
Efficacy and safety of nanoparticle-albumin-bound paclitaxel compared with conventional taxanes in women with breast cancer: a systematic review and meta-analysis
Introduction
According to the World Cancer Report 2020, breast cancer has surpassed lung cancer to become the most common malignant tumor in women (1). However, the decline of breast cancer mortality may be partly related to developments in chemotherapy (2). Taxanes are considered the cornerstone drugs in many cancers, including ovarian cancer, lung cancer, pancreatic cancer, and breast cancer. In China, approved taxanes include paclitaxel, docetaxel, liposomal paclitaxel, and nanoparticle-albumin-bound paclitaxel (Nab-P). Notably, the water solubility of conventional taxanes, including paclitaxel and docetaxel, is extremely poor, which makes intravenous administration challenging (3). Additionally, conventional taxanes have poor selectivity for the site of action, weak targeting, poor drug delivery efficiency, and low tissue availability, and the cosolvents can also cause various adverse reactions, including allergic reactions, neurotoxicity (4,5), and even drug resistance (6,7). Additionally, the tiny particles formed in the blood circulation encapsulate the effective drugs, hindering the effects and limiting the extensive application of conventional paclitaxel.
Following the continuous development and application of new formulations of paclitaxel, Nab-P was first approved by the United States (US) Food and Drug Administration (FDA) in 2005 for the treatment of advanced breast cancer. In 2009, Nab-P was approved for marketing in China, and at the same time, the 1st nanotechnology drug delivery application for breast cancer was approved by the FDA. This novel nano-drug delivery system comprises albumin, which is a carrier of natural fat-soluble molecules. The characteristics of hydrophobic albumin molecules, via the transcellular albumin-binding glycoprotein (GP60) pathway, and the secreted protein acidic rich in cysteine (SPARC) pathway in the extracellular matrix of tumors, are used to increase the concentration of the extra-tumor drug. This novel delivery system also increases the speed at which paclitaxel enters tumor cells and allows for the effective redistribution of the drug. Taken together, these design features avoid the problems and toxicity associated with solvents (8).
Nab-P has shown better efficacy and safety than conventional taxanes in early studies but the superiority of Nab-P has failed to be verified in a phase III study (9). Further more, the conclusions of toxicity profiles comparison between Nab-P and conventional taxanes are inconsistent in previous meta-analysis studies (10,11). Thus, we conducted a meta-analysis of the therapeutic outcomes and safety of Nab-P and conventional taxanes in breast cancer to gather more information for reference in clinical practice. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-690/rc) (12).
Methods
Protocol and guidance
The protocol was registered in PROSPERO (CRD42020190984).
Search strategy
The PubMed, Web of Science, Embase, Scopus, and the Cochrane Library databases were searched to retrieve eligible studies from their inception to December 31, 2020. The Medical Subject Heading (MeSH) terms of breast neoplasms, Nab-P, and corresponding keywords were used. The search strategy is detailed in Appendix 1. To maximize the search for relevant articles, reference lists from the eligible articles and systematic reviews were also checked.
Inclusion criteria
Studies were considered eligible if they enrolled adults (age ≥18) with breast cancer, compared Nab-P (at any dose) to conventional paclitaxel or docetaxel, provided information on survival data, the response rate, or adverse events (Aes), were randomized controlled trials (RCTs), case-control studies (CCSs), or cohort studies (CSs), and were published in English (including those published online, ahead of the print publication).
Exclusion criteria
Studies were excluded if they were case reports, case series, single-arm clinical trials, animal studies, gray literature (including meeting abstracts), did not contain hazard ratios (HRs) or odd ratios (Ors) with their 95% confidence intervals (95% Cis), included any neoplasm other than breast cancer, and had been reported in multiple publications.
Outcomes
The efficacy outcomes included the overall response rate (ORR), pathologic complete response (pCR), progression-free survival (PFS), and overall survival (OS). The safety outcomes included any grade Aes and grade 3/4 Aes.
