
Efficacy and safety of acellular dermal matrix versus connective tissue graft for root coverage of Miller’s Class I and II gingival recession: a systematic review and meta-analysis
Introduction
Gingival recession is defined as the exposure of the root surface following an apical shift in the position of the gingiva beyond the cementoenamel junction (CEJ) (1). It can locally or generally affect one or more tooth surfaces, and is more common in adults (2,3). As a highly prevalent condition worldwide, gingival recession can increase the risk of root caries and affect patient comfort and esthetics (4). In addition, progressive gingival recession has also been found to be associated with an increased risk of tooth loss secondary to clinical attachment loss (CAL) (5). It is universally recognized that anatomical abnormalities, inflammation, trauma, and iatrogenic factors including placement of orthodontic appliances and improper denture design can lead to gingival recession (6). Although reducing these causes is conducive to decreasing its incidence and severity, it is very important to implement practical management and preventative strategies in clinic.
In recent years, numerous techniques have been developed to treat gingival recessions. The subepithelial connective tissue graft (CTG) is considered the gold standard for localized recession defects because of its predictability in increasing the width of keratinized gingiva and in achieving root coverage (7). This technique requires tissue to be harvested from the palate and then placed over an appropriate recession defect with coronal advancement of the flap over the donor graft (8). However, harvest tissue from the palatal area is time consuming and increases the postoperative morbidity of patients, such as pain, bleeding, and hyposensitivity (9,10). In case of a lack of sufficient donor material, an increased number of staged surgeries may be needed for patients with multiple recessions. Acellular dermal matrix (ADM) was developed as a substitute for autogenous CTG and has presented a potential alternative to thicken soft tissues and cover multiple gingival recessions (11). Al-Hamdan et al. found that the use of ADM with the coronally advanced flap resulted in a significant increase in keratinized tissue (KT) and percent root coverage (PRC) (12). Nevertheless, CTG may be slightly superior to ADM if the gain of keratinized mucosal width is taken as a main goal (13). Another study (14) evaluating the 9-year assessment of treated isolated gingival recessions and their adjacent untreated sites reported that ADM-treated sites displayed recession relapse from 1 to 9 years. However, Maluta et al. demonstrated that both treatments were effective for treating multiple gingival recessions (15). Barros et al. also reported that ADM presents consistent levels of root coverage when compared with CTG (16). This may be due to inconsistent differences in study design, such as search sources, results measurements, and sample sizes. Meta-analysis is a statistical analysis method based on evidence-based medicine. The purpose of meta-analysis is to comprehensively analyze the research results of multiple small samples of the same subject, so as to increase the sample size, improve the research efficiency of the original results, and make the conclusions more representative. A meta-analysis based on the randomized controlled trials (RCTs) that comprehensively analyze the efficacy and safety of ADM versus CTG for root coverage in patients with gingival recession is needed.
The current meta-analysis was conducted to comprehensively analyze the efficacy and safety of these two procedures for root coverage among patients presenting with gingival recession. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-656/rc).
Methods
Search strategy
This procedure of search was conducted by two of the researchers (Min Zhang and Mengxi Wang). Databases including PubMed, Embase, Cochrane Library, and Web of Science were searched for articles until 15 May 2020. Terms used for search in various combinations included ‘acellular dermis’, ‘connective tissue graft’, ‘root coverage’, and ‘gingival recession’.
Inclusion and exclusion criteria
Study inclusion and exclusion criteria was conducted by Min Zhang. The inclusion criteria in a patient/population, intervention, comparison, and outcomes (PICOS) format were as follows: (I) population (P): adults with gingival recession; (II) intervention (I): ADM; (III) comparison (C): CTG; (IV) outcome (O): PRC as the primary outcome, clinical attachment level (CAL), KT, probing depth (PD), recession width (RW), and recession depth (RD) as secondary outcomes; and (V) study design (S): RCTs with the study length of 3 months or above. Studies from which we were unable to extract the valid data, those not published in English, as well as meta-analyses, reviews, case reports, and animal experiments were all excluded.
Literature bias risk and quality evaluation
The risk of bias and quality of in RCTs were assessed by the Cochrane Risk of Bias 2.0 Tool according to the Cochrane Handbook for Systematic Reviews of Interventions (17). The literature quality evaluation was completed by two reviewers (Min Zhang and Mengxi Wang) independently. In case of any disagreement, the third reviewer (Chunli Zhang) would be invited to resolve the disagreement by arbitration.
Data extraction
A list of articles was compiled by two researchers (Min Zhang and Mengxi Wang). After filtering out duplicate articles, initial screening by reading article titles and abstracts was performed. Further screening by reading the full text to screen articles based on inclusion and exclusion criteria. If two researchers disagreed during the extraction process, a third person was consulted for arbitration. The extracted data contained the first author, year of publication, country, study length, participants, Miller classification, surgical methods, postoperative intervention, number of sites and patients, gender, age, and quality assessment.
