
Prevalence of chronic obstructive pulmonary disease among 48,061 digestive tract cancer patients in Europe
Introduction
Both digestive tract cancers and chronic obstructive pulmonary disease (COPD) represent major global health burden associated with a dismal prognosis in many patients. Importantly, digestive tract cancers and COPD share many different risk factors including alcohol and tobacco abuse, western diet and specific genetic aberrations. In the past, the relationship between digestive tract cancer and COPD has been extensively studied. As an example, it was suggested that cancer is more frequent in patients with COPD than patients without COPD (1-4); HR 2.8 (95% CI: 2.6–3.1). Lung, liver, colorectal, breast, prostate and stomach were the most frequent malignancies overrepresented in COPD (1). In addition, COPD is a relevant driver for the development of complications in the context of necessary anti-cancer treatments (5,6). Just recently, elevate arterial blood pressure, myocardial infarction, COPD and asthma were comorbidities with a high risk for increased mortality in patients with colorectal cancer receiving surgery (7). Finally, besides other factors, COPD was identified as a factor reducing patients’ adherence to cancer screening programs, potentially preventing cancer diagnosis in early disease stages (8). Despite these strong data showing an association between COPD and digestive tract cancers incidence as well as cancer-related morbidity, no comparative analyses regarding frequencies of COPD in digestive tract cancer patients from different countries have been published so far. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-200/rc).
Methods
Database
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study features a cross-sectional design. We analyzed the IQVIA’s Oncology Dynamics (OD) database, which has been extensively described elsewhere (9-11). This database is an anonymized cross-sectional survey of oncologists using a standardized online questionnaire. This survey contains only questions about drug-treated cancer patients.
Patient selection and study outcome
Surveys were conducted in Germany, France, United Kingdom (UK), Spain, and Italy. Included were patients with one of the four gastrointestinal tumors: esophageal (ICD-10: C15), stomach (ICD10: C16), colon (ICD-10: C18), and rectum (ICD-10: C20), filled in the time between January 1st 2017 and March 31st 2021. The outcome of the study was the proportion of cancer patients with a documentation of COPD diagnosis (ICD-10: J40, J41, J42, J43, J44).
Statistical analysis
The prevalence of COPD was calculated as the proportion of patients with COPD on all patients. Multivariable logistic regression was used to evaluate the association between cancer type and country and COPD diagnosis adjusted for age, sex and facility. Cancers of colon and rectum were analyzed separately and additionally as one group (colorectal). For further details see (9-11).
Results
Baseline characteristics
Overall, 48,061 people with a malignancy of the digestive tract (4,229 esophageal, 7,568 stomach, 27,300 colon, and 8,964 rectal cancer) documented by 811 physicians were analysed (Table 1). There was a significant difference in age from 64.1 years in patients with esophagus cancer to 66.0 years in colon cancer patients. Esophageal cancer was associated with highest frequency of males (73.7%), lowest rates were found in subjects having colon cancer (61.0%).
Table 1
| Variable | Esophagus | Stomach | Colon | Rectum | P value |
|---|---|---|---|---|---|
| N | 4,229 | 7,568 | 27,300 | 8,964 | |
| Age, year (mean, SD) | 64.1 (9.3) | 64.4 (10.9) | 66.0 (10.6) | 64.8 (10.7) | <0.001 |
| Males (%) | 73.7 | 64.5 | 61.0 | 62.5 | <0.001 |
| Facility (%) | |||||
| Hospital | 78.7 | 51.7 | 51.7 | 58.2 | <0.001 |
| Office based oncologist | 8.9 | 12.8 | 12.6 | 18.4 | |
| Unknown | 12.4 | 35.5 | 35.7 | 23.4 | |
| Country (%) | |||||
| Germany | 21.0 | 23.0 | 18.0 | 25.4 | <0.001 |
| France | 17.0 | 13.0 | 15,2 | 14.2 | <0.001 |
| Italy | 12.4 | 35.5 | 35.7 | 23.4 | <0.001 |
| Spain | 13.2 | 15.0 | 16,4 | 22.4 | <0.001 |
| UK | 36.4 | 13.5 | 14..6 | 14.6 | <0.001 |
SD, standard deviation.
