
The efficacy and safety of biliary stenting alone versus stenting combined with iodine-125 seed strand implantation for the treatment of cholangiocarcinoma with malignant obstructive jaundice: a prospective, nonrandomized, controlled clinical study
Introduction
Cholangiocarcinoma is a malignant tumor involving the intrahepatic and perihilar regions and distal biliary tracts (1). Progressive cholangiocarcinoma is often accompanied by malignant obstructive jaundice (MOJ) and surgery is difficult to perform (2), and thus the tumor has a very poor prognosis. For these patients, effective bile drainage should be implemented as soon as possible (3) to prevent the continuous deterioration of liver function. It is also necessary to actively treat the tumor.
Currently, commonly used interventional treatments for jaundice include percutaneous transhepatic cholangial drainage (PTCD) and percutaneous transhepatic insertion of a biliary stent (PTIBS). As a brachytherapy technique, iodine-125 seed implantation has been tried to use in the biliary tract in recent years and has achieved satisfactory clinical results in some patients (4). However, there is no high-level evidence-based medical evidence that the treatment is safe and effective so far. In this study, we compared the safety and efficacy of biliary stenting alone and stenting combined with iodine-125 seed strand implantation to provide a reference for clinical practice. We present the following article in accordance with the TREND reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-676/rc).
Methods
Materials
Research subjects
This study was a prospective, nonrandomized, concurrent controlled trial. Patients with biliary malignancies combined with MOJ who were treated at the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital) and the Second Affiliated Hospital of Wenzhou Medical University between January 2018 and September 2020 were included in this trial. After being fully informed of the conditions and risk, patients who voluntarily treated with iodine-125 seeds were included in the study group (PTCD + biliary stent implantation + iodine-125 seed implantation, n=30), and the remaining patients were included in the control group (PTCD + biliary stent implantation, n=30). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Zhejiang Cancer Hospital’s medical ethics committee (Ethical Approval No. IRB-2020-6). The Second Affiliated Hospital of Wenzhou Medical University was informed and agreed the study. Signed informed consent was taken from all the patients.
- The inclusion criteria were: age ≥18 and ≤75 years; pathological diagnosis of cholangiocarcinoma; no indications for surgery; platelet (PLT) count ≥60×109/L, hemoglobin (HB) level ≥80 g/L, and prolonged prothrombin time (PT) <4 seconds; and performance status (PS) score of 0 to 1.
- The exclusion criteria were: age <18 or >75 years; poor general condition (PS score ≥2); dysfunction of important organs such as the heart, lung, liver, and kidney; patients expected to be unable to tolerate the intervention; diffuse biliary strictures where stents cannot pass; PLT count <60×109/L, HB level <80 g/L, and prolonged PT ≥4 seconds; extensive systemic tumor metastasis; and an expected survival time of 3 months.
Materials and devices
- Iodine-125 seeds were provided by Beijing Zhibo Hi-tech Biotechnology Co., Ltd. (Beijing, China; National Drug Approval Number H20083013). The seed activity was 0.6–0.8 mCi, the average energy was 27–35 keV, and the half-life was 59.4 days.
- The biliary self-expanding metal stents used included Niti-S (Taewoong, Busan, South Korea), HANAROSTENT (M.I. Tech, Pyeongtaek, South Korea), Zilver (Cook Medical, Bloomington, IN, USA), and WallFlex (Boston Scientific, Natick, MA, USA). The diameter of the stents was 6–8 mm, and the length was 60–100 mm.
- Other equipment included an iodine-125 seed implantation device, treatment planning system (TPS; Fitian Zhaoye Technology Co., Ltd., Beijing, China), PTCD puncture and drainage kit (Argon Medical Devices, Plano, TX, USA), and an interventional catheter and guidewire.
Study methods
Preoperative preparation
All patients were required to complete routine blood, liver and kidney function tests, and coagulation function tests, along with enhanced computed tomography (CT) and/or magnetic resonance cholangiopancreatography (MRCP) before operation. The degree of biliary stricture was assessed based on imaging findings. All patients underwent PTCD first, and biliary stent implantation or biliary stent combined with iodine-125 seed strand implantation was performed at the same time or within 2 weeks of operation. Signed informed consent was obtained before operation.
Preparation of iodine-125 seed strand
The TPS was used to formulate seed implantation plans before operation. Total activity and number of seeds required for operation were calculated. The specific long diameter of the implanted seeds was calculated using the following formula: length of obstructed segment (mm)/4.5 + 2. The number of seeds was increased or decreased as appropriate according to the patient’s condition. The 4F tube in the PTCD drainage kit was cut to the required length, and the distal end was heated and sealed. The seeds required for the plan were individually inserted into the tube, and the proximal lumen was sealed with a gelatin sponge strip to ensure the seeds were in a fixed position.
Interventional operation
- PTCD: under ultrasound guidance, the intrahepatic bile duct was punctured with a 22-G puncture needle followed by cholangiography. Under fluoroscopy, the appropriate biliary tract branch was selected, and the target bile duct was punctured again. A 6-F triple set was implanted after successful puncture, through which a 0.035-inch supersmooth guidewire was placed into the common bile duct in a sheath, and an 8F drainage tube was imported. Angiography was performed again to confirm the position of the drainage tube and to exclude spillage of the contrast agent.