Statistical analysis
All studies were statistically analyzed using Stata 15.1 software (Stata Corporation, College Station, TX, USA). Weighted mean difference (WMD) was used as the statistics for measurement data and the effect sizes were expressed as 95% confidence intervals (CI). Heterogeneity tests were performed for each effect size, and random effects models were adopted when I2≥50%, otherwise the fixed effects model was applied. A difference was considered statistically significant at P<0.05. When I2≥50% and P<0.05, subgroup analysis was performed according to the study length and quality of literature. Publication bias was tested by Begg’s test.
Results
Baseline information of included studies
According to the search strategy, 24 RCTs (13,16,18-39) were finally included in this meta-analysis (Figure 1). A total of 587 patients with 1,315 gingival recession sites were involved in the study. There were 724 loci in the ADM group and 591 loci in the CTG group. Table 1 shows the baseline information of included studies.
Table 1
| Study | Study length | Participants/Miller classification | Surgical methods | Postoperative intervention | Groups | No. of sites | No. of patients | Male/female | Age (years) |
|---|---|---|---|---|---|---|---|---|---|
| Harris (21), 2000 America | 3 months | 50 participants; Miller Class I or II recessions of at least 2 mm | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | Routine postoperative instructions; dressing; suture removal at 14 days | ADM | 25 | 25 | 12/13 | 40.8±12.2 |
| CTG | 25 | 25 | 10/15 | 39.6±11.7 | |||||
| Aichelmann-Reidy (18), 2001 America | 6 months | 22 participants; Miller Class I or II recessions of at least 2 mm | ADM vs. CTG (CT side toward tooth); releasing incisions; no root conditioning | NSAID and amoxicillin (dose and duration not provided); dressing; suture removal at 10 days; no CHX | ADM | 22 | 22 | 7/15 | 47.2 [24–67]* |
| CTG | 22 | 22 | 7/15 | 47.2 [24–67]* | |||||
| Henderson (22), 2001 America | 12 months | 10 participants; Miller Class I or II recessions of at least 3 mm | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | Systemic doxycycline hyclate 50mg once a day for 14 days; Dexamethasone, taking 3 mg for 3 days, 2 mg for 3 days, then 1 mg for 3 days, one dose daily; Naproxen 375 mg every 12 hours for7 days;0.12% CHX | ADM | 10 | 10 | 5/5 | 42.2±16.7 |
| CTG | 10 | 10 | 5/5 | 42.2±16.7 | |||||
| Novaes (27), 2001 Brazil | 6 months | 9 participants; Miller Class I or II | ADM vs. CTG (CT side toward flap); releasing incisions; root conditioning with tetracycline | Amoxicillin (500 mg tid for 7 days); dressing; suture removal at 15 days; 0.12% CHX | ADM | 15 | 9 | 2/7 | 42±9.42 |
| CTG | 15 | 9 | 2/7 | 42±9.42 | |||||
| Paolantonio (28), 2002, Italy | 12 months | 30 participants; Miller Class I or II recessions of at least 3 mm | ADM vs. CTG (CT side toward tooth); releasing incisions when needed; no root conditioning | Analgesics; no dressing; suture removal at 15 days; 0.12% CHX | ADM | 15 | 15 | 11/19 | 34.5±5.2 |
| CTG | 15 | 15 | 34.5±5.2 | ||||||
| Hirsch (23), 2005 Israel | 24 months | 166 participants; Miller Class I or II | ADM vs. CTG (CT side toward tooth); releasing incisions; no root conditioning | Amoxicillin (500 mg tid) and ibuprofen (400 mg ×2) were prescribed. Chlorhexidine gluconate mouth rinses (0.2% bid) were also prescribed. Sutures were removed after 10 days in most cases | ADM | 262 | 101 | 37/64 | 24.0±0.5 |
| CTG | 169 | 65 | 22/43 | 25.4±2.3 | |||||
| Rahmani (29), 2006, Iran | 6 months | 14 participants; Miller Class I or II recessions | ADM vs. CTG (CT side toward tooth); releasing incisions; no root conditioning | No prescriptions mentioned; dressing; suture removal not specified; 0.2% CHX (starting 2 days prior; the duration of use not specified) | ADM | 10 | 14 | 8/6 | 41.7 [23–62]* |
| CTG | 10 | 14 | 8/6 | 41.7 [23–62]* | |||||
| Joly (25), 2007 Brazil | 6 months | 10 participants; Miller Class I or II recessions of at least 3 mm | ADM vs. CTG (orientation not provided); no releasing incisions; conditioned with tetracycline | NSAID and acetaminophen; no dressing; suture removal at 14 days; 0.12% CHX | ADM | 10 | 10 | 6/4 | [27–51]* |
| CTG | 10 | 10 | 6/4 | [27–51]* | |||||
| Haghighati (20), 2009 Iran | 6 months | 16 participants; Miller Class I or II recessions of at least 2 mm | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | NSAID; dressing; suture removal at 10 days; 0.2% CHX | ADM | 16 | 16 | 8/8 | NA |