Prevalence of COPD
Rates of COPD were highest among patients with esophageal (25.5%) and lowest in patients with rectal malignancies (9.8%). Patients with stomach and colon cancer had a COPD prevalence of 13.4% and 11.0%, respectively. In a multivariate regression model, esophageal cancer (OR: 3.88, 95% CI: 3.50–4.30, P<0.001), stomach cancer (OR: 1.49, 95% CI: 1.35–1.64, P<0.001) and colon cancer (OR: 1.12, 95% CI: 1.03–1.21, P=0.008) were significantly linked with a higher odd of COPD compared to rectal cancer as reference group (Table 2). In the sensitivity analyses using colorectal cancer as reference group, esophageal cancer (OR: 3.50, 95% CI: 3.22–3.78, P<0.001), stomach cancer (OR: 1.36, 95% CI: 1.26–1.46, P<0.001) were also linked to a higher odd of COPD.
Table 2
| Variable | Proportion of patients with COPD (%) | OR (95% CI) | P value |
|---|---|---|---|
| Age, year | |||
| ≤50 | 3.3 | Reference | |
| 50–60 | 8.0 | 2.34 (1.94–2.84) | <0.001 |
| 60–70 | 12.4 | 3.93 (3.28–4.70) | <0.001 |
| 70–80 | 17.2 | 6.02 (5.02–7.21) | <0.001 |
| >80 | 20.6 | 8.05 (6.61–9.80) | <0.001 |
| Sex | |||
| Male | 15.0 | 1.87 (1.76–2.00) | <0.001 |
| Female | 8.2 | Reference | |
| Cancer type | |||
| Esophagus | 25.5 | 3.88 (3.50–4.30) | <0.001 |
| Stomach | 13.4 | 1.49 (1.35–1.64) | <0.001 |
| Colon | 11.0 | 1.12 (1.03–1.21) | 0.008 |
| Rectum | 9.8 | Reference | |
| Country | |||
| Germany | 13.4 | 1.95 (1.76–2.16) | <0.001 |
| France | 11.9 | 1.65 (1.47–1.84) | <0.001 |
| Italy | 11.9 | 1.77 (1.60–1.96) | <0.001 |
| Spain | 16.8 | 2.65 (2.39–2.94) | <0.001 |
| UK | 8.4 | Reference |
GI, gastrointestinal; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Geographical variation
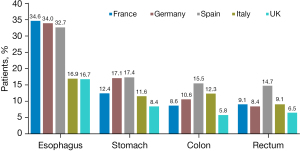
Among all cancer types, COPD prevalence was highest in Spain (16.8%; Figure 1), followed by Germany (13.4%). The highest COPD prevalence was among patients with esophageal cancer (34.6% in France, 34.0% in Germany, and 32.7% in Spain). The lowest prevalence of COPD was among rectum cancer patients with 14.7% in Spain, 9.1% in France, and 8.4% in Germany. In multivariate regression analysis, the odds for COPD was at highest in Spain (OR: 2.65, 95% CI: 2.39–2.94, P<0.001), followed by Germany (OR: 1.95, 95% CI: 1.76–2.16, P<0.001), Italy (OR: 1.77, 95% CI: 1.60–1.96, P<0.001) and France (OR: 1.65, 95% CI: 1.47–1.84, P<0.001), when compared to the UK (Table 2).
Discussion
In our analyses, we show that COPD is not not equally distributed between different tumor entities and largely varies between different countries and age groups. This ads a country-specific component to the previously available results from epidemiologic and clinical analyses, allowing the identification of previously unrecognized aspects regarding the role of COPD in patients with digestive tract cancer.
Digestive tract cancers belong to the most important malignancies worldwide with continuously rising prevalence rates (12). Similarly, COPD is an extremely common disease, causing a significant morbidity and mortality in almost all countries worldwide (11). COPD and digestive tract cancers share a variety of features and risk factors such as smoking and alcohol abuse. We therefore used the IQVIA Oncology Dynamics (OD) database to analyze potential associations between these cancers and COPD in five different countries. We clearly show that COPD rates are highest in patients with esophageal cancer, while COPD prevalence was lowest among patients with rectal cancer. Unfortunately, our database does not allow to distinguish between the different histological entities of esophageal cancer. However, given the relatively low COPD rates in gastric cancer and the widely overlapping risk profiles of COPD and squamous cell carcinoma (SCC) of the esophagus (13), it seems likely that the effect is driven by this cancer subtype rather than adenocarcinoma of the esophagus. Notably, the distribution of COPD was very heterogeneous between different European countries. Interestingly, Spain and Germany displayed higher frequencies of COPD in digestive tract cancer patients while the association was rather weak in the UK. On the one hand, this finding might reflect the different distribution of COPD in the different countries, on the other hand it might also reflect the different relevance of COPD as a risk factor in the different countries. In addition, these data also raise the question of other risk factors in the context of COPD that could influence the role as a factor in the development of cancer. Conceivable, for example, would be certain genetic alterations that are distributed differently in different countries. In line with this hypothesis, we demonstrate that the cancer promoting effect of COPD varies between male and female patients as well as different age groups. Finally, differences between the respective health care systems could also be conceivable as specific factors that could explain the observed differences.
