
A retrospective study: screening failure analysis of 1,058 healthy volunteers in phase I clinical trials
Introduction
Phase I clinical trials are a type of preliminary clinical pharmacology and health safety evaluation study. Their purpose is to observe the degree of human tolerance and the pharmacokinetic characteristics of new drugs, and to provide a basis for subsequent drug delivery plans (1-4). The vast majority of phase I clinical trials randomly enroll healthy volunteers, except for some toxicity trials which recruit patients to participate, providing safety and ethics for the people who take the drugs. Healthy volunteers are defined as people who volunteer to take part in research with no serious mental and physical diseases, as well as no history of drug and alcohol abuse. They are also qualified in the screening examination, including the assessment of vital signs, physical examinations, laboratory examinations, imaging examinations, and electrocardiogram (ECG), among others (5,6). Qualified healthy volunteers will minimize the damage of adverse drug reactions and help to acquire experimental scientific data. The quality of healthy subjects largely determines the quality of clinical trials (7). However, there are no expected therapeutic benefits for healthy volunteers who randomly participate in phase I trials and they will face potential uncertain health risks when participating in these studies (8). Moreover, it is difficult to define healthy subjects, as the screening standards of various research centers are not unified. As a result, the screening success ratio in phase I clinical trials is low, and the screening process of the trials consumes a significant amount of human and material resources, but the results are unsatisfactory (9). To our knowledge, there is still no large survey and complete cause analysis and also annual analysis to date of screening failure in healthy subjects in phase I clinical trials in China. In our study, we present the current situation of screening failure of a total of 1,058 healthy volunteers in 11 phase I clinical trials from October 2018 to June 2021 in our hospital. The issues that affect volunteer screening failure are discussed. This may help investigators and sponsors plan protocols and recruitment plans better, optimize the screening process, define normal ranges with reasonable variations, and develop more pragmatic targets. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-767/rc).
Methods
We retrospectively surveyed a total of 1,466 healthy volunteers in 11 phase I clinical trials from October 2018 to June 2021 in our hospital, of which 1,058 subjects failed the screening.
Inclusion criteria
Healthy volunteers who conformed to the following criteria were included: (I) be able to understand and be willing to strictly abide by the clinical trial, complete the trial, and sign the informed consent; (II) men and women aged 18–65; (III) males who weighed ≥50.0 kg and females who weighed ≥45.0 kg, and body mass index (BMI) was in the range of 19.0–26.0 kg/m2 (in some trials the range was 19.0–28.0 kg/m2); (IV) in good health, without a history of serious and chronic diseases such as respiratory system, circulatory system, digestive system, urinary system, blood system, endocrine system, immune system, nervous system, and psychiatric diseases, among others; (V) subjects (including partners) had no pregnancy plan from 2 weeks before the screening period to 3 months after the last administration, and took appropriate contraceptive measures.
Exclusion criteria
Healthy volunteers who conformed to the following criteria were excluded: (I) subjects with specific allergic history (asthma, urticaria, etc.) or allergic constitution (such as those allergic to drugs or food such as milk and pollen), or allergic to drug components or analogues; (II) those who have special dietary requirements and cannot accept a unified diet; (III) those who cannot tolerate venipuncture and have a history of needle fainting and blood fainting; (IV) for physical examination, vital sign detection, ECG, laboratory examinations, and imaging examinations the investigator’s judgment was abnormal and clinically significant; (V) hepatitis B surface antigen, Treponema pallidum specific antibody, human immunodeficiency virus antibody, and hepatitis C virus antibody were clinically significant; (VI) regular drinkers within 6 months before screening, i.e. drinking more than 14 units of alcohol per week (1 unit = 360 mL beer or 45 mL spirits with 40% alcohol or 150 mL wine); (VII) smoking ≥5 cigarettes per day within 3 months before screening; (VIII) blood donation or massive blood loss (≥400 mL) within 3 months before screening; (IX) those who had taken the study drug or participated in any clinical trial within 3 months before screening; (X) those who had undergone surgery within 30 days before screening or planned surgery during the study; (XI) those who had taken any prescription drugs, over-the-counter drugs, health care products, vitamins, and Chinese herbal medicines within 14 days before check-in; (XII) those who had eaten grapefruit, pitaya, mango, and other fruits or related products that affect metabolic enzymes within 7 days before check-in; (XIII) drinking too much tea, coffee, and/or caffeinated drinks (more than 8 cups per day, 1 cup =250 mL) within 7 days before check-in, and consuming chocolate or any food containing caffeine or xanthine within 48 hours before administration; (XIV) those who had consumed any alcohol products within 48 hours before check-in, or the alcohol test result was >0 mg/100 mg; (XV) female subjects were lactating or had positive serum pregnancy results; (XVI) persons with a history of drug abuse or positive urine drug screening; (XVII) subjects who may not be able to complete the study for other reasons or the investigators thought they should not be included.
Subjects who conformed to the above inclusion criteria and did not meet the exclusion criteria were randomly included in the trials.