| CTG | 16 | 16 | 8/8 | NA | |||||
| Sadat Mansouri (30), 2010 Iran | 6 months | 5 participants; Miller Class I or II recessions of at least 2 mm | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | NSAID and amoxicillin; dressing; suture removal at 15 days; 0.2% CHX | ADM | 9 | 5 | 2/3 | 37.6±8.26 |
| CTG | 9 | 5 | 2/3 | 37.6±8.26 | |||||
| Moslemi (26), 2011 Iran | 5 years | 15 participants; Miller Class I or II recessions of at least 2 mm | ADM vs. CTG (CT side toward tooth); releasing incisions; no root conditioning | NSAID; dressing; suture removal at 10 days; 0.2% CHX | ADM | 15 | 15 | 7/8 | 39.4±5.2 |
| CTG | 15 | 15 | 7/8 | 39.4±5.2 | |||||
| Koudale (39), 2012 India | 6 months | 10 participants; Miller Class I or II recessions of at least 2 mm | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | NSAID, acetaminophen, and amoxicillin (500 mg tid for 7 days); dressing; suture removal at 14 days; 0.12% CHX | ADM | 5 | 5 | NA | 22.5±8.23 |
| CTG | 5 | 5 | NA | 22.5±8.23 | |||||
| Gholami (38), 2013 India | 6 months | 16 participants; Miller Class I or II recessions | ADM vs. CTG (CT side toward flap); double papillary flap; root conditioning with tetracycline | Acetaminophen and amoxicillin (500 mg tid for 7 days); dressing; suture removal at 10 days;0.12% CHX | ADM | 16 | 16 | NA | NA |
| CTG | 16 | 16 | NA | NA | |||||
| Shori (37), 2013 India | 6 months | 20 participants; Miller Class I or II recessions of at least 3 mm | ADM vs. CTG (CT side toward tooth); releasing incisions; no root conditioning | NSAID, acetaminophen, and amoxicillin (500 mg tid for 7 days); dressing; suture removal not specified; 0.2% CHX | ADM | 10 | 10 | NA | 29.7±4.35 |
| CTG | 10 | 10 | NA | 29.7±4.35 | |||||
| Thomas (33), 2013 India | 6 months | 10 participants; Miller Class I or II recessions | ADM vs. CTG (CT side toward tooth); releasing incisions; no root conditioning | NSAID and amoxicillin (500 mg tid for 5 days); dressing; suture removal at 14 days; 0.2% CHX | ADM | 10 | 10 | NA | 34 [18–50]* |
| CTG | 10 | 10 | NA | 34 [18–50]* | |||||
| Goyal (36), 2014 India | 6 months | 30 participants; Miller Class II recessions of at least 4 mm | ADM vs. CTG (orientation not provided); releasing incisions; no root conditioning | NSAID and doxycycline hyclate (200 mg on 1st day and 100 mg/day for 7 days); no dressing; suture removal at 10 days; 0.2% CHX | ADM | 15 | 15 | NA | NA |
| CTG | 15 | 15 | NA | NA | |||||
| Barros (16), 2015 Brazil | 12 months | 15 participants; Miller Class I or II recessions of at least 3 mm | ADM vs. CTG (CT side toward tooth); releasing incisions and extended flap technique; root conditioning with EDTA | NSAID and amoxicillin (500 mg tid for 7 days); no dressing; suture removal at 15 days; 0.12% CHX | ADM | 15 | 15 | 5/10 | [23–54]* |
| CTG | 15 | 15 | 5/10 | [23–54]* | |||||
| Taiyeb Ali (32), 2015 Malaysia | 6 months | 6 participants; Miller Class I or II recessions of at least 3 mm | ADM vs. CTG (CT side toward tooth); releasing incisions; root conditioning with tetracycline | 0.12% CHX; No other postoperative details | ADM | 4 | 3 | 3/3 | 37.8 [23–58]* |
| CTG | 4 | 3 | 37.8 [23–58] | ||||||
| Thakare (35), 2015 India | 6 months | 20 participants; Miller Class I or II recessions of at least 2 mm | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | NSAID and acetaminophen; dressing; suture removal at 14 days; 0.2% CHX | ADM | 21 | 10 | NA | [18–50]* |
| CTG | 23 | 10 | NA | [18–50]* | |||||
| Hutton (24), 2018 America | 4 months | 20 participants; Miller Class I or II recessions | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | Amoxicillin 500 mg for 7 days, Clindamycin 300 mg for 7 days. An NSAID (Ibuprofen 600 mg every 6-8 hours for 3 to 5 days) and a narcotic pain reliever (hydrocodone/APAP 5/325 mg every 6–8 hours as needed for pain, up to 4 days) | ADM | 10 | 10 | 6/4 | 59.7±10.9 |
| CTG | 10 | 10 | 5/5 | 51.2±11.0 | |||||
| Vreeburg (34), 2018 America | 6 months | 24 participants; Miller Class I, II, or III recessions of at least 2 mm | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | 500 mg amoxicillin, 3 times daily for 7 days, or 300 mg clindamycin, 3 times daily for 7 days if the patient was allergic to penicillin. 50 mg Tramadol was prescribed for post-operative analgesia. Ice pack application was used immediately after surgery on an intermittent basis for the first 3 to 4 hours at the surgical sites. 0.2% CHX for 2 weeks | ADM | 11 | 11 | 9/15 | 50.5 [26–78]* |
