Direct nonmedical costs, indirect costs, and hidden costs were not incorporated into the calculations due to measurement difficulties. Therefore, this study included only direct medical costs. Because the treatment time of platelet-elevating drugs in the two groups was balanced, hospitalization and nursing expenses were not included. This study included the cost of rhIL-11 or rhTPO drugs, the cost of platelet transfusion, the cost of hemostasis, and the cost of other platelet-elevating drugs. The cost of drugs was based on the bid price of the drugs on the Sunshine Procurement Platform in 2022. The bid price for rhTPO was 789 yuan (1.5×104 U/tube), and for rhIL-11, which has two specifications, it was 125.73 yuan (1.5 mg/tube) and 218.45 yuan (3 mg/tube).
Study time and discounting
In this study, platelet-elevating treatment was a short-term process. The study time extended from the initiation of rhTPO and rhIL-11 platelet-elevating treatment to the 14th day after continuous treatment. Thus, there was no involvement of discounting.
Economic evaluation methods
If no significant difference in effectiveness was identified between the two groups, the total cost of the treatment regimens in the two groups was directly compared using the minimum cost analysis method to determine the economy of the treatment regimens.
If a significant difference in effectiveness was found between the two groups, the CEA method was used, and the incremental cost-effectiveness ratio (ICER) was used as the evaluation indicator (21).
Statistical methods
The XGBoost algorithm was used for modeling (22). The importance of each variable’s impact on drug efficacy was calculated and sorted in descending order. Important variables were selected as covariates, including basic patient information, CIT grade before target drug use, target drug use time, length of hospital stay, sex, age, disease classification, and chemotherapy regimen. Furthermore, 1:1 PSM was performed using R software, and the caliper value was set to 0.1 to control for confounding factors between the rhIL-11 and rhTPO groups.
Univariate sensitivity analysis was used to evaluate the impact of changes in factors such as drug price and clinical efficacy on economic results. At the same time, in order to evaluate the uncertainty effect of all variables simultaneously, Monte Carlo simulation was used to conduct probabilistic sensitivity analysis. This study assumed that the cost was normally distributed, the effect followed a triangular distribution, and the probability followed a beta distribution. The simulation was run with 1,000 samples and the results of the uncertainty analysis were represented using cost-effectiveness acceptability curves.
The quantitative indicators did not satisfy a normal distribution, and the median and upper and lower quartiles were calculated. The Wilcoxon rank sum test was used to compare index values between the two groups. The frequency and its percentage were used in the statistical description of qualitative indicators or grade indicators. Unordered categorical indicators were compared using the χ2 test or Fisher’s method. All statistical tests were performed using two-sided tests, and P<0.05 was considered statistically significant.
Results
Basic information
In this study, a total of 1,571 patients met the inclusion and exclusion criteria, and 476 patients were included after 1:1 PSM, including 238 patients in the rhIL-11 group and 238 patients in the rhTPO group. After PSM, no significant difference in any of the basic data was noted between the two groups (Table 1).