Screening design and administration
Our study and the 11 trials in our analysis were conducted in accordance with the Declaration of Helsinki (as revised in 2013), and the 11 trials were approved by ethics board of Cangzhou Central Hospital. In this study, since we just collected the past trial information, ethical approval was waived by ethics board of Cangzhou Central Hospital. Individual consent for this retrospective analysis was waived. The volunteers were recruited through the internet, posted advertisements, and word of mouth from the investigators and contacting recruitment companies. During the trial recruitment, all candidate subjects had sufficient consideration and selection time, and the phase I clinical trial department offered telephone consultation. All subjects who voluntarily participated in the 11 trials signed the informed consent form, and they retained a valid copy of the informed consent form at the same time (10,11). The subjects were considered to be in good health, and their personal height and weight, recent smoking history, drinking history, diet, and other basic conditions met the trial’s requirements when they signed the informed consent form. Volunteers who did not obtain informed consent and did not sign the informed consent form were not allowed to enter the trial. After signing the informed consent form, the subjects underwent a trial duplication check, demographic information collection, height and weight measurement, vital sign examination (one retest opportunity), medical history inquiry, physical examination, and ECG. After passing the above non-invasive examinations, the volunteers underwent laboratory tests [including blood routine, blood biochemistry, human chorionic gonadotropin (hCG), infectious markers, and routine urine tests], imaging examinations (feasible only if the serum pregnancy results of female subjects were normal), urine test for drug abuse, and alcohol abuse among others. According to the research protocols, coagulation marker tests, routine fecal tests, X-ray, ultrasonic examination, and nicotine detection would be added as appropriate. During the screening process, no subsequent inspection would be conducted if any inspection failed. Subjects could withdraw from the project at any time without giving any reasons. For the evaluation of abnormal results of all laboratory tests, clinicians with rich screening experience judged the medical decision level (MDL) of each index, and researchers carefully analyzed and comprehensively judged whether it had clinical significance (12). Qualified volunteers would be selected in the order of screening number from small to large when the number of volunteers exceeded the requirements. Reference values of the main laboratory examinations are listed in Table 1.
Table 1
| Test item | Reference value* |
|---|---|
| Blood routine | |
| Total white blood cells, 109/L | 3.5–10.0 |
| Red blood cell count, 1012/L | 3.50–5.50 |
| Hemoglobin, g/L | 110–160 |
| Platelet count, 109/L | 100–300 |
| Neutrophil count, 109/L | 1.8–6.4 |
| Percentage of neutrophils, % | 40.0–75.0 |
| Lymphocyte count, 109/L | 1.0–3.3 |
| Percentage of lymphocytes, % | 18.0–40.0 |
| Eosinophil count, 109/L | 0.05–0.50 |
| Percentage of eosinophils, % | 0.0–5.0 |
| Basophil count, 109/L | 0.0–0.1 |
| Percentage of basophils, % | 0.0–1.0 |
| Monocyte count, 109/L | 0.2–1.0 |
| Percentage of monocytes, % | 3.5–10.0 |
| Blood biochemistry | |
| Alanine aminotransferase, U/L | 7.0–50.0 |
| Aspartate aminotransferase, U/L | 13.0–40.0 |
| γ-glutamyltransferase, U/L | 7–60 |
| Alkaline phosphatase, U/L | 35–135 |
| Total bilirubin, μmol/L | 3.4–23.0 |
| Direct bilirubin, μmol/L | 0.0–6.8 |
| Urea nitrogen, mmol/L | 2.6–9.5 |
| Creatinine, μmol/L | 41–111 |
| Uric acid, μmol/L | 155–428 |
| Blood glucose, mmol/L | 3.90–6.10 |
| Total cholesterol, mmol/L | <5.20 |
| Triglyceride, mmol/L | 0–1.7 |
| Low density lipoprotein cholesterol, mmol/L | <3.12 |
| High density lipoprotein cholesterol, mmol/L | 1.04–1.55 |
| Potassium, mmol/L | 3.5–5.3 |
| Phosphorus, mmol/L | 0.85–1.51 |
| Calcium, mmol/L | 2.15–2.52 |
| Sodium, mmol/L | 137.0–147.0 |
| Chlorine, mmol/L | 96–110 |
| Creatine kinase, U/L | 40–310 |
| Creatine kinase isoenzyme, U/L | 0.0––25.0 |
| Coagulation routine | |
| Fibrinogen, g/L | 1.8–4 |
| Prothrombin time, s | 10–14 |
| Thrombin time, s | 14–21 |
| Partial thromboplastin time, s | 23.3–32.5 |
| Urinary routine | |
| Urinary leukocyte | Negative |
| Occult blood, mg/dL | Negative |
| Protein, mg/dL | Negative |
| Glucose, mg/dL | Normal |
| Red blood cell count, /uL | 0–17 |
| Total white blood cells, /uL | 0–28 |
| Fecal occult blood | Negative |
*, part of the reference value range fluctuates slightly with the change of the test kits.
Statistical analysis
Data on all participants who failed the screening for the study were analyzed and reasons for their non-randomization were classified with SPSS 25.0 (descriptive analysis), as well as the differences between the four screening years [2018–2021].
Results
Screening
This study collected the project information of 1,058 volunteers who failed screening in 11 clinical trials in the phase I clinical trial laboratory of our hospital from October 2018 to June 2021. A total of 1,466 healthy volunteers were screened in 11 clinical trials, and 408 of them were successfully enrolled. The total screening success ratio was 27.8%, of which the highest screening success ratio was 38.5% and the lowest was only 18.2% (Figure 1).
Demographic information
The average age of the 1,058 subjects who failed the screening was 34±8.57 years old, and 766 of them were under 40 years old (including 40 years old), while 292 were over 40 years old. A total of 705 volunteers were males and 353 were females. Height and weight information was collected from 1,039 subjects, including 792 cases with BMI in the range of 19.0–26.0 kg/m2 and 247 cases with BMI greater than 26.0 kg/m2. Physical information was collected from 1,012 subjects, including 111 manual workers and 901 non-manual workers (Table 2).
Table 2
| 2018, n | 2019, n | 2020, n | 2021, n | Total, n | |
|---|---|---|---|---|---|
| Age, years | |||||
| 18–40 | 143 | 166 | 267 | 190 | 766 |
| >40 | 116 | 43 | 75 | 58 | 292 |
| Sex | |||||
| Male | 159 | 154 | 221 | 171 | 705 |
| Female | 100 | 55 | 121 | 77 | 353 |
| BMI (kg/m2) | |||||
| 19–26 | 137 | 170 | 282 | 203 | 792 |
| >26 | 119 | 38 | 49 | 41 | 247 |
| Physical labor | |||||
| Yes | 23 | 33 | 17 | 38 | 111 |
| No | 233 | 175 | 314 | 179 | 901 |
BMI, body mass index.