Study selection
The study selection was performed independently by 2 authors; any discrepancies were reviewed by a 3rd author and resolved by consensus. The study selection steps were as follows: (I) 1 author removed duplicate articles; (II) 2 independent authors screened all the titles and abstracts and reached a consensus with each other; and (III) the full texts were obtained, and the articles were further screened to identify eligible studies. Any disagreements were resolved by consensus.
Data extraction
The data collection was performed independently by 2 authors, who used a standard data extraction form to collect the data from the included studies. The following data were extracted: the first author’s last name, year of publication, study design, race of patients, number of patients, treatment lines, study region, study treatment (name, dosage, schedule, and combination drug if any), efficacy outcomes (i.e., PFS, OS, ORR, and pCR), and AE outcomes. When a study mentioned an outcome of interest without estimates, the data were obtained by calculation and transformation. If a study only stated the survival curve, the curve was imported into Engauge Digitizer to obtain the original data by drawing the outline of the curve and deriving the data to calculate the HR and the CI of the curve according to a previously describe method (13).
Assessment of risk of bias and quality of evidence
The risk of bias for each included RCT was assessed using the Cochrane Collaboration tool (14). The risk of bias for each included CCS or CS was assessed by the Newcastle-Ottawa scale (15). The quality of evidence for the outcomes was assessed using the grading of recommendations assessment, development, and evaluation (GRADE) approach (16).
Statistical analysis
Data synthesis
We performed the statistical analyses using the “meta” package in R (version 3.6.1, R Foundation for Statistical Computing, Vienna, Austria). We input the outcome data of each included study in the meta-analysis (17). The pooled HRs and RRs were reported with 95% Cis; all tests were 2-sided, and a probability level of 0.05 was used to determine statistical significance. Heterogeneity among the studies was assessed using the I2 statistic. If significant heterogeneity was not present (I2<50%), a fixed-effects model was used to pool the outcomes, and otherwise, a random-effects model was used. Publication bias was assessed by funnel plots and eggers test
Subgroup analysis
Subgroup analyses were performed to examine the interactions and explain the heterogeneity according to lines of treatment (1st-line or 2nd-line), treatment frequency [every week (QW) or every 3 weeks (Q3W)], the control drug (paclitaxel or docetaxel), the combination drug (with or without), race of patients (Asian or Western), and tumor stage (early or advanced) when the relevant data were available.
Sensitivity analyses
We conducted sensitivity analyses by excluding trials with high or unknown risks of bias, excluding the largest trials, using random-effect models, and excluding trials with male patients.
Results
Characteristics of the included studies
A total of 4,340 articles were initially retrieved. Next, the titles and abstracts of the articles were carefully read, and any duplicate and irrelevant articles were excluded. After reading the full text of the articles, and based on the aforementioned inclusion and exclusion criteria, 20 studies (22 articles, including 2 studies that had reported the outcomes of interest in separate articles) were ultimately included in the meta-analysis (9,17-37). Figure 1 shows the screening process. The characteristics of the included studies are shown in Table 1.