Table 1
| Variables | rhIL-11 group (n=238) | rhTPO group (n=238) | P value |
|---|---|---|---|
| Sex, n (%) | 0.270 | ||
| Male | 135 (56.7) | 123 (51.7) | |
| Female | 103 (43.3) | 115 (48.3) | |
| Age (years), mean (25th–75th) | 41.0 (29.0–51.0) | 41.0 (31.0–51.0) | 0.619 |
| BMI (kg/m2), mean (25th–75th) | 22.7 (20.5–24.9) | 22.5 (20.6–24.5) | 0.682 |
| Disease classification, n (%) | 0.099 | ||
| Leukemia | 194 (81.5) | 198 (83.2) | |
| Nonleukemia* | 44 (18.5) | 40 (16.8) | |
| Chemotherapy regimen#, n (%) | 0.921 | ||
| ≤3 | 165 (69.3) | 164 (68.9) | |
| >3 | 73 (30.7) | 74 (31.1) | |
| CIT classification, n (%) | 0.374 | ||
| I | 16 (6.7) | 8 (3.4) | |
| II | 27 (11.3) | 32 (13.4) | |
| III | 89 (37.4) | 90 (37.8) | |
| IV | 106 (44.5) | 108 (45.4) | |
| Target drug application time (days), mean (25th–75th) | 12.0 (8.0–15.0) | 12.0 (10.0–15.0) | 0.557 |
| Length of hospital stay (days), mean (25th–75th) | 29.0 (25.0–34.0) | 29.0 (26.0–35.0) | 0.104 |
| Liver function, n (%) | 0.270 | ||
| Normal | 134 (56.3) | 122 (51.3) | |
| Abnormal | 104 (43.7) | 116 (48.7) | |
| Renal function, n (%) | 0.338 | ||
| Normal | 221 (92.9) | 226 (95.0) | |
| Abnormal | 17 (7.1) | 12 (5.0) |
*, includes lymphoma, multiple myeloma, and myelodysplastic syndrome; #, refers to the number of drugs with high bleeding risk included in the chemotherapy regimen. PSM, propensity score matching; BMI, body mass index; CIT, chemotherapy-induced thrombocytopenia; rhIL-11, recombinant human interleukin-11; rhTPO, recombinant human thrombopoietin.
Clinical efficacy
Analysis of overall efficacy
The platelet compliance rates of the two groups of patients after platelet-elevating treatment are shown in Table 2. The results showed no significant difference in the platelet compliance rate between the two groups in the first week after treatment (7.1% vs. 9.7%, P=0.322). The platelet compliance rate was higher in the rhTPO group than in the rhIL-11 group 2 weeks after treatment, and the difference was statistically significant.
Table 2
| Platelet compliance | rhIL-11 group (n=238), n (%) | rhTPO group (n=238), n (%) | P value |
|---|---|---|---|
| Week 1 | 0.322 | ||
| Yes | 17 (7.1) | 23 (9.7) | |
| No | 221 (92.9) | 215 (90.3) | |
| Week 2 | 0.016 | ||
| Yes | 59 (24.8) | 83 (34.9) | |
| No | 179 (75.2) | 155 (65.1) |
rhIL-11, recombinant human interleukin-11; rhTPO, recombinant human thrombopoietin.
The bleeding rates of the two groups of patients within 2 weeks of treatment is shown in Table 3. The results showed that the proportion of patients bleeding in the rhIL-11 group was significantly higher than that in the rhTPO group.
Table 3
| Bleeding | rhIL-11 group (n=238), n (%) | rhTPO group (n=238), n (%) | P value |
|---|---|---|---|
| Yes | 55 (23.1) | 32 (13.4) | 0.006 |
| No | 183 (76.9) | 206 (86.6) |
rhIL-11, recombinant human interleukin-11; rhTPO, recombinant human thrombopoietin.
Efficacy analysis of patients with different CIT grades
A total of 83 patients had grade I–II CIT. No significant difference in the platelet compliance rate was found between the two groups at the first and second week after platelet-elevating treatment (Table 4).
Table 4
| Platelet compliance | rhIL-11 group (n=43), n (%) | rhTPO group (n=40), n (%) | P value |
|---|---|---|---|
| Week 1 | 0.435 | ||
| Yes | 5 (11.6) | 2 (5.0) | |
| No | 38 (88.4) | 38 (95.0) | |
| Week 2 | 0.657 | ||
| Yes | 10 (23.3) | 11 (27.5) | |
| No | 33 (76.7) | 29 (72.5) |
CIT, chemotherapy-induced thrombocytopenia; rhIL-11, recombinant human interleukin-11; rhTPO, recombinant human thrombopoietin.
A total of 393 patients had grade III–IV CIT. After the first week of platelet-elevating treatment, no significant difference in the platelet compliance rate was noted between the two groups of patients. However, the platelet compliance rate at week 2 was significantly higher in the rhTPO group than in the rhIL-11 group (Table 5).