Reasons for failure in volunteer screening
Of the 1,058 volunteers, we obtained a total of 1,196 failure reasons for some participators failed for more than one reason. A total of 247 subjects (23.3%) had abnormal blood biochemistry examination results, including 176 males and 71 females. A total of 204 cases (19.3%) were unqualified in terms of their vital signs (blood pressure, pulse, and respiration), including 144 males and 60 females. ECG was abnormal in 176 subjects (16.6%), and 148 subjects (14.0%) had abnormal blood routine examination. A total of 102 subjects (9.6%) failed the screening due to unqualified BMI, including 73 males and 29 females. A total of 92 subjects (8.7%) had abnormal urine/fecal examination results, of which 84 cases had unqualified urine test results. Additionally, 80 (7.6%) subjects voluntarily withdrew from the trial due to personal reasons, 36 subjects (3.4%) had abnormal chest X-ray, 29 (2.7%) subjects failed in consultation, and 19 subjects (1.8%) failed in routine coagulation examination results. Seventeen subjects (1.6%) failed the duplicate test, in that they had participated in other trials within 3 months before participating in this trial screening in our hospital. Fourteen subjects (1.3%) were not included in the trial due to the full number of qualified candidates being reached and 9 (0.8%) failed in routine coagulation examination. Six subjects (0.5%) failed nicotine detection, 5 subjects failed in physical examination, and 5 subjects (0.4%) were considered by the investigators to be unsuitable to participate in the trial. There were 4 female volunteers (0.3%) who had a hCG positive result. Only 1 person failed in a previous visit inquiry, alcohol detection, and urine drug detection (Figure 2).
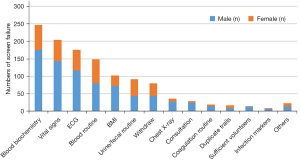
Blood biochemistry is the first cause of screening failure. The main items include liver function, renal function, blood lipid, blood glucose, electrolytes, and myocardial enzymes (Table 3). There were 77 cases of abnormal liver function, including 61 male and 16 females, aged 32.6±8.4 years, with a BMI of 23.9±2.1 kg/m2. Of these, a total of 74 cases had their physical labor information collected, including 7 cases of physical labor and 67 cases of non-physical labor. There were 59 cases of abnormal renal function, 44 cases of elevated uric acid (up to 626 mmol/L), and 6 cases of decreased creatinine. Among the 59 subjects, 45 were male and 14 were female, aged 32.3±8.1 years, with a BMI of 23.9±2.0 kg/m2. All cases had physical labor information collected, including 8 cases of physical labor and 51 cases of non-physical labor. There were 58 cases of dyslipidemia, including 40 males and 18 females, aged 32.6±8.4 years, with a BMI of 23.9±2.0 kg/m2. Of these, there were 9 cases of manual labor and 49 cases of non-manual labor. There were 39 cases of abnormal blood glucose, including 28 males and 11 females, aged 32.8±8.5 years, with a BMI of 23.9±2.0 kg/m2. Of these, there were 38 cases who had physical labor information collected, including 5 cases of physical labor and 33 cases of non-physical labor. There were 21 cases with abnormal electrolytes, 10 cases with abnormal blood potassium, and 7 cases with abnormal blood phosphorus. Among the 21 subjects, there were 14 males and 7 females, aged 32.6±8.4 years, with a BMI of 23.9±2.0 kg/m2. Of these, there were 2 cases of manual labor and 19 cases of non-manual labor. There were 14 cases with abnormal myocardial enzymes, including 10 males and 4 females, aged 32.7±8.4 years, with a BMI of 24.0±2.1 kg/m2. Of these, there was 1 case of manual labor and 13 cases of non-manual labor. Some volunteers had 2 or more biochemical abnormalities, including 7 cases of liver function abnormalities and dyslipidemia, while 6 cases had liver function and renal function abnormalities.
Table 3
| Liver function, n | Renal function, n | Blood lipids, n | Blood glucose, n | Electrolytes, n | Myocardial enzymes, n | Total, n* | |
|---|---|---|---|---|---|---|---|
| Age, years | |||||||
| 18–40 | 66 | 49 | 36 | 24 | 15 | 12 | 202 |
| >40 | 11 | 10 | 22 | 15 | 6 | 2 | 66 |
| Sex | |||||||
| Male | 61 | 45 | 40 | 28 | 14 | 10 | 198 |
| Female | 16 | 14 | 18 | 11 | 7 | 4 | 70 |
| BMI (kg/m2) | |||||||
| 19–26 | 70 | 45 | 50 | 26 | 20 | 13 | 224 |
| >26 | 7 | 14 | 8 | 13 | 1 | 1 | 44 |
| Physical labor | |||||||
| Yes | 7 | 8 | 9 | 5 | 2 | 1 | 32 |
| No | 67 | 51 | 49 | 33 | 19 | 13 | 232 |
*, some volunteers had 2 or more abnormal biochemical items, and some volunteers did not accept the height and weight measurement. BMI, body mass index.
In our study, vital signs were the second cause of screening failure. A total of 180 volunteers failed due to high blood pressure, including 133 males and 47 females, aged 37.9±8.5 years, with a BMI of 25.2±2.0 kg/m2. Of these, 176 had physical labor information collected, including 27 cases of physical labor and 149 cases of non-physical labor. Among the volunteers with high blood pressure, 33 cases had a fast pulse, including 17 males and 16 females. There were 47 volunteers with a fast pulse, including 22 males and 25 females, aged 33.9±8.8 years, with a BMI of 25.3±2.5 kg/m2. Of these, there were 4 cases of manual labor and 43 cases of non-manual labor. Five volunteers failed the screening due to low blood pressure, including 1 males and 4 females. Five volunteers failed the screening because of a slow pulse, and all of them were non-manual workers.