Table 1
| Author | Publication year | Country | Research type | Sample size | Cancer type | Triple-negative breast cancer | Dosage and frequency | Control |
|---|---|---|---|---|---|---|---|---|
| Gradishar | 2005 | Russia, USA, UK | RCT | 454 | Metastatic | Not mentioned | 260Q3W/175Q3W | sb-pc |
| Davidson | 2008 | – | RCT | 351 | Metastatic | Not mentioned | 260Q3W/175Q3W | sb-pc |
| Gradishar | 2009 | Russia, USA | RCT | 148 | Metastatic | Not mentioned | 100QW, 150QW, 300Q3W/100Q3W | doc |
| Guan | 2009 | China | RCT | 210 | Metastatic | Not mentioned | 260Q3W/175Q3W | sb-pc |
| Pippen | 2011 | – | RCT | 197 | Early | Not mentioned | 260Q3W/175Q3W | sb-pc |
| Zhang | 2012 | China | RCT | 52 | Not mentioned | Not mentioned | 260/175 | sb-pc |
| Gradishar | 2012 | Russia, USA | RCT | 148 | Metastatic | Not mentioned | 100QW, 150QW, 300Q3W/100Q3W | doc |
| Rugo | 2015 | USA | RCT | 554 | Metastatic | Not mentioned | 150QW/90QW | sb-pc |
| Huang | 2015 | China | RCT | 120 | Advanced | Not mentioned | 125QW/80QW | sb-pc |
| Untch | 2016 | Germany | RCT | 1,206 | Primary | Not mentioned | 125QW/80QW | sb-pc |
| Tamura | 2017 | Japan | RCT | 197 | Metastatic | Not mentioned | 150QW/73Q3W | doc |
| Cortes | 2018 | Russia, USA, UK | RCT | 454 | Metastatic | Not mentioned | 260Q3W/175Q3W | sb-pc |
| Gianni | 2018 | Spain, Australia, Singapore | RCT | 672 | Not mentioned | Not mentioned | 125QW/90QW | sb-pc |
| Mahtan | 2018 | USA | OS | 925 | Metastatic | Not mentioned | – | sb-pc |
| Kuwayama | 2018 | Japan | RCT | 152 | Early | Not mentioned | 100QW/75Q3W | doc |
| Ciruelos | 2019 | Spain | RCT | 20 | Not mentioned | Not mentioned | 100QW, 150QW, 150Q2W/80QW | sb-pc |
| Xie | 2019 | China | OS | 162 | Early | Not mentioned | 260Q2W/175Q2W | sb-pc |
| Untch | 2019 | Germany | RCT | 1,206 | Primary | Not mentioned | 125QW/80QW | sb-pc |
| Luhn | 2019 | USA | OS | 200 | Not mentioned | Triple-negative breast cancer | – | sb-pc |
| Bachelot | 2019 | France | OS | 1,436 | Metastatic | Not mentioned | – | doc |
| Yang | 2019 | China | OS | 50 | Not mentioned | Not mentioned | 260Q3W/175Q3W | sb-pc |
| Han | 2020 | China | OS | 95 | Advanced | Not mentioned | 260Q3W/175Q3W | sb-pc |
RCT, randomized controlled trials; Q3W, every 3 weeks; QW, every week; sb-pc, solvent-based paclitaxel; doc, docetaxel; OS, observational study.
Quality evaluation of the included studies
The quality of the 20 included studies was evaluated according to the study type. In this meta-analysis, 2 quality evaluation scales were used; that is, the Cochrane risk of bias assessment form for the 14 included RCTs and the Newcastle-Ottawa scale (NOS) for the 6 included CCSs and CSs. The included studies were found to have moderate risks of bias (see Appendix 2).
Outcome indicators of the included literature
As Appendix 3 shows, the indicators extracted from the included articles included PFS, OS, ORR, pCR, and other indicators related to the research objectives. Additionally, data on adverse outcomes, such as allergy, leukopenia, neurotoxicity, and neutropenia, were extracted.
Meta-analysis of therapeutic efficacy
ORR
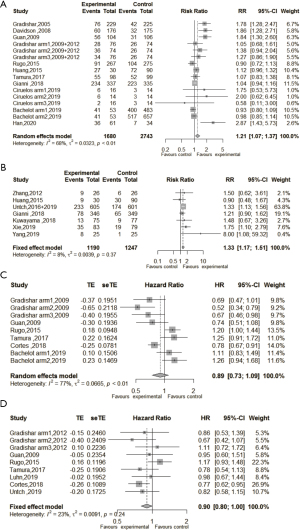
The ORRs were available in 11 studies (9,18-21,24-26,28,31,35,36). The heterogeneity test results for those studies were I2=68% (P<0.01); thus, a random-effects model was used for the analysis. The results showed that the ORR of the Nab-P group was 21% higher than that of the control group (RR =1.21, 95% CI: 1.07–1.37, and P=0.003, see Figure 2A for further details). The meta-regression analysis showed that the dosage of the drug affected the combined effect size. The heterogeneity among the studies was not due to the region of the study, number of doses, controls, study design, or tumor type (see Appendix 4). In the subgroup analyses, the ORRs of the patients in the Nab-P groups who received 2 treatment lines, had a treatment frequency of Q3W, received the single-administration treatment mode, and were Asian were significantly higher than those of the control groups (see Appendix 5).