Table 5
| Platelet compliance | rhIL-11 group (n=195), n (%) | rhTPO group (n=198), n (%) | P value |
|---|---|---|---|
| Week 1 | 0.112 | ||
| Yes | 12 (6.2) | 21 (10.6) | |
| No | 183 (93.8) | 177 (89.4) | |
| Week 2 | 0.016 | ||
| Yes | 49 (25.1) | 72 (36.4) | |
| No | 146 (74.9) | 126 (63.6) |
CIT, chemotherapy-induced thrombocytopenia; rhIL-11, recombinant human interleukin-11; rhTPO, recombinant human thrombopoietin.
Economic analysis
CEA
In this study, the total platelet compliance rate in the second week after platelet-elevating treatment was used as the efficacy indicator, and the cost-effectiveness method was used to evaluate the economy of the two drugs in the treatment of CIT in patients with hematological tumors. Because the treatment time of platelet-elevating drugs in the two groups was balanced, the hospitalization and nursing expenses of the two groups of patients were not included in the cost statistics. The costs in this study included the cost of rhIL-11 or rhTPO drugs, the cost of platelet transfusion, the cost of hemostasis, and the cost of other platelet-elevating drugs.
Table 6 shows that the actual clinical treatment costs in the rhTPO group and the rhIL-11 group were 27,695.0 and 4,806.8 yuan, respectively. The effectiveness values of the rhTPO group and rhIL-11 group were 0.349 and 0.248, respectively. The ICER was 226,615.84, indicating that patients in the rhTPO group paid a further 226,615.80 yuan for each additional increase in effectiveness value compared to the rhIL-11 group.
Table 6
| Group | C (yuan) | ΔC (yuan) | E | ΔE | ICER |
|---|---|---|---|---|---|
| rhIL-11 group | 4,806.8 | 22,888.2 | 0.248 | 0.101 | 226,615.8 |
| rhTPO group | 27,695.0 | 0.349 |
CEA, cost-effectiveness analysis; CIT, chemotherapy-induced thrombocytopenia; rhIL-11, recombinant human interleukin-11; rhTPO, recombinant human thrombopoietin; C, cost; ΔC, incremental cost; E, effectiveness; ΔE, incremental effect; ICER, incremental cost-effectiveness ratio.
Univariate sensitivity analysis
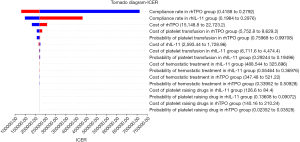
Univariate sensitivity analysis was used to evaluate the impact of changes in factors such as drug price and clinical efficacy on economic results. A sensitivity analysis was performed based on the assumption that all factor changes were within the upper and lower 20% range. As shown in Figure 1, the ICER value was sensitive to the platelet compliance rate in the rhTPO group and the rhIL-11 group, the cost of rhTPO, the cost of platelet transfusion in the rhTPO group, and the probability of platelet transfusion in the rhTPO group.
Probabilistic sensitivity analysis
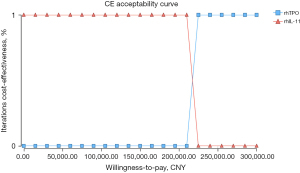
The results of probabilistic sensitivity analysis of 1,000 simulation samples are shown in Figure 2. When the willingness to pay was less than approximately 220,000 yuan, rhIL-11 economy was 100% better than that of rhTPO. When the willingness to pay was greater than approximately 220,000 yuan, rhTPO economy was 100% better than that of rhIL-11.
Discussion
rhTPO is a genetically engineered protein drug with good clinical tolerance and safety. It can increase the number of peripheral platelets by stimulating platelet formation and promote the recovery of platelets after chemotherapy. The effectiveness can be achieved within 1 week of application (19,23). rhIL-11 is secreted by human bone marrow stromal cells and interstitial cells. By increasing the number of peripheral platelets and maintaining their functions, rhIL-11 shortens the duration of platelet reduction and accelerates the recovery of platelets to normal levels. Since it acts only in the early stages of megakaryocyte differentiation, the onset of action should be approximately 3 weeks after application (24). The “Consensus on the clinical diagnosis, treatment, and prevention of chemotherapy-induced thrombocytopenia in China” recommends that both rhTPO and rhIL-11 be used as drugs for the treatment of CIT (1).