ECG abnormality was the third cause of screening failure in this study, mainly including sinus bradycardia, wave form abnormality, PR interval abnormality, QT/QTc abnormality, and conduction block, among others. There were 50 cases of sinus bradycardia (with heart rate ranging from 47 to 59 bpm), including 40 males and 10 females, aged 33.7±8.7 years, with a BMI of 23.6±2.2 kg/m2. Of these, 49 cases had physical labor information collected, including 8 cases of physical labor and 41 cases of non-physical labor. There were 40 cases of abnormal wave form (elevation or low level), including 19 males and 18 females, aged 33.6±8.8 years, with a BMI of 23.8±2.2 kg/m2. Of these, there were 9 cases of manual labor and 28 cases of non-manual labor. The PR interval was abnormal in 29 cases, prolonged in 7 cases (insufficient to diagnose conduction block), and shortened in 22 cases (99–119 ms). The QT/QTc interval was abnormal in 23 cases, prolonged in 8 cases (432–478 ms), and shortened in 15 cases (270–339 ms). Different types of conduction block occurred in 9 cases, including 4 cases of complete right bundle branch block, 2 cases of left anterior branch conduction block, and 3 cases of first degree atrioventricular block. In addition, some volunteers had pre-excitation syndrome, frequent atrial premature beats, and left ventricular high voltage, among other conditions.
Although blood routine is only the fourth cause of screening failure, it is still a common cause of screening failure in clinical trials. In our study, 148 subjects failed due to blood routine examination results. According to the number of cases, the items in order were elevated platelet count, elevated lymphocyte percentage/count, abnormal hemoglobin, abnormal neutrophil count/percentage, abnormal leukocyte, elevated absolute value/percentage of eosinophils, abnormal absolute value of monocytes, and elevated percentage of basophils. The platelet counts of 51 subjects increased, including 12 cases above 400×109/L, 21 cases of 350×109/L–400×109/L, and 18 cases of 330×109/L–350×109/L. There were 38 patients with elevated lymphocyte percentage/count, including 9 patients with abnormal neutrophil count/percentage. There were 30 cases of abnormal hemoglobin, 13 cases of elevated hemoglobin (178–188 g/L), and 17 cases of decreased hemoglobin (85–110 g/L). There were 23 cases of abnormal neutrophils, of which 7 cases had abnormal leukocyte count. The absolute value/percentage of eosinophils increased in 11 subjects, the absolute value of monocytes was abnormal in 4 cases, and the percentage of basophils increased in only 3 cases.
Distribution of unqualified examinations in screening
In our study, 1,044 subjects (98.9%) failed the screening examination due to less than 2 examinations (including 2 examinations). Among them, 936 (88.5%) subjects failed 1 examination, including 235 in 2018, 170 in 2019, 303 in 2020, and 228 in 2021. A total of 107 subjects (10.4%) failed 2 tests, including 22 in 2018, 29 in 2019, 36 in 2020, and 20 in 2021. Most of the 2 unqualified examinations were blood routine and urine routine, blood routine and blood biochemistry, and blood biochemistry and urine routine. A total of 14 cases (1.0%) failed 3 screening examinations, only 1 case failed in 4 screening examinations, and no subjects failed more than 4 examinations (Table 4).
Table 4
| Unqualified examination numbers | 2018, n | 2019, n | 2020, n | 2021, n | Total (%) |
|---|---|---|---|---|---|
| 1 | 235 | 170 | 303 | 228 | 936 (88.5) |
| 2 | 22 | 29 | 36 | 20 | 107 (10.4) |
| 3 | 1 | 10 | 3 | 0 | 14 (1.0) |
| 4 | 1 | 0 | 0 | 0 | 1 (0.1) |
| Total | 259 | 209 | 342 | 248 | 1,058 (100.0) |
Annual distribution of screening failure reasons
A total of 259 healthy volunteers failed screening in 2018. The top 3 causes of screening failure were vital signs, ECG, and blood biochemistry. Among them, 75 cases (29.0%) failed due to vital signs, 49 cases (18.9%) failed due to ECG, and 38 cases (14.7%) failed due to blood biochemistry. In 2019, 209 healthy volunteers failed screening. Blood biochemistry, ECG, and blood routine were the top 3 causes. Among them, 51 cases (24.4%) failed due to blood biochemical screening, 44 cases (21.1%) failed due to ECG screening, and 43 cases (20.6%) failed due to blood routine screening. In 2020, 342 healthy volunteers failed screening. The top 3 causes of screening failure were blood biochemistry, vital signs, and ECG. Among them, 99 cases (28.9%) failed due to blood biochemistry screening, 55 cases (16.1%) failed due to vital sign screening, and 54 cases (15.8%) failed due to ECG screening. In 2021, 248 healthy volunteers failed screening. The top 3 causes of screening failure were blood biochemistry, vital signs, and blood routine. Among them, 59 cases (23.8%) failed due to blood biochemical screening, 48 cases (19.4%) failed due to vital sign screening, and 39 cases (15.7%) failed due to blood routine screening (Figure 3).