| CTG | 13 | 13 | 50.5 [26–78]* | ||||||
| Gürlek (19), 2020 Turkey | 18 months | 12 participants; Miller Class I or II recessions | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | The patient was prescribed a nonsteroidal anti-inflammatory medication postoperatively and instructed not to brush the teeth in the operation areas | ADM | 41 | 12 | 4/8 | 31.41±13.32 |
| CTG | 41 | 12 | 4/8 | 31.41±13.32 | |||||
| Kroiss (13), 2019 Germany | 5 years | 39 participants; Miller Class I or II recessions | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | All patients were placed on 0.12% CHX twice a day for 1 min for 2 weeks, and NSAID and analgesic medication (ibuprofen 400 mg) was prescribed | ADM | 141 | 20 | 5/15 | 46.6 [25–69]* |
| CTG | 97 | 19 | 2/17 | 43.6 [24–64]* | |||||
| Suzuki (31), 2020 Brazil | 6 months | 18 participants; Miller Class I or II recessions | ADM vs. CTG (CT side toward flap); releasing incisions; no root conditioning | 0.12% CHX twice a day for the first 15 days. Amoxicillin (500 mg) three times daily for 7 days-starting 24 h before surgery-ibuprofen (600 mg) three times daily for 5 days, and dipyrone sodium (500 mg) four times daily for 3 days-both beginning before surgery | ADM | 16 | 18 | 9/9 | 34.5±7.5 |
| CTG | 16 | 18 | 9/9 | 34.5±7.5 |
*, the extreme value. Data are shown as median, range, and interquartile range or mean ± SD. ADM, acellular dermal matrix; CTG, connective tissue graft; CT, connective tissue; NSAID, nonsteroidal anti‑inflammatory drug; CHX, chlorhexidine; EDTA, ethylene diamine tetraacetic acid; NA, data deficient.
Bias risk evaluation of included literature
Firstly, the bias risk assessment tool recommended by the Cochrane systematic review manual was used to evaluate the bias of the included literature. The results are shown in Table S1. The five included studies showed the largest percentage of “low risk”, indicating that the included studies met the requirements of the analysis.
PRC
A total of 10 RCTs reported the PRC, and the pooled results showed that there was no difference in PRC between the ADM group and the CTG group (WMD: −1.61, 95% CI: −3.49 to 0.28, P=0.094; I2=86.0%) (Table 2, Figure 2).
Table 2
| Characteristics | WMD (95% CI) | P value | I2 (%) |
|---|---|---|---|
| PRC (%) | |||
| Overall | −1.61 (−3.49, 0.28) | 0.094 | 86.0 |
| Publication bias | Z=0.55 | 0.583 | |
| Study length | |||
| <6 months | −0.19 (−1.38, 4.01) | 0.931 | 0.0 |
| 6–12 months | −0.52 (−5.78, 4.74) | 0.845 | 44.5 |
| >12 months | 0.84 (−8.26, 9.95) | 0.856 | 20.2 |
| CAL (mm) | |||
| Overall | 0.25 (0.03, 0.47) | 0.026 | 54.8 |
| Publication bias | Z=−0.20 | 0.843 | |
| Study length | |||
| <6 months | 0.31 (0.04, 0.58) | 0.027 | 0.0 |
| 6–12 months | 0.29 (0.18, 0.39) | <0.001 | 59.5 |
| >12 months | 0.39 (0.16, 0.63) | 0.001 | 0.0 |
| KT (mm) | |||
| Overall | −0.44 (−0.63, −0.25) | <0.001 | 56.8 |
| Publication bias | Z=−0.02 | 0.986 | |
| Study length | |||
| <6 months | −0.29 (−0.50, −0.07) | 0.009 | 18.9 |
| 6–12 months | −0.36 (−0.53, −0.19) | <0.001 | 40.2 |
| >12 months | −0.78 (−1.07, −0.48) | <0.001 | 32.7 |
| Quality assessment | |||
| High quality | −0.35 (−0.50, −0.20) | <0.001 | 51.2 |
| Low quality | −0.77 (−1.23, −0.30) | 0.001 | 42.8 |
| PD (mm) | |||
| Overall | 0.07 (−0.01, 0.14) | 0.067 | 39.9 |
| Publication bias | Z=−0.26 | 0.795 | |
| Study length | |||
| <6 months | 0.11 (−0.01, 0.23) | 0.078 | 71.1 |
| 6–12 months | −0.00 (−0.07, 0.07) | 0.978 | 0.0 |
| >12 months | 0.04 (−0.08, 0.17) | 0.495 | 68.9 |
| RW (mm) | |||
| Overall | 0.07 (−0.10, 0.23) | 0.437 | 32.5 |
| Publication bias | Z=−0.09 | 0.928 | |
| Study length | |||
| <6 months | −0.08 (−0.49, 0.33) | 0.716 | 6.3 |
| 6–12 months | 0.07 (−0.10, 0.23) | 0.434 | 23.7 |
| >12 months | 0.43(−0.02, 0.87) | 0.059 | 79.9 |
| RD (mm) | |||
| Overall | 0.11(−0.10, 0.31) | 0.294 | 52.1 |
| Publication bias | Z=0.41 | 0.680 | |
| Study length | |||
| <6 months | −0.20 (−0.66, 0.26) | 0.385 | 72.7 |
| 6–12 months | 0.06 (−0.24, 0.36) | 0.686 | 66.4 |
| >12 months | −0.089 (−0.81, 0.63) | 0.809 | 72.9 |
PRC, percent root coverage; CAL, clinical attachment level; KT, keratinized tissue; PD, probing depth; RW, recession width; RD, recession depth.