pCR
The pCRs after the neoadjuvant treatment of Nab-P and solvent-based paclitaxel of early breast cancer patients were available in 7 studies (17,23,25,28,30,32,33,36). The heterogeneity test results for those studies were I2=8% (P=0.37); thus a fixed-effects model was used for the analysis. The results showed that the pCR of the neoadjuvant treatment was 33% higher in the Nab-P breast cancer group than the control group (RR =1.33, 95% CI: 1.17–1.51; P<0.001). Thus, Nab-P had some advantages in the treatment of early breast cancer (see Figure 2B for further details).
PFS
Data on the PFS of patients with breast cancer in the Nab-P and conventional taxanes groups were available in 6 studies (9,20,21,26,27,35). The heterogeneity test results for those studies were I2=77% (P<0.01); thus, a random-effects model was used for the analysis. The results showed that the risk of disease progression or death was 11% lower in the Nab-P treatment group than the control group (HR =0.89, 95% CI: 0.73–1.09; P=0.269; see Figure 2C). The meta-regression analysis showed that the region of study, dosage, number of doses, controls, and study design did not cause heterogeneity among the studies (see Appendix 6). In the subgroup analysis, the risk of disease progression or death of the patients in the Nab-P groups who received 2 treatment lines and had a treatment frequency of Q3W was significantly lower than that of patients in the control groups. No significant difference was found between the 2 groups in terms of race (Western or Asian). Additionally, different administration modes (single or combination) had the opposite effect on PFS between the 2 groups (see Appendix 7).
OS
Data on patients’ OS after Nab-P and conventional taxanes treatments for breast cancer were available in 7 studies (9,21,24,26,27,33,34). The heterogeneity test results were I2=23% (P=0.24); thus, a fixed-effects model was applied. The results indicated that the risk of death among breast cancer patients in the Nab-P treatment group was 10% less than that of patients in the control group, but no significant difference was found between the groups (P=0.478; see Figure 2D for further details). In the subgroup analysis, the risk of death of patients in the Nab-P groups who received two treatment lines, had a treatment frequency of Q3W, and received a single-administration treatment mode was significantly lower than that of patients in the control groups. No significant difference was reported in the subgroups as stratified by race, control group, or disease stage (see Appendix 8).
Meta-analysis of drug safety
Allergic reactions
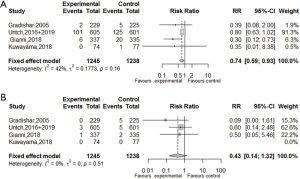
Data on the incidence of allergic reactions were available in 4 studies (17,18,20,30,33). The pooled results showed that the risk of allergic reactions of any grade in the Nab-P group were 26% lower than those of patients in the control group (RR =0.74, 95% CI: 0.59–0.93; P=0.009). The risk of grade ≥3 allergic reactions in the Nab-P group was 57% lower than that in the control group, but no significant difference was found between the 2 groups (RR =0.43, 95% CI: 0.14–1.32; P=0.14, see Figure 3 for further details).