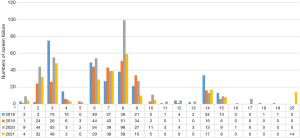
Abnormal blood biochemistry was the main reason for screening failure between 2019 and 2021, except in 2018 in which it was the second reason. Compared with 2018, the proportion of vital sign abnormalities decreased in the most recent 3 years, but there were still small-scale fluctuations (29.0%, 12.4%, 16.1%, and 19.4% in turn). From 2018 to 2021, ECG decreased by 18.9%, 21.1%, 15.8%, and 11.7%, in respectively, and the withdrawn proportion decreased (13.1%, 7.2%, 3.8%, and 6.9%, respectively). Chest X-ray had small-scale fluctuations of 5.0%, 2.9%, 2.6%, and 3.2% respectively, and the proportions of consulting abnormalities were 5.8%, 2.9%, 1.5%, and 1.2%, respectively. Blood routine failure fluctuated by 10.4%, 20.6%, 11.4%, and 15.7% respectively. The number of volunteers met the requirements in 2021 only, and some qualified subjects did not participate in the trials.
Discussion
To our knowledge, this is the largest survey and complete cause analysis to date of screening failure in healthy subjects in phase I clinical trials in China. The purpose of phase I clinical trials is to determine the safety and toxicity of the study drug or scheme and determine the recommended phase II dose (RP2D), which plays an important role in the follow-up of clinical trials and even the drug registration and marketing. The success or failure of screening of healthy subjects largely determines the quality and success of phase I clinical trials. Our results show that when screening failure of a large number of volunteers occurs, the research centers face losses of time and human and material resources. The 3 main reasons for screening failure are abnormalities in blood biochemistry tests, vital signs, and ECG. These results emphasize the need to continuously design new strategies in protocols enrolling volunteers to screen out qualified subjects. It is ethically and scientifically important to protect the safety of participants and the integrity of the trial data.
Our analysis showed that the total success ratio of 11 trials from 2018 to 2021 was only 27.8%, of which the highest was 38.5% and the lowest was only 18.2%, with an average of about 1/3. This is basically consistent with Li et al.’s research (13). Our screening success ratio was slightly higher than 21.8% of Wang’s study in Josephine Ford Cancer Center (14). It is accepted that most of the screening work of Wang’s study is carried out around tumor patients. Strict screening standards, patients’ own physique, disease changes, and other reasons will lead to screening failure. In addition, a study in India screened 156 volunteers, with a success ratio of 47.4% (15). To our knowledge, less than half of the screenings worldwide have been successful, and various research centers have consumed a significant amount of time and human and material resources.
We found that the proportion of screening failure due to 1–2 reasons was 98.9%, and more than 3 reasons accounted for only 1.1%, which was in line with our previous screening experience. To the best of our knowledge, there are no such large studies which have analyzed the number of failures. Among the reasons for screening failure, the proportion of non-laboratory and laboratory examinations was roughly similar. Among the non-laboratory examinations, the top 3 were vital signs, ECG, and BMI, while the top 3 laboratory examinations were blood biochemistry, blood routine, and urine/fecal routine.
In our study, blood biochemical examination ranked first among the causes of screening failure, except that it ranked second in 2018. From 2019 to 2021, blood biochemical examination ranked first, mainly including liver function, kidney function, and blood lipid, which is in line with some previous studies (16,17). The failure of blood inspection is inevitable. Firstly, the influencing factors of different indicators are mixed. For example, recent diets, work and rest time, activity intensity, and the stress state of volunteers may affect the results of liver function, kidney function, blood glucose, blood lipid, electrolyte, and muscle enzyme tests (18-21). In addition, hemolysis of samples may affect lactate dehydrogenase, aspartate transaminase, and kalemia test (22,23). Secondly, problems such as different testing equipment and reagents in different regions and different races will lead to different reference ranges for laboratory tests (22,23). As a result, it is difficult for researchers in different research centers to define the scope of no clinical significance (NCS). Also, there are no guidelines in place defining acceptable normal ranges for key safety parameters permitting enrollment of a healthy subject into a phase I clinical trial. Ideally, we prefer to recommend unified judgment according to the laboratory reference range, but this will inevitably lead to a very high screening failure ratio. At present, most researchers prefer to determine an allowable fluctuation range according to the laboratory reference range. Those within the fluctuation range are determined as NCS. They believe that these slight abnormal changes may be caused by many confounding factors such as physiological changes rather than the abnormalities of the volunteers themselves (24-29). However, whether these volunteers that investigators determined NCS are healthy has always been a controversial issue. Thus, the assessment of healthy volunteers to determine their eligibility for clinical trials can never be a simple tick box approach but always remains a complex medical decision which requires the clinical judgement of adequately trained and experienced investigators (30). Considering the above problems, the authors suggest that investigators can strengthen the adequacy of notification and fully inform volunteers of the benefits of a healthy diet, regular work and rest, and appropriate activities, among others. At the same time, significant attention should be paid to the process of sample collection and transportation to reduce problems such as sample hemolysis and contamination. For slightly abnormal results, it is recommended to formulate a unified NCS standard in combination with the requirements of the project, the characteristics of the study drug, and the overall situation of the enrolled population, so as to avoid bias in the research data as far as possible and reduce the consumption of resources. At present, different NCS standards are not recommended for different volunteers in the same trial. This judgment can better take into account the individual factors of different volunteers, but the subjective factors of investigators and the bias of data are inevitable. Although blood routine examination is the fourth cause of screening failure, it also involves the above problems.