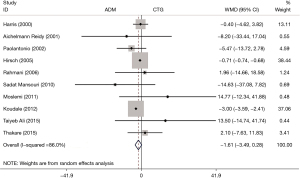
Gain in CAL
The CAL was mentioned in 17 RCTs. The pooled analysis demonstrated that patients who underwent ADM treatment had a higher gain in CAL than those who underwent CTG (WMD: 0.25, 95% CI: 0.03 to 0.47, P=0.026; I2=54.8%) (Table 2, Figure 3). Subgroup analysis also showed a higher gain in CAL of the ADM group than that of the CTG group regarding the study length (<6 months, WMD: 0.31, 95% CI: 0.04 to 0.58, P=0.027; 6–12 months, WMD: 0.29, 95% CI: 0.18 to 0.39, P<0.001; >12 months, WMD: 0.39, 95% CI: 0.16 to 0.63, P=0.001) (Table 2).
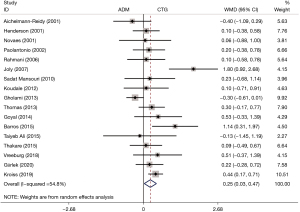
Gain in KT width
A total of 21 studies referenced the KT width. Patients who received the ADM treatment showed a smaller gain in KT width than those that underwent CTG (WMD: −0.44, 95% CI: −0.63 to −0.25, P<0.001; I2=56.8%) (Table 2, Figure 4). Subgroup analysis exhibited the same results in both the study length (<6 months, WMD: −0.29, 95% CI: −0.50 to −0.07, P=0.009; 6–12 months, WMD: −0.36, 95% CI: −0.53 to −0.19, P<0.001; >12 months, WMD: −0.78, 95% CI: −1.07 to −0.48, P<0.001) and quality assessment (high quality, WMD: −0.35, 95% CI: −0.50 to −0.20, P<0.001; low quality, WMD: −0.77, 95% CI: −1.23 to −0.30, P=0.001) (Table 2).
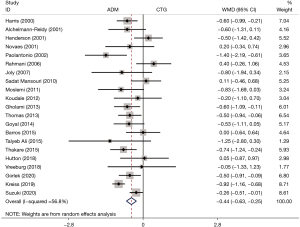
PD
The post-treatment PD was reported in 18 RCTs. No difference in PD was found between the ADM group and the CTG group (WMD: 0.07, 95% CI: −0.01 to 0.14, P=0.067; I2=39.9%) (Table 2, Figure 5).
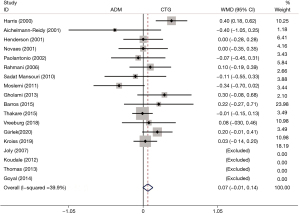
RW
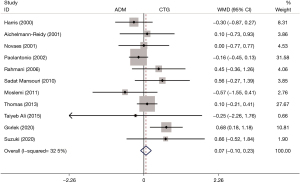
A total of 11 RCTs mentioned the post-treatment RW. There was no difference in RW between the two groups (WMD: 0.07, 95% CI: −0.10 to 0.23, P=0.437; I2=32.5%) (Table 2, Figure 6).
RD
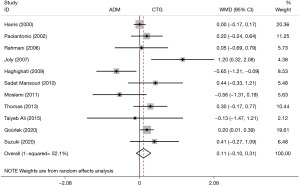
The outcome of RD was reported in 11 RCTs. The pooled analysis did not detect a difference in RD between patients who underwent ADM and those receiving CTG (WMD: 0.11, 95% CI: −0.10 to 0.31, P=0.294; I2=52.1%) (Table 2, Figure 7).