Leukopenia
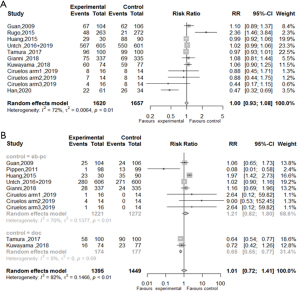
Data on the incidence of leukopenia were available in 9 studies (9,21,25,26,28,30,31,33,37). No significant difference in the risk of leukopenia of any grade was found between the 2 treatment groups (RR =1.00, 95% CI: 0.93–1.08; P=0.991, see Figure 4A for further details). Compared to the conventional taxanes group, the risk of grade ≥3 leukopenia in patients with breast cancer in response to Nab-P treatment was increased by 1% (RR =1.01, 95% CI: 0.93–1.08; P=0.955, see Figure 4B for further details), but the difference was not significant. Compared to docetaxel treatment, the risk of grade ≥3 leukopenia in the Nab-P treatment group was significantly decreased by 35% (RR =0.65, 95% CI: 0.55–0.77; P<0.001). However, no significant difference was observed in the risk of grade ≥3 leukopenia between the conventional solvent-based paclitaxel group and Nab-P group (RR =1.21, 95% CI: 0.82–1.80; P>0.05, see Figure 4B).
Neutropenia
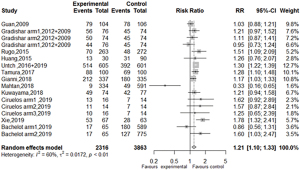
Data on the incidence of neutropenia were available in 13 studies (9,17,20,21,23-26,28-31,33,35,36). The risk of neutropenia of any grade in the Nab-P group was 8% higher than that of the conventional taxanes group (RR =1.08, 95% CI: 1.02–1.14; P=0.009, see Figure 5A). Compared to docetaxel treatment, the risk of grade ≥3 neutropenia in the Nab-P treatment group was significantly decreased by 46% [RR =0.54 (0.37, 0.79)], but no significant difference in the risk of grade ≥3 neutropenia was found between the conventional solvent-based paclitaxel treatment and the Nab-P treatment groups (see Figure 5B for further details). The risk of grade ≥ 4 neutropenia in the Nab-P group was significantly lower than that of the conventional taxanes group [RR =0.39 (0.20, 0.77); P=0.007; see Figure 5C for further details].

Sensory neuropathy
Data on the incidence of sensory neuropathy were available in 12 studies (9,17,20,21,24-26,28-33,35). The risk of sensory neuropathy was 21% higher in the Nab-P group than the conventional taxanes group [RR =1.21 (1.10, 1.33); P<0.001; see Figure 6 for further details].
Publication bias and sensitivity analysis
No significant publication bias was observed based on a visual inspection of the funnel plots for PFS and OS (see Appendix 9). A sensitivity analysis of the primary outcome indicators was conducted by excluding each study, one by one. Our results showed that each study had a negligible effect on the outcomes, which was not changed as a result of eliminating a specific study; thus, the results of this meta-analysis were relatively stable (see Appendix 10).
Discussion
Albumin is an endogenous protein that does not have an opsonizing effect. A previous study demonstrated that albumin could be used as a drug carrier to prepare nanoparticles and reduce the affinity of nanoparticles to macrophages (38), thereby prolonging the circulating period and improving the targeted efficacy of the drug. The results of this meta-analysis showed that the pCRs and ORRs of breast cancer patients treated with Nab-P were better than those of breast cancer patients treated with solvent paclitaxel. This is likely because the nano-drug delivery system binds paclitaxel to albumin, thereby reducing the dose of paclitaxel and the need for toxic solvents, such as polyoxyethylene castor oil (39). The albumin transport pathway (gp60-caveolin-SPARC) may facilitate the transportation of Nab-P (40). We found that the application of Nab-P in late lines of treatment had better effects than conventional taxanes on all indicators of therapeutic efficacy in patients with advanced breast cancer. This may be due less interference of anti-tumor therapies n the late-line group than front-line group, which usually involves monotherapy rather than combination therapy. Our results provide further evidence that Nab-P has more beneficial effects in breast cancer treatment than conventional taxanes.