We also found that abnormal vital signs have always been a common cause of screening failure in clinical trials. The main problems are high blood pressure and fast pulse, which may be mainly caused by the nervousness of volunteers participating in the trials. It has not been ruled out that some people may experience the white coat effect. Volunteers from other places may not have had a good rest and others may sleep less due to long-term work, rest habits, and professional night shifts. It may also be that they have high blood pressure and fast pulse and fail to find them in time in the early stage, resulting in the early development of hypertension or nodal tachycardia. As vital sign measurement is easy to operate, feasible, and non-invasive, we strongly recommended that investigators arrange the examination as soon as possible to quickly find unqualified volunteers and save human and material resources to the greatest extent. Given that blood pressure is greatly affected by emotion and activity, it is recommended to have a full rest before the examination and provide a re-test opportunity. We are pleased to find that compared with 2018, the proportion of screening failures caused by abnormal vital signs has decreased in recent years, possibly because researchers have paid more and more attention to the role of being informed early and early education. At the same time, due to the gradual progress of clinical trial science popularization, more and more volunteers understand and even participate in trials. In addition, although there are relatively few cases of low blood pressure and slow pulse, it still needs the attention of researchers. These patients are often more prone to suffer adverse reactions. For the problem of body temperature, while considering the ambient temperature of the screening site, it is still necessary to pay attention to the current epidemic situation and the high incidence of seasonal influenza to reduce unnecessary infection.
ECG also plays an irreplaceable role in the evaluation of volunteers’ heart condition, and is a necessary tool in almost all clinical trials. The volunteers had ECG abnormalities in a variety of situations, mainly sinus bradycardia, which constituted the third cause of screening failure in our study. Communicating with most volunteers, we learned that these people often spent more time performing daily exercise, and some of them were even sports athletes or fitness coaches. Although the heart rate of these people was lower than the low limit of normal value, it did not mean that their heart condition was abnormal. It has been reported in a previous study that sinus bradycardia is not uncommon in healthy subjects and may possibly be due to physiological changes in vagal tone, diurnal variations, or the effect of food intake (31). In another survey, researchers observed normal sinus rhythm in only 13% of 156 healthy volunteers throughout the entire observation period during 24-hour ambulatory ECG recordings (32). We suggest that when judging the results of this part of the examination, the researchers can appropriately relax the standards according to the trial, but pay special attention to the drugs that can cause slow heart rate or low blood pressure, and adopt more strict standards to guard against unnecessary injury. According to the data analysis in the past 4 years, the proportion of ECG screening failure decreased, and there were fewer and fewer ECG pathological abnormalities, which is benefited from people’s attention to the annual physical examination.
In addition to the failure of the above screening examination due to objective reasons, some subjective factors in the screening process also warrant attention. Firstly, the major incentive for most participants has been repeatedly shown to be monetary. There is a mountain of evidence that some of these healthy volunteers, especially professional volunteers, might misrepresent their medical histories to enroll in clinical trials and that these participants would not qualify otherwise (33-38). Secondly, qualified volunteers voluntarily quit, due to participating in healthy examination free of charge, time arrangement, long distance, group effect, peer participation, and dissatisfaction with trial compensation (39,40). Thirdly, the researchers may decide that it is not suitable for the subjects to participate in the trial. In addition to the comprehensive evaluation of the volunteers’ health, judging compliance also plays an important role. Since this judgment is often subjective, it is suggested that multiple researchers reach an agreement.
Demographic information such as the age and BMI of volunteers may deem them as unqualified, which may be related to failure to clearly understand the project requirements when signing the informed consent. For failure due to infectious diseases, although there were not many cases in our study, attention should be paid to this reason. For volunteers who suffered certain diseases and did not know in advance, attention should be paid to privacy protection and volunteers should be informed in time. Four female volunteers had positive pregnancy test results in this study. Before the screening work, we considered the possibility of this situation. The imaging screening of all female volunteers was arranged after confirming that the blood pregnancy test was negative to reduce unnecessary injury. However, due to the limitations of current laboratory methods, it is impossible to determine very early pregnancy. Therefore, investigators still need to fully educate and inform both male and female volunteers who are of childbearing age. In the past, we also found that the identity card of volunteers was lost or expired and they could not be identified. It is necessary to inform volunteers during the recruitment process in detail. It is worth noting that there is a problem of obtaining a sufficient number of qualified volunteers, which also causes a waste of resources. Therefore, it is recommended to develop a comprehensive expected screening plan before the trial, give subjects sufficient information, and consider giving certain compensation if necessary.
There are still some limitations in our study. Firstly, this study only evaluated failure from the perspective of researchers in the screening of healthy volunteers in phase I clinical trials. Comprehensive analysis of the causes from the perspectives of the pharmaceutical industry, contract research organizations, academia, ethics committees, and other competent authorities is necessary. Secondly, the inclusion and exclusion criteria of clinical trials involved in this study were slightly different. Some trials do not require blood lipid examination, coagulation function testing, stool routine testing, smoking examination, and physical labor collection, among other factors, which may lead to a lack of research results, but it does not mean that these examinations are not important in the statistics of screening failure. Thirdly, only the physical health of the volunteers is assessed before clinical trials, and there is little to no attention paid to their psychological health. This is not only related to volunteer compliance, but also to adverse events, and can even affect the blood parameters in terms pharmacokinetics and eventually influence the final results of the clinical trial (41-46). In addition, the determination of abnormal conditions in different trials is inconsistent. The investigators comprehensively determine the scope of NCS according to the metabolic mechanism of the test drug and possible adverse reactions, resulting in the screening failure criteria of different clinical trials not being completely consistent. Lastly, although we collect case data as much as possible, the number of female volunteers in studies is still insufficient, as fewer female volunteers want to participate in clinical trials worldwide. Our results only provide a trend or direction for follow-up researchers, and the exact results still need to be verified by large sample studies.
Conclusions
Our study confirms the high screening failure ratio of healthy volunteers in phase I clinical trials and shows the distribution of the failure reasons. The three main reasons for screening failure are abnormalities in blood biochemistry tests, vital signs, and ECG. Consequently, screening failure is a burdensome issue which various clinical trial sites must contend with (14,47). Urgent efforts should be made to better predict a patient’s likelihood of failing screening for any study. Investigators can still take some effective measures by strengthening the in-depth understanding of informed consent, paying attention to the quality of test samples, a correcting definition of NCS. Also, low-cost and non-invasive examinations can be arranged first to better protect the volunteers and reduce the screening costs of clinical trials (48,49). To our delight, we find people’s attention to the annual physical examination may help to screen healthy volunteers. Overall, this study shows that it is crucial and professional to develop a research plan to minimize the resultant impact on timelines and budgets of phase I clinical trials enrolling healthy volunteers.