Publication bias
No publication bias was found in PRC (Z=0.55, P=0.583), gain in CAL (Z=−0.20, P=0.843), gain in KT width (Z=−0.02, P=0.986), PD (Z=−0.26, P=0.795), RW (Z=−0.09, P=0.928), and RD (Z=0.41, P=0.680) (Table 2).
Discussion
The prevalence of gingival recession is high worldwide. The objective of the present meta-analysis was to make a comparison between ADM and the gold standard, CTG, for root coverage in patients with gingival resection. A total of 24 RCTs including 587 participants were included, and the results showed that patients who accepted ADM had a higher gain in CAL but a smaller gain in KT width than those receiving CTG; no differences were found between these two techniques in PRC, PD, RW, and RD. These findings suggest that patients with gingival recession may experience a benefit from the ADM treatment that is comparable to the CTG as the gold standard, especially in gaining CAL, but CTG may have a significant advantage over ADM in gaining KT width.
Both ADM and CTG have good efficacy in gaining CAL during root coverage procedures (13,38). In contrast to the previous studies that showed no significant differences in gaining CAL between different treatment modalities (7,40), our study exhibited that patients who underwent ADM treatment had a higher gain in CAL than those who receiving CTG, as supported by the Kroiss et al. study results at the time of 6-month examination (13).
Cieślik-Wegemund et al. found a similar gain in KT width between ADM and CTG after the tunnel technique (41). Joly et al. also obtained similar results (25). The results of the meta-analysis performed by Gallagher et al. were in favor of ADM regarding gain of KT width (42). They speculated that the reason for keratinizing effects might primarily result from the dermal origin of the graft or, more probably, from the migration of host cells, which were likely to trigger the keratinization of the overlying epithelium (36,37). On the contrary, our study found that patients who received the ADM treatment had a smaller gain in KT width than those who received CTG treatment, which was in line with the results reported by Shori et al. and by Harris that CTG had an advantage in gaining KT width (37,43). An RCT also demonstrated that the KT showed a significant increase after 3 and 6 months in both groups (31). It can be hypothesized from the results above that the gain in KT width after root coverage treatments may be influenced by several factors such as source of graft materials and the flap design.
The development of keratinized epithelium can only be induced by the cells from periodontal ligament and gingival connective tissue (44). The induction characteristic of ADM grafts will rely on the percentage of colonization of non-vital graft by the host cell deriving from these tissues which can induce keratinization. In contrast, CTGs are entirely composed of the tissues capable of inducing the epithelial keratinization. A significantly higher PRC after CTG treatment was reported by Cieślik-Wegemund et al. (41) and Pietruska et al. (45). However, no significant difference was found in PRC between the ADM group and the CTG group, and neither in PD, RW, or RD, which were in accordance with the previous studies (16,19,40).
The major superiority of our meta-analysis was that more studies and outcomes (KT width, PD, RW, and RD) were included compared with the previous meta-analysis (42), which made our results more reliable and convincing. However, there were several limitations that should be interpreted cautiously. First, the description of the randomization method was inadequate in some studies, which may have influenced the accuracy of the results. Second, only a small number of included studies involved long-term follow-up, leading to the limited applicability to clinical situations. Third, other data sources, which are not considered in this study, may increase the quantity and quality of RCTs retrieved. Due to the small number of studies included, the results should be interpreted with caution. In the future, more well-designed studies with long-term follow-up need to be conducted to further verify our findings.