Additionally, in the advanced setting, patients treated with Q3W Nab-P benefited more than those with QW Nab-P in the ORR, PFS and OS when compared with conventional taxanes. It may be that the patients who received Q3W dosing also received corresponding preventive and supportive therapy (e.g., granulocyte-colony stimulating factor) to ensure full-dosage chemotherapy and the entire course of treatment. Thus, patients’ compliance with Q3W Nab-P is expected to be better than their compliance with a weekly regimen. We also compared the efficacy of Nab-P and conventional taxane treatment under different modes of administration in relation to the ORR, PFS, and OS of patients with advanced breast cancer. Our results demonstrated that Nab-P monotherapy had more beneficial effects on the ORR, PFS, and OS of patients, which may be because the combination of drugs dilute the efficacy of Nab-P, which in turn resulted in findings of non-significant differences in comparisons of Nab-P to conventional taxanes. Additionally, while combination therapy may improve short-term curative effects, it may also increase the risk of toxicity, which could in turn result in a failure to complete the entire course of chemotherapy. Thus, in later-line treatments, Nab-P monotherapy may be better than conventional taxanes for patients with advanced breast cancer.
The adverse reactions caused by Nab-P and conventional taxanes in the treatment of breast cancer were also explored. We found that there was no significant difference between the 2 treatments in relation to allergic reactions and leukopenia. However, the incidences of peripheral neurotoxicity and neutropenia caused by Nab-P were significantly higher than those caused by conventional taxanes. The relative higher dosage of paclitaxel in Nab-P compared to those of standard paclitaxel in the control groups may be the major cause of higher incidence of treatment related adverse events Additionally, the administration time of Nab-P was decreased to 30 minutes due to the removal of organic solvents, which is another reason for the increase in allergic reactions and neurotoxicity. In addition to the solvent, the dose of each injection, time of administration, treatment duration, and cumulative dose are also known to influence the occurrence of peripheral neuropathy (41).
This study had several limitations. First, the included RCTs provided little information on the risk assessment items, and thus we were unable to properly evaluate the quality of the studies. However, in sensitivity analyses, no significant changes were found after excluding studies of high and unknown quality. Second, the sample sizes of the included studies were relatively small in relation certain outcomes; thus, further research needs to be conducted using large sample-sized studies to extend findings on these outcomes. Finally, since we have not enrolled non-English language literature databases that the language bias might exist.
In conclusion, the pCR rate and the response rate was significantly higher in patients treated with neoadjuvant nab-paclitaxel than those with conventional taxanes. The PFS and OS of advanced breast cancer patients were comparable between the groups; however, Nab-P produced fewer side effects than conventional taxanes, including allergic reactions, which suggests that Nab-P has a unique mechanism of action and offers considerable advantages in the treatment of breast cancer. Nab-P has promising efficacy and tolerance in the treatment of advanced breast cancer patients, and it is expected to be widely used in breast cancer patients.
Acknowledgments
Funding: Special funding for clinical research was provided by the Wu Jieping Medical Foundation (No. 320675012292), the Zhejiang Public Welfare Technology Research Program (No. LGJ20H160001), Health General Program of Zhejiang Provinces (No. 2021KY088), the Zhejiang Traditional Chinese Medicine Science Fund Project (No. 2020ZB037), and the Scientific Research Foundation of Zhejiang Medical Association (No. 2016ZYC-A06).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist Available at https://apm.amegroups.com/article/view/10.21037/apm-22-690/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-690/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization (2020). World cancer report 2020. Available online: https://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-Cancer-Research-For-Cancer-Prevention-2020. Accessed26 July 2021.
- Burton R, Bell R. The global challenge of reducing breast cancer mortality. Oncologist 2013;18:1200-2. [Crossref] [PubMed]
- Ma P, Mumper RJ. Paclitaxel Nano-Delivery Systems: A Comprehensive Review. J Nanomed Nanotechnol 2013;4:1000164. [Crossref] [PubMed]
- Biganzoli L, McCartney A. Neoadjuvant nab-paclitaxel in breast cancer: who stands to benefit? Chin Clin Oncol 2020;9:42. [Crossref] [PubMed]
- Sang D, Zhang YR, Ding MX, et al. Experience of rescuing 4 patients with anaphylactic shock caused by paclitaxel. Clin Med J 2017;15:62-4.