Acknowledgments
We thank the valuable contributions of all the phase I clinical trial volunteers and the research staff in Cangzhou Central Hospital, Hebei Province, China for their invaluable support in collecting the data. We also thank research nurse Xinyue Huang who helped with the data entry.
Funding: This research was funded by Cangzhou key R&D program (No. 213106025) and Tianjin Natural Science Foundation (No. 20JCQNJC00190).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-767/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-767/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-767/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). In this study, since we just collected the past trial information, ethical approval was waived by ethics board of Cangzhou Central Hospital. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Drug Registration Regulations. Beijing: National Medical Products Administration, 2020-03-30. Available online: https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/fgwj/bmgzh/20200330180501220.html
- Karakunnel JJ, Bui N, Palaniappan L, et al. Reviewing the role of healthy volunteer studies in drug development. J Transl Med 2018;16:336. [Crossref] [PubMed]
- Young TC, Srinivasan S, Vetter ML, et al. A Systematic Review and Pooled Analysis of Select Safety Parameters Among Normal Healthy Volunteers Taking Placebo in Phase 1 Clinical Trials. J Clin Pharmacol 2017;57:1079-87. [Crossref] [PubMed]
- Pasqualetti G, Gori G, Blandizzi C, et al. Healthy volunteers and early phases of clinical experimentation. Eur J Clin Pharmacol 2010;66:647-53. [Crossref] [PubMed]
- Research on healthy volunteers. A report of the Royal College of Physicians. J R Coll Physicians Lond 1986;20:243-57. [PubMed]
- Griffin JP, Posner J, Barker GR. editors. The Textbook of Pharmaceutical Medicine. 7th Edition. John Wiley & Sons, 2013.
- Oh J, Yi S, Gu N, et al. Utility of Integrated Analysis of Pharmacogenomics and Pharmacometabolomics in Early Phase Clinical Trial: A Case Study of a New Molecular Entity. Genomics Inform 2018;16:52-8. [Crossref] [PubMed]
- Joubert P, Rivera-Calimlim L, Lasagna L. Commentary. The normal volunteer in clinical investigation: how rigid should selection criteria be? Clin Pharmacol Ther 1975;17:253-7. [Crossref] [PubMed]
- Blenkowski RS, Goldfarb NM. Screen failures in clinical trials: financial Roulette or the cost of doing business? J Clin Res Best Pract 2008. Available online: https://www.wcgclinical.com/magi/
- Koyfman SA, Reddy CA, Hizlan S, et al. Informed consent conversations and documents: A quantitative comparison. Cancer 2016;122:464-9. [Crossref] [PubMed]
- Guo B, Li D, Yuan Y. SPIRIT: A seamless phase I/II randomized design for immunotherapy trials. Pharm Stat 2018;17:527-40. [Crossref] [PubMed]
- Pravettoni G, Mazzocco K, Gorini A, et al. Understanding cognitive processes behind acceptance or refusal of phase I trials. Crit Rev Oncol Hematol 2016;100:69-73. [Crossref] [PubMed]
- Li B, Zhang Q, Liu Y, et al. Analysis of the reasons for screening failure in phase I clinical trials in China: a retrospective study of the clinical trials screening process. Ann Transl Med 2021;9:1564. [Crossref] [PubMed]
- Wang D, Pearce T, Cobani V, et a1. Lessons from the other side of clinical trial accrual: Screen failures at the Josephine Ford Cancer Center/Henry Ford Health System in 2010. J Clin Oncol 2011;29:Abstr 16624.
- Gogtay NJ, Thatte UM, Kulkarni PS. Frequency and causes for exclusion from randomization of healthy volunteers screened for a phase 1 study in India. Natl Med J India 2012;25:18-20. [PubMed]
- Wang JY, Li X, Chen C, et al. Analysis of Failure Reasons of Screening for Healthy Subjects in Phase I Clinical Trials of New Drug. Chinese Journal of Medicinal Guide 2018;20:760-4.