Using of data from a large cohort of patients from different European countries, helped us to better understand the intercountry variation of COPD rates in digestive tract cancer. However, our study has important limitations that needs to be considered in the interpretation of the results. Most importantly, the original questionnaire was intended for use as a research tool. Moreover, different factors that may influence carcinogenesis in COPD patients such as the genetic and/or socioeconomic status as well as smoking behavior and alcohol were not part of the questionnaire. Next, due to the cross-sectional design, there is no information on the order of event enabling to identify if cancer or COPD was diagnosed first, therefore no causal relationships but only associations can be given from the data presented here. Finally, no data regarding lung function were recording within the database. Nevertheless, the database was used for many different studies and has demonstrated a cancer type distribution similar to the comparator sources (14).
In summary, our data demonstrate that COPD is differentially associated with digestive tract cancers. Notably, despite showing some variation, this association was observed in five different European countries, underlining the role of COPD as a potential cancer promoting factor in gastrointestinal malignancies. Results from this study clearly demonstrate that along with pulmonary cancers also digestive tract cancers should be considered in patients with COPD to improve long-term prognosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-200/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-200/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-200/coif). Karel Kostev and Laura Hoyer are employees of IQVIA. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). An individual consent form was not obtained following the national and European legislation, as the used database contains only fully anonymized data.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ho CH, Chen YC, Wang JJ, et al. Incidence and relative risk for developing cancer among patients with COPD: a nationwide cohort study in Taiwan. BMJ Open 2017;7:e013195. [Crossref] [PubMed]
- Ahn SV, Lee E, Park B, et al. Cancer development in patients with COPD: a retrospective analysis of the National Health Insurance Service-National Sample Cohort in Korea. BMC Pulm Med 2020;20:170. [Crossref] [PubMed]
- Menon S, Nightingale P, Trudgill N. Chronic Obstructive Pulmonary Disease and the Risk of Esophagitis, Barrett's Esophagus, and Esophageal Adenocarcinoma: A Primary Care Case-Control Study. J Clin Gastroenterol 2019;53:e451-5. [Crossref] [PubMed]
- Mascalchi M, Luconi M. Lung Cancer Screening, Emphysema, and COPD. Chest 2021;159:1699-700. [Crossref] [PubMed]
- Gonçalves Pereira R, Branco J, Narciso Rocha F, et al. MAJOR PULMONARY SURGERY IN PATIENTS WITH COMPROMISED LUNG FUNCTION. Port J Card Thorac Vasc Surg 2021;28:25-32. [PubMed]
- Quintana JM, Anton-Ladislao A, Lázaro S, et al. Effect of comorbidities on long-term outcomes of colorectal cancer patients. Eur J Cancer Care (Engl) 2022;31:e13561. [Crossref] [PubMed]
- van den Bosch T, Warps AK, de Nerée Tot Babberich MPM, et al. Predictors of 30-Day Mortality Among Dutch Patients Undergoing Colorectal Cancer Surgery, 2011-2016. JAMA Netw Open 2021;4:e217737. [Crossref] [PubMed]
- Samuel G, Kratzer M, Asagbra O, et al. Facilitators and barriers to colorectal cancer screening in an outpatient setting. World J Clin Cases 2021;9:5850-9. [Crossref] [PubMed]
- Zhao Z, Pelletier E, Barber B, et al. Major surgery in patients with metastatic colorectal cancer in Western europe. J Gastrointest Cancer 2012;43:456-61. [Crossref] [PubMed]
- Marchetti P, Maass N, Gligorov J, et al. Patient database analysis of fulvestrant 500 mg in the treatment of metastatic breast cancer: A European perspective. Breast 2017;32:247-55. [Crossref] [PubMed]
- Chambers P, Man KKC, Lui VWY, et al. Understanding Molecular Testing Uptake Across Tumor Types in Eight Countries: Results From a Multinational Cross-Sectional Survey. JCO Oncol Pract 2020;16:e770-8. [Crossref] [PubMed]
- Xie Y, Shi L, He X, et al. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep (Oxf) 2021;9:91-104. [Crossref] [PubMed]
- Lagergren J, Smyth E, Cunningham D, et al. Oesophageal cancer. Lancet 2017;390:2383-96. [Crossref] [PubMed]
- Alymova S, Kostev K, Casey V, et al. Evaluation of the representativeness of the German Oncology Dynamics dataset. Int J Clin Pharmacol Ther 2022;60:207-16. [Crossref] [PubMed]