- Gelderblom H, Verweij J, Nooter K, et al. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer 2001;37:1590-8. [Crossref] [PubMed]
- Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol 1990;8:1263-8. [Crossref] [PubMed]
- Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, Nab-P, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res 2006;12:1317-24. [Crossref] [PubMed]
- Rugo HS, Barry WT, Moreno-Aspitia A, et al. Randomized Phase III Trial of Paclitaxel Once Per Week Compared With Nanoparticle Albumin-Bound Nab-Paclitaxel Once Per Week or Ixabepilone With Bevacizumab As First-Line Chemotherapy for Locally Recurrent or Metastatic Breast Cancer: CALGB 40502/NCCTG N063H (Alliance). J Clin Oncol 2015;33:2361-9. [Crossref] [PubMed]
- Lee H, Park S, Kang JE, et al. Efficacy and safety of nanoparticle-albumin-bound paclitaxel compared with solvent-based taxanes for metastatic breast cancer: A meta-analysis. Sci Rep 2020;10:530. [Crossref] [PubMed]
- Liu M, Liu S, Yang L, et al. Comparison between nab-paclitaxel and solvent-based taxanes as neoadjuvant therapy in breast cancer: a systematic review and meta-analysis. BMC Cancer 2021;21:118. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [Crossref] [PubMed]
- Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto—GBG 69): a randomised, phase 3 trial. Lancet Oncol 2016;17:345-56. [Crossref] [PubMed]
- Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle Nab-P compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J Clin Oncol 2005;23:7794-803. [Crossref] [PubMed]
- Davidson N, Tjulandin S, O'Shaughnessy J, et al. Overall survival analysis of a randomized phase III trial comparing nab-paclitaxel with solvent-based paclitaxel in patients with metastatic breast cancer previously treated with anthracycline. Eur J Cancer Suppl 2008;6:218. [Crossref]
- Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol 2009;27:3611-9. [Crossref] [PubMed]
- Guan ZZ, Li QL, Feng F, et al. Superior efficacy of a Cremophor-free Nab-P compared with solvent-based paclitaxel in Chinese patients with metastatic breast cancer. Asia Pac J Clin Oncol 2009;5:165-74. [Crossref]
- Pippen J, Paul D, Vukelja S, et al. Dose-dense doxorubicin and cyclophosphamide followed by dose-dense Nab-P plus bevacizumab is safe as adjuvant therapy in patients with early stage breast cancer. Breast Cancer Res Treat 2011;130:825-31. [Crossref] [PubMed]
- Zhang J, Zhang S, Liu L, et al. A phase II study of Nab-P combined with epirubicin and cyclophosphamide as neo-adjuvant therapy in breast cancer women. Ann Oncol 2012;23:ix141. [Crossref]
- Gradishar WJ, Krasnojon D, Cheporov S, et al. Phase II trial of nab-paclitaxel compared with docetaxel as first-line chemotherapy in patients with metastatic breast cancer: final analysis of overall survival. Clin Breast Cancer 2012;12:313-21. [Crossref] [PubMed]
- Huang L, Chen S, Yao L, et al. Phase II trial of weekly nab-paclitaxel and carboplatin treatment with or without trastuzumab as nonanthracycline neoadjuvant chemotherapy for locally advanced breast cancer. Int J Nanomedicine 2015;10:1969-75. [PubMed]
- Tamura K, Inoue K, Masuda N, et al. Randomized phase II study of nab-paclitaxel as first-line chemotherapy in patients with HER2-negative metastatic breast cancer. Cancer Sci 2017;108:987-94. [Crossref] [PubMed]