Conclusions
Our results suggested that the ADM treatment for patients with gingival recession might be superior to CTG in gaining CAL, but CTG might have a significant advantage over ADM in gaining KT width.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-656/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-656/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fageeh HN, Meshni AA, Jamal HA, et al. The accuracy and reliability of digital measurements of gingival recession versus conventional methods. BMC Oral Health 2019;19:154. [Crossref] [PubMed]
- George SG, Kanakamedala AK, Mahendra J, et al. Treatment of gingival recession using a coronally-advanced flap procedure with or without placental membrane. J Investig Clin Dent 2018;9:e12340. [Crossref] [PubMed]
- Yan J, Zhang J, Zhang Q, et al. Effectiveness of laser adjunctive therapy for surgical treatment of gingival recession with flap graft techniques: a systematic review and meta-analysis. Lasers Med Sci 2018;33:899-908. [Crossref] [PubMed]
- Shkreta M, Atanasovska-Stojanovska A, Dollaku B, et al. Exploring the Gingival Recession Surgical Treatment Modalities: A Literature Review. Open Access Maced J Med Sci 2018;6:698-708. [Crossref] [PubMed]
- Merijohn GK. Management and prevention of gingival recession. Periodontol 2000 2016;71:228-42. [Crossref] [PubMed]
- Mythri S, Arunkumar SM, Hegde S, et al. Etiology and occurrence of gingival recession - An epidemiological study. J Indian Soc Periodontol 2015;19:671-5. [Crossref] [PubMed]
- Chambrone L, Chambrone D, Pustiglioni FE, et al. Can subepithelial connective tissue grafts be considered the gold standard procedure in the treatment of Miller Class I and II recession-type defects? J Dent 2008;36:659-71. [Crossref] [PubMed]
- Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol 1985;56:715-20. [Crossref] [PubMed]
- Liu CL, Weisgold AS. Connective tissue graft: a classification for incision design from the palatal site and clinical case reports. Int J Periodontics Restorative Dent 2002;22:373-9. [PubMed]
- Cordioli G, Mortarino C, Chierico A, et al. Comparison of 2 techniques of subepithelial connective tissue graft in the treatment of gingival recessions. J Periodontol 2001;72:1470-6. [Crossref] [PubMed]
- Agarwal C, Purohit P, Sharma SK, et al. Modified Approach of Double Papillae Laterally Positioned Flap Technique using Alloderm® for Root Coverage. J Clin Diagn Res 2014;8:ZD25-7. [Crossref] [PubMed]
- Al-Hamdan K. Long-term predictability of allogenic dermal matrix for root coverage: Three years observation period on 15 consecutive cases. Saudi Dent J 2021;33:99-104. [Crossref] [PubMed]
- Kroiss S, Rathe F, Sader R, et al. Acellular dermal matrix allograft versus autogenous connective tissue grafts for thickening soft tissue and covering multiple gingival recessions: a 5-year preference clinical study. Quintessence Int 2019;50:278-85. [PubMed]
- Barootchi S, Tavelli L, Gianfilippo RD, et al. Acellular dermal matrix for root coverage procedures: 9-year assessment of treated isolated gingival recessions and their adjacent untreated sites. J Periodontol 2021;92:254-62. [Crossref] [PubMed]
- Maluta R, Monteiro MF, Peruzzo DC, et al. Root coverage of multiple gingival recessions treated with coronally advanced flap associated with xenogeneic acellular dermal matrix or connective tissue graft: a 6-month split-mouth controlled and randomized clinical trial. Clin Oral Investig 2021;25:5765-73. [Crossref] [PubMed]
- Barros RR, Macedo GO, de Queiroz AC, et al. A modified surgical flap for root coverage in association with grafting materials. J Esthet Restor Dent 2015;27:84-91. [Crossref] [PubMed]
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [Crossref] [PubMed]
- Aichelmann-Reidy ME, Yukna RA, Evans GH, et al. Clinical evaluation of acellular allograft dermis for the treatment of human gingival recession. J Periodontol 2001;72:998-1005. [Crossref] [PubMed]
- Gürlek Ö, Gümüş P, Nizam N, et al. Coronally advanced flap with connective tissue graft or xenogeneic acellular dermal matrix in the treatment of multiple gingival recessions: A split-mouth randomized clinical trial. J Esthet Restor Dent 2020;32:380-8. [Crossref] [PubMed]
- Haghighati F, Mousavi M, Moslemi N, et al. A comparative study of two root-coverage techniques with regard to interdental papilla dimension as a prognostic factor. Int J Periodontics Restorative Dent 2009;29:179-89. [PubMed]
- Harris RJ. A comparative study of root coverage obtained with an acellular dermal matrix versus a connective tissue graft: results of 107 recession defects in 50 consecutively treated patients. Int J Periodontics Restorative Dent 2000;20:51-9. [PubMed]
- Henderson RD, Greenwell H, Drisko C, et al. Predictable multiple site root coverage using an acellular dermal matrix allograft. J Periodontol 2001;72:571-82. [Crossref] [PubMed]
- Hirsch A, Goldstein M, Goultschin J, et al. A 2-year follow-up of root coverage using sub-pedicle acellular dermal matrix allografts and subepithelial connective tissue autografts. J Periodontol 2005;76:1323-8. [Crossref] [PubMed]
- Hutton CG, Johnson GK, Barwacz CA, et al. Comparison of two different surgical approaches to increase peri-implant mucosal thickness: A randomized controlled clinical trial. J Periodontol 2018;89:807-14. [Crossref] [PubMed]