This study was a retrospective analysis. To make the samples as close as possible to the results generated by randomization, PSM was used to select appropriate cases, and differences in the efficacy and economy of the two drugs were analyzed. The XGBoost model achieves the best predictive modeling among machine learning models and traditional regression models (22). The strengths of this study included the use of the XGBoost calculation model to analyze the importance of each variable in the two groups of patients to the efficacy of the drug and the scientific selection of covariates by PSM to effectively control confounding factors between the rhIL-11 group and the rhTPO group while minimizing sample loss (20). Ultimately, a total of 476 cases were included in this study after PSM, which is greater than the case numbers of previous retrospective studies.
The results of this study showed that at 2 weeks after platelet-elevating treatment, the platelet compliance rate of the CIT patients in the rhTPO group was 34.9%, which was 1.4 times that of the rhIL-11 group (24.8%), and the difference was statistically significant (P=0.016). At the same time, the bleeding rate of patients in the rhTPO group was 42.0% lower than that in the rhIL-11 group (23.1% vs. 13.4%, P=0.006), indicating that in patients with hematological tumors, the efficacy and safety of rhTPO in the treatment of CIT were better, which was similar to the results of previous studies (14,25,26). Subgroup analysis showed no significant difference in the efficacy of the 2 drugs in patients with grade I–II CIT (23.3% vs. 27.5%, P>0.05). However, in patients with grade III–IV CIT, the platelet compliance rate in the rhTPO group was 36.4%, which was significantly higher than that in the rhIL-11 group (25.1%). These patients also had a higher platelet compliance rate than patients with grade I–II CIT (27.5%), which confirmed the results of Chen et al. (21), suggesting that rhTPO may have better therapeutic effectiveness on platelet elevation in patients with severe CIT, which may be related to the effect of rhTPO on improving platelet function. Notably, however, in this study, the platelet compliance rate of patients with hematological tumors after 2 weeks of CIT treatment with rhTPO and rhIL-11 was still lower than 40%, which was much lower than that of patients with other solid tumors. This finding may be related to the disease characteristics of hematological diseases themselves (26).
Although the efficacy and safety of rhTPO in the treatment of CIT in patients with hematological tumors are superior to those of rhIL-11, due to the high price of rhTPO, its clinical application is still subject to certain limitations (16,17). In this study, the platelet compliance rate at 2 weeks of treatment was used as an efficacy indicator. The CEA showed that the actual clinical treatment costs in the rhTPO group and the rhIL-11 group were 27,695.0 yuan and 4,806.8 yuan, respectively. The effectiveness values in the rhTPO group and rhIL-11 group were 0.349 and 0.248, respectively. The ICER was 226,615.8. Despite a temporary lack of payment threshold, this value was nearly 3 times higher than China’s gross domestic product (GDP) per capita in 2021 (80,976 yuan), which seemed to imply that under current conditions, rhTPO economy is poor. However, this study used the platelet compliance rate as an indicator of efficacy, and although it has a certain correlation with long-term prognosis, survival time, and quality of life of cancer patients, quantitative analyses are lacking. Therefore, further investigation is needed to determine which is the more economic treatment regimen of the two. The results of this study can provide a reference for the clinical treatment of patients in the real world. Chen et al. (21) also analyzed the economy of CIT treatment with rhIL-11 and rhTPO in patients with tumors based on retrospective data using the least-cost method and the cost-effectiveness method. The results showed comparable therapeutic effectiveness for both drugs in patients with mild CIT (grade I–II), and rhIL-11 had a greater economic advantage, while rhTPO had a cost-effectiveness advantage over rhIL-11 in patients with grade III–IV CIT. However, this conclusion was based on the assumption that the average total cost of the two drugs is used as the willingness-to-pay threshold. Whether this is a scientific assumption is not known.