- Cortes J, Pérez-García J, Whiting S, et al. Quality-Adjusted Survival With nab-Paclitaxel Versus Standard Paclitaxel in Metastatic Breast Cancer: A Q-TWiST Analysis. Clin Breast Cancer 2018;18:e919-26. [Crossref] [PubMed]
- Gianni L, Mansutti M, Anton A, et al. Comparing neoadjuvant nab-paclitaxel vs paclitaxel both followed by anthracycline regimens in women with ERBB2/HER2-negative breast cancer—The Evaluating Treatment with Neoadjuvant Abraxane (ETNA) Trial: A randomized phase 3 clinical trial. JAMA Oncol 2018;4:302-8. [Crossref] [PubMed]
- Mahtani RL, Parisi M, Glück S, et al. Comparative effectiveness of early-line nab-paclitaxel vs. paclitaxel in patients with metastatic breast cancer: a US community-based real-world analysis. Cancer Manag Res 2018;10:249-56. [Crossref] [PubMed]
- Kuwayama T, Nakamura S, Hayashi N, et al. Randomized Multicenter Phase II Trial of Neoadjuvant Therapy Comparing Weekly Nab-paclitaxel Followed by FEC With Docetaxel Followed by FEC in HER2- Early-stage Breast Cancer. Clin Breast Cancer 2018;18:474-80. [Crossref] [PubMed]
- Ciruelos E, Apellániz-Ruiz M, Cantos B, et al. A Pilot, Phase II, Randomized, Open-Label Clinical Trial Comparing the Neurotoxicity of Three Dose Regimens of Nab-Paclitaxel to That of Solvent-Based Paclitaxel as the First-Line Treatment for Patients with Human Epidermal Growth Factor Receptor Type 2-Negative Metastatic Breast Cancer. Oncologist 2019;24:e1024-33. [Crossref] [PubMed]
- Xie F, Chen R, Zhang L, et al. Efficacy of two-weekly nanoparticle Nab-P as neoadjuvant chemotherapy for breast cancer. Nanomedicine 2019;14:1595-1603. [Crossref] [PubMed]
- Untch M, Jackisch C, Schneeweiss A, et al. NAB-Paclitaxel Improves Disease-Free Survival in Early Breast Cancer: GBG 69-GeparSepto. J Clin Oncol 2019;37:2226-34. [Crossref] [PubMed]
- Luhn P, Chui SY, Hsieh AF, et al. Comparative effectiveness of first-line nab-paclitaxel versus paclitaxel monotherapy in triple-negative breast cancer. J Comp Eff Res 2019;8:1173-85. [Crossref] [PubMed]
- Bachelot T, Ciruelos E, Schneeweiss A, et al. Preliminary safety and efficacy of first-line pertuzumab combined with trastuzumab and taxane therapy for HER2-positive locally recurrent or metastatic breast cancer (PERUSE). Ann Oncol 2019;30:766-73. [Crossref] [PubMed]
- Yang M, Qu H, Liu A, et al. Efficacy and safety of nanoparticle Nab-P as neoadjuvant chemotherapy in HER2-negative breast cancer. J Cancer Res Ther 2019;15:1561. [Crossref] [PubMed]
- Han X, Wang Z. Efficacy of Nab-P in the treatment of advanced refractory breast cancer and its effect on serum resistin. J BUON 2020;25:681-7. [PubMed]
- Torchilin VP, Berdichevsky VR, Barsukov AA, et al. Coating liposomes with protein decreases their capture by macrophages. FEBS Lett 1980;111:184-8. [Crossref] [PubMed]
- Henderson IC, Bhatia V. Nab-paclitaxel for breast cancer: a new formulation with an improved safety profile and greater efficacy. Expert Rev Anticancer Ther 2007;7:919-43. [Crossref] [PubMed]
- Kudlowitz D, Muggia F. Nanoparticle Nab-P (nab-paclitaxel): extending its indications. Expert Opin Drug Saf 2014;13:681-5. [PubMed]
- Park SB, Goldstein D, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin 2013;63:419-37. [Crossref] [PubMed]