- Deiteren A, Coenen E, Lenders S, et al. Data driven evaluation of healthy volunteer characteristics at screening for phase I clinical trials to inform on study design and optimize screening processes. Clin Transl Sci 2021;14:2450-60. [Crossref] [PubMed]
- Cheng SQ, Zhang JF, Zhang ZF, et al. Influence of diet intake on liver function test. World J Gastroenterol 1997;3:250. [Crossref] [PubMed]
- Delanaye P, Cavalier E, Pottel H. Serum Creatinine: Not So Simple! Nephron 2017;136:302-8. [Crossref] [PubMed]
- Riby LM, Lai Teik Ong D, Azmie NBM, et al. Impulsiveness, postprandial blood glucose, and glucoregulation affect measures of behavioral flexibility. Nutr Res 2017;48:65-75. [Crossref] [PubMed]
- Jackson IM, McKiddie MT, Buchanan KD. Influence of blood-lipid levels and effect of prolonged fasting on carbohydrate metabolism in obesity. Lancet 1971;2:450-2. [Crossref] [PubMed]
- Hayashi K, Hitosugi T, Kawakubo Y, et al. Influence of measurement principle on total hemoglobin value. BMC Anesthesiol 2020;20:81. [Crossref] [PubMed]
- Heireman L, Van Geel P, Musger L, et al. Causes, consequences and management of sample hemolysis in the clinical laboratory. Clin Biochem 2017;50:1317-22. [Crossref] [PubMed]
- Grossfeld GD, Litwin MS, Wolf JS Jr, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology 2001;57:604-10. [Crossref] [PubMed]
- Leeflang MMG, Allerberger F. How to: evaluate a diagnostic test. Clin Microbiol Infect 2019;25:54-9. [Crossref] [PubMed]
- Jacobsen FK, Christensen CK, Mogensen CE, et al. Pronounced increase in serum creatinine concentration after eating cooked meat. Br Med J 1979;1:1049-50. [Crossref] [PubMed]
- Mercke Odeberg J, Andrade J, Holmberg K, et al. UGT1A polymorphisms in a Swedish cohort and a human diversity panel, and the relation to bilirubin plasma levels in males and females. Eur J Clin Pharmacol 2006;62:829-37. [Crossref] [PubMed]
- Pocock SJ, Ashby D, Shaper AG, et al. Diurnal variations in serum biochemical and haematological measurements. J Clin Pathol 1989;42:172-9. [Crossref] [PubMed]
- Costongs GM, Janson PC, Bas BM, et al. Short-term and long-term intra-individual variations and critical differences of clinical chemical laboratory parameters. J Clin Chem Clin Biochem 1985;23:7-16. [Crossref] [PubMed]
- Association of the British Pharmaceutical Industry (ABPI). (2012) Guidelines for phase I clinical trials. Available online: https://www.abpi.org.uk
- Hingorani P, Natekar M, Deshmukh S, et al. Morphological abnormalities in baseline ECGs in healthy normal volunteers participating in phase I studies. Indian J Med Res 2012;135:322-30. [PubMed]
- Hingorani P, Karnad DR, Natekar M, et al. Baseline and new-onset morphologic ECG abnormalities in healthy volunteers in phase I studies receiving placebo: changes over a 6-week follow-up period. J Clin Pharmacol 2014;54:776-84. [Crossref] [PubMed]
- Edelblute HB, Fisher JA. Using "clinical trial diaries" to track patterns of participation for serial healthy volunteers in U.S. phase I studies. J Empir Res Hum Res Ethics 2015;10:65-75. [Crossref] [PubMed]
- Tishler CL, Bartholomae S. Repeat participation among normal healthy research volunteers: professional guinea pigs in clinical trials? Perspect Biol Med 2003;46:508-20. [Crossref] [PubMed]
- Elliott C. Guinea-Pigging: Healthy Human Subjects for Drug-Safety Trials are in Demand. But Is It a Living? The New Yorker 2008 January 7;36–41.
- Abadie R. The professional guinea pig big pharma and the risky world of human subjects. Durham: Duke University Press, 2010. doi:
10.1215/9780822393245 10.1215/9780822393245 - Iltis AS. Payments to normal healthy volunteers in phase 1 trials: avoiding undue influence while distributing fairly the burdens of research participation. J Med Philos 2009;34:68-90. [Crossref] [PubMed]
- Gelinas L, Lynch HF, Bierer BE, et al. When clinical trials compete: prioritising study recruitment. J Med Ethics 2017;43:803-9. [Crossref] [PubMed]
- Fisher JA, McManus L, Wood MM, et al. Healthy Volunteers’ Perceptions of the Benefits of Their Participation in Phase I Clinical Trials. J Empir Res Hum Res Ethics 2018;13:494-510. [Crossref] [PubMed]
- Stunkel L, Grady C. More than the money: a review of the literature examining healthy volunteer motivations. Contemp Clin Trials 2011;32:342-52. [Crossref] [PubMed]
- Wei Y, Li H, Wang H, et al. Psychological Status of Volunteers in a Phase I Clinical Trial Assessed by Symptom Checklist 90 (SCL-90) and Eysenck Personality Questionnaire (EPQ). Med Sci Monit 2018;24:4968-73. [Crossref] [PubMed]
- Sundquist J, Palmér K, Johansson LM, et al. The effect of mindfulness group therapy on a broad range of psychiatric symptoms: A randomised controlled trial in primary health care. Eur Psychiatry 2017;43:19-27. [Crossref] [PubMed]
- Wang Z, Wang J, Maercker A. Program Use and Outcome Change in a Web-Based Trauma Intervention: Individual and Social Factors. J Med Internet Res 2016;18:e243. [Crossref] [PubMed]
- Lee SS, Allen J, Black DW, et al. Quetiapine’s effect on the SCL-90-R domains in patients with borderline personality disorder. Ann Clin Psychiatry 2016;28:4-10. [PubMed]
- Alcocer-Gómez E, Cano-García FJ, Cordero MD. Effect of coenzyme Q10 evaluated by 1990 and 2010 ACR Diagnostic Criteria for Fibromyalgia and SCL-90-R: four case reports and literature review. Nutrition 2013;29:1422-5. [Crossref] [PubMed]
- Herrick LM, Camilleri M, Schleck CD, et al. Effects of Amitriptyline and Escitalopram on Sleep and Mood in Patients With Functional Dyspepsia. Clin Gastroenterol Hepatol 2018;16:401-6.e2. [Crossref] [PubMed]
- Pressler TR, Yen PY, Ding J, et al. Computational challenges and human factors influencing the design and use of clinical research participant eligibility pre-screening tools. BMC Med Inform Decis Mak 2012;12:47. [Crossref] [PubMed]
- Li K, Zhang JY, Ji L, et al. Optimization of screening process for healthy subjects in bio-equivalence trials. Chin J Clin Pharmacol. 2021;37:298-301.
- Breithaupt-Groegler K, Coch C, Coenen M, et al. Who is a ‘healthy subject’?-consensus results on pivotal eligibility criteria for clinical trials. Eur J Clin Pharmacol 2017;73:409-16. [Crossref] [PubMed]