This study had the following limitations. First, fewer than 60% of the patients with hematological tumors did not reach the platelet standard after 2 weeks of treatment. Therefore, during the study period, calculating the time required for the platelets to return to normal in all patients and the duration after reaching the standard was impossible. Therefore, the 2-week platelet compliance rate was used as an indicator of efficacy. Because this study was a retrospective analysis, analyzing adverse reactions such as fatigue, fever, chills, and edema caused by CIT treatment was difficult. Therefore, the treatment cost of adverse reactions was not included in the cost calculation. Notably, however, these adverse effects are mostly self-reversible, and the cost of treatment is largely negligible. However, this cost may affect the results of an economic evaluation of drugs (7,27). In addition, the cost and effectiveness evaluations of this study were all defined by the treatment time of the study drug, and a follow-up of the patients was not performed. It was worth noting that the conclusions in this paper were based on the PSM-matched study samples, which might not be extended to the patients, not matched in PSM, who might also be a group of clinical concern. The sample was obtained from one hospital only was also a limitation of this study.
In summary, in the treatment of CIT in patients with hematological tumors, rhTPO elevated platelets more effectively than rhIL-11, especially for severe CIT (grade III–IV). However, CEA showed that compared to patients receiving rhIL-11, patients in the rhTPO group needed pay a further 226,615.80 yuan for each additional increase in effectiveness value, which was nearly 3 times higher than China’s GDP per capita in 2021 (80,976 yuan). This finding seemed to imply that the rhTPO regimen is less economical under current conditions. The platelet compliance rate in the two groups, the cost of rhTPO, the cost of platelet transfusion in the rhTPO group, and the probability of platelet transfusion in the rhTPO group had a significant impact on economics. However, many of the limitations mentioned above still existed in this paper. Therefore, the status of the two drugs in the treatment of CIT in patients with hematologic tumors needs to be further demonstrated in real-world studies with larger sample sizes from multiple centers.
Acknowledgments
Funding: This study was funded by the Suzhou Science and Technology Development Plan (Science and Technology for People’s Livelihood-Basic Research on Medical and Health Applications) (No. SYSD2020181) and the Bethune Public Welfare Foundation’s “Bethune Pursuit-Pharmaceutical Research Capacity Building” Project (No. B-19-H-20200622).
Footnote
Reporting Checklist: The authors have completed the CHEERS reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-880/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-880/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-880/coif). RWW is from Beijing Medicinovo Technology Co., Ltd., Beijing, China. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). As a retrospective study, the patients did not provide informed consent. The study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Soochow University (Ethics Approval No. 258, 2022).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The Society of Chemotherapy, Chinese Anti-Cancer Association. Committee of Neoplastic Supportive-Care (CONS), China Anti-Cancer Association. Consensus on the clinical diagnosis, treatment, and prevention of chemotherapy-in-duced thrombocytopenia in China (2019 version). Chinese Journal of Clinical Oncology 2019;46:923-9.
- Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol 2001;19:1137-46. [Crossref] [PubMed]
- Ten Berg MJ, van den Bemt PM, Shantakumar S, et al. Thrombocytopenia in adult cancer patients receiving cytotoxic chemotherapy: results from a retrospective hospital-based cohort study. Drug Saf 2011;34:1151-60. [Crossref] [PubMed]
- Castaman G, Pieri L. Management of thrombocytopenia in cancer. Thromb Res 2018;164:S89-93. [Crossref] [PubMed]
- Weycker D, Hatfield M, Grossman A, et al. Risk and consequences of chemotherapy-induced thrombocytopenia in US clinical practice. BMC Cancer 2019;19:151. [Crossref] [PubMed]
- Xu Y, Song X, Du F, et al. A Randomized Controlled Study of rhTPO and rhIL-11 for the Prophylactic Treatment of Chemotherapy-Induced Thrombocytopenia in Non-Small Cell Lung Cancer. J Cancer 2018;9:4718-25. [Crossref] [PubMed]
- Chinese Society of Clinical Oncology (CSCO) Anti-Lymphoma Alliance. Hematology Branch of Chinese Medical Association. China experts consensus of recombinant human interleukin-11 in the treatment of thrombocytopenia. Chinese Clinical Oncology 2018;23:260-6.
- Wu QR, Zhao YQ, Chu DT, et al. Effectiveness and safety of recombinant human thrombopoietin on treating chemotherapy-induced thrombocytopenia: data pool analysis of phase II/III and complement multi-center clinical trials. Chinese Journal of Cancer Biotherapy 2013;20:645-53.