- Joly JC, Carvalho AM, da Silva RC, et al. Root coverage in isolated gingival recessions using autograft versus allograft: a pilot study. J Periodontol 2007;78:1017-22. [Crossref] [PubMed]
- Moslemi N, Mousavi Jazi M, Haghighati F, et al. Acellular dermal matrix allograft versus subepithelial connective tissue graft in treatment of gingival recessions: a 5-year randomized clinical study. J Clin Periodontol 2011;38:1122-9. [Crossref] [PubMed]
- Novaes AB Jr, Grisi DC, Molina GO, et al. Comparative 6-month clinical study of a subepithelial connective tissue graft and acellular dermal matrix graft for the treatment of gingival recession. J Periodontol 2001;72:1477-84. [Crossref] [PubMed]
- Paolantonio M, Dolci M, Esposito P, et al. Subpedicle acellular dermal matrix graft and autogenous connective tissue graft in the treatment of gingival recessions: a comparative 1-year clinical study. J Periodontol 2002;73:1299-307. [Crossref] [PubMed]
- Rahmani ME, Lades MA. Comparative clinical evaluation of acellular dermal matrix allograft and connective tissue graft for the treatment of gingival recession. J Contemp Dent Pract 2006;7:63-70. [Crossref] [PubMed]
- Sadat Mansouri S, Ayoubian N, Eslami Manouchehri M. A comparative 6-month clinical study of acellular dermal matrix allograft and subepithelial connective tissue graft for root coverage. J Dent (Tehran) 2010;7:156-64. [PubMed]
- Suzuki KT, de Jesus Hernandez Martinez C, Suemi MI, et al. Root coverage using coronally advanced flap with porcine-derived acellular dermal matrix or subepithelial connective tissue graft: a randomized controlled clinical trial. Clin Oral Investig 2020;24:4077-87. [Crossref] [PubMed]
- Taiyeb Ali TB, Shapeen IM, Ahmed HB, et al. Efficacy of acellular dermal matrix and autogenous connective tissue grafts in the treatment of gingival recession defects among Asians. J Investig Clin Dent 2015;6:125-32. [Crossref] [PubMed]
- Thomas LJ, Emmadi P, Thyagarajan R, et al. A comparative clinical study of the efficacy of subepithelial connective tissue graft and acellular dermal matrix graft in root coverage: 6-month follow-up observation. J Indian Soc Periodontol 2013;17:478-83. [Crossref] [PubMed]
- Sean K. Vreeburg GRG, Rossmann JA. A Comparative Study of Root Coverage using OrACELL™ Versus Subepithelial Connective Tissue Graft: A Randomized Controlled Trial. The Open Dentistry Journal 2018;12:977-86. [Crossref]
- Thakare P, Baliga V, Bhongade ML. Comparative evaluation of the effectiveness of acellular dermal matrix allograft and subepithelial connective tissue to coronally advanced flap alone in the treatment of multiple gingival recessions: A clinical study. J Indian Soc Periodontol 2015;19:537-44. [Crossref] [PubMed]
- Goyal N, Gupta R, Pandit N, et al. Analysis of patient acceptance following treatment of Miller's class II gingival recession with acellular dermal matrix and connective tissue graft. J Indian Soc Periodontol 2014;18:352-6. [Crossref] [PubMed]
- Shori T, Kolte A, Kher V, et al. A comparative evaluation of the effectiveness of subpedicle acellular dermal matrix allograft with subepithelial connective tissue graft in the treatment of isolated marginal tissue recession: A clinical study. J Indian Soc Periodontol 2013;17:78-81. [Crossref] [PubMed]
- Gholami GA, Saberi A, Kadkhodazadeh M, et al. Comparison of the clinical outcomes of connective tissue and acellular dermal matrix in combination with double papillary flap for root coverage: A 6-month trial. Dent Res J (Isfahan) 2013;10:506-13. [PubMed]
- Koudale SB, Charde PA, Bhongade ML. A comparative clinical evaluation of acellular dermal matrix allograft and sub-epithelial connective tissue graft for the treatment of multiple gingival recessions. J Indian Soc Periodontol 2012;16:411-6. [Crossref] [PubMed]
- Chambrone L, Sukekava F, Araújo MG, et al. Root-coverage procedures for the treatment of localized recession-type defects: a Cochrane systematic review. J Periodontol 2010;81:452-78. [Crossref] [PubMed]
- Cieślik-Wegemund M, Wierucka-Młynarczyk B, Tanasiewicz M, et al. Tunnel Technique With Collagen Matrix Compared With Connective Tissue Graft for Treatment of Periodontal Recession: A Randomized Clinical Trial. J Periodontol 2016;87:1436-43. [Crossref] [PubMed]
- Gallagher SI, Matthews DC. Acellular dermal matrix and subepithelial connective tissue grafts for root coverage: A systematic review. J Indian Soc Periodontol 2017;21:439-48. [PubMed]
- Harris RJ. A short-term and long-term comparison of root coverage with an acellular dermal matrix and a subepithelial graft. J Periodontol 2004;75:734-43. [Crossref] [PubMed]
- Karring T, Lang NP, Löe H. The role of gingival connective tissue in determining epithelial differentiation. J Periodontal Res 1975;10:1-11. [Crossref] [PubMed]
- Pietruska M, Skurska A, Podlewski Ł, et al. Clinical evaluation of Miller class I and II recessions treatment with the use of modified coronally advanced tunnel technique with either collagen matrix or subepithelial connective tissue graft: A randomized clinical study. J Clin Periodontol 2019;46:86-95. [Crossref] [PubMed]
(English Language Editor: J. Jones)