- Ventola CL. Big Data and Pharmacovigilance: Data Mining for Adverse Drug Events and Interactions. P T 2018;43:340-51. [PubMed]
- Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol 2017;2:230-43. [Crossref] [PubMed]
- Meskó B, Hetényi G, Győrffy Z. Will artificial intelligence solve the human resource crisis in healthcare? BMC Health Serv Res 2018;18:545. [Crossref] [PubMed]
- Barlas S. The 21st Century Cures Act: FDA Implementation One Year Later: Some Action, Some Results, Some Questions. P T 2018;43:149-79. [PubMed]
- Tang G, Wang XM, Meng JX, et al. Efficacy of Recombinant Human Thrombopoietin and Recombinant Human Interleukin 11 for Treatment of Chemotherapy Induced Thrombocytopenia in Acute Myeloid Leukaemia Patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018;26:234-8. [PubMed]
- Zhu Q, Yang S, Zeng W, et al. A Real-World Observation of Eltrombopag and Recombinant Human Thrombopoietin (rhTPO) in Lymphoma Patients With Chemotherapy Induced Thrombocytopenia. Front Oncol 2021;11:701539. [Crossref] [PubMed]
- Chuang JL, Tan JY, Tang M, et al. Meta-analysis of recombinant human thromobopoietin and recombinant human interleukin11 in the treatment of chemotherapy induced thrombocytopenia. Practical Journal of Clinical Medicine 2016;13:110-4.
- Chen FJ, Zhang D, Wang Z, et al. Economic Evaluation of rhIL-11 (I) and rhTPO in the Treatment of Thrombocytopenia Caused by Chemotherapy. China Pharmacist 2015;18:250-2.
- Yang F, Li LG, Xuan JW. The cost-effectiveness analysis of rhTPO versus IL-11 on the treatment of chemotherapy-induced thrombocytopenia patients in China. China Journal of Pharmaceutical Economics 2018;13:11-6.
- Austin PC. The performance of different propensity-score methods for estimating differences in proportions (risk differences or absolute risk reductions) in observational studies. Stat Med 2010;29:2137-48. [Crossref] [PubMed]
- Beal SJ, Kupzyk KA. An introduction to propensity scores: what, when, and how. J Early Adolesc 2014;34:66-92. [Crossref]
- Ali MS, Prieto-Alhambra D, Lopes LC, et al. Propensity Score Methods in Health Technology Assessment: Principles, Extended Applications, and Recent Advances. Front Pharmacol 2019;10:973. [Crossref] [PubMed]
- Chen L, Lin LL, Lin S, et al. Cost-effectiveness Analysis of rhTPO and Interleukin-11 in the Treatment of Chemotherapy-related Thrombocytopenia. Chinese Journal of Modern Applied Pharmacy 2019;36:2445-9.
- Davagdorj K, Pham VH, Theera-Umpon N, et al. XGBoost-Based Framework for Smoking-Induced Noncommunicable Disease Prediction. Int J Environ Res Public Health 2020;17:6513. [Crossref] [PubMed]
- Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood 2002;100:3457-69. [Crossref] [PubMed]
- Ma SS, Ho SH, Ma SY, et al. The pharmacokinetic and pharmacodynamic properties of site-specific pegylated genetically modified recombinant human interleukin-11 in normal and thrombocytopenic monkeys. Eur J Pharm Biopharm 2017;119:185-91. [Crossref] [PubMed]
- Zou LL, Guo KL, Li L. Therapeutic effect of rhTPO and rhIL-11 in treatment of thrombocytopenia caused by chemotherapy of AML. Journal of Qiqihar University of Medicine 2016;37:4293-4.
- Wu RH, Su WY, Xing Z, et al. Study on effect and economic evaluation of rhTPO in treating thrombocytopenia caused by XELOX/SOX scheme chemotherapy. Chongqing medicine 2020;49:4070-4.
- Hou M, Li M, Jin Z. Post-marketing study of recombinant human thrombopoietin injection for adverse drug reaction monitoring. Chinese Journal of New Drugs and Clinical Remedies 2015;34:642-6.