- Biliary stent implantation: the required length of the stent was determined by cholangiography, a 0.038-inch super rigid exchange guidewire was used to cross the stenosis segment, and the distal end of the common bile duct or distal part opening into the duodenum was located. The stent system was implanted under the guidance of a long sheath such that the marked points at both ends were at least 1 cm above the obstructed segment, and then the stent was released. The effect was confirmed by cholangiography again.
- Iodine-125 seed strand implantation: the prepared iodine-125 seed strand was placed into the biliary tract through the 8F biliary drainage tube. The seed strand and the end of the drainage tube were then fixed to each other and connected with a 3-way tube so that they could still be used for bile drainage. The seed strand and the stent were placed into the stenosis segment at the same time, and the guidewire was withdrawn while the stent was released such that the seed strand was fixed between the bile duct wall and the stent, and the irradiation range of the seeds covered the obstructed segment (Figure 1).
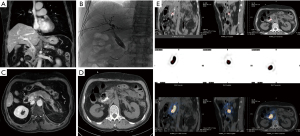 Figure 1 A 67-year-old man who had a recurrence of gallbladder cancer after operation. The tumor involved the lower segment of the common bile duct, resulting in obstructive jaundice. The process for the implantation of biliary stents and iodine-125 seed strand. (A,B) The patient experienced recurrence of gallbladder cancer and obstructive jaundice after operation. PTCD was performed. The repeated abdominal MRI showed that the intrahepatic bile duct was slightly dilated. A nodular shadow was observed behind the head of the pancreas, measuring approximately 2.5 cm × 1.7 cm, with indistinct boundaries with the head of the pancreas and the adjacent portal vein. Uneven enhancement and involvement of the lower end of the common bile duct were observed. (C,D) Cholangiography confirmed the obstruction of the lower segment of the common bile duct. A biliary stent (8 mm× 60 mm) was implanted, and an iodine-125 seed strand (0.7 mCi, 10 pieces) was implanted through the PTCD tube. (E) On the second day after operation, iodine-125 whole-body scan and SPECT were performed, suggesting the presence of a strip-shaped radioactive aggregation shadow in the abdominal hepatobiliary area and no significant radioactive aggregation throughout other tissues in the body. PTCD, percutaneous transhepatic cholangial drainage; MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography.
Figure 1 A 67-year-old man who had a recurrence of gallbladder cancer after operation. The tumor involved the lower segment of the common bile duct, resulting in obstructive jaundice. The process for the implantation of biliary stents and iodine-125 seed strand. (A,B) The patient experienced recurrence of gallbladder cancer and obstructive jaundice after operation. PTCD was performed. The repeated abdominal MRI showed that the intrahepatic bile duct was slightly dilated. A nodular shadow was observed behind the head of the pancreas, measuring approximately 2.5 cm × 1.7 cm, with indistinct boundaries with the head of the pancreas and the adjacent portal vein. Uneven enhancement and involvement of the lower end of the common bile duct were observed. (C,D) Cholangiography confirmed the obstruction of the lower segment of the common bile duct. A biliary stent (8 mm× 60 mm) was implanted, and an iodine-125 seed strand (0.7 mCi, 10 pieces) was implanted through the PTCD tube. (E) On the second day after operation, iodine-125 whole-body scan and SPECT were performed, suggesting the presence of a strip-shaped radioactive aggregation shadow in the abdominal hepatobiliary area and no significant radioactive aggregation throughout other tissues in the body. PTCD, percutaneous transhepatic cholangial drainage; MRI, magnetic resonance imaging; SPECT, single-photon emission computed tomography.
Observation indicators
Changes in various biochemical indicators were detected before and within 1 week of operation. Clinical symptoms of the patients were observed, and adverse reactions were determined according to the grading standard for common adverse reactions from the National Cancer Institute (version 3.0). Individualized follow-up antitumor treatment was administered according to the patient’s condition.
The biochemical indicators were examined again at 4 weeks after operation, and a serum total bilirubin level that was 20% lower than the baseline level within 4 weeks of stent implantation was defined as clinical success. At the same time, the upper abdominal CT or magnetic resonance imaging (MRI) scan was repeated, and the images were read by 2 attending physicians with more than 5 years of experience in abdominal imaging diagnosis. Biliary patency was evaluated, and the short-term efficacy of the tumor treatment was evaluated according to the modified response evaluation criteria in solid tumors (mRECIST) criteria. The objective response rate (ORR) and disease control rate (DCR) of the patients were analyzed.
Regular outpatient visits and telephone follow-ups were performed. The occurrence of in-stent restenosis was determined based on the decrease and then subsequent increase in the bilirubin index combined with the CT/MRI examination. Stent patency time was defined as the time interval between stent implantation and in-stent restenosis or the time of last follow-up or death of patients without restenosis. Survival was defined as the time interval between stent implantation and death from any cause or the last follow-up. If death was due to other causes, the patient was classified as lost to follow-up. For patients who were lost to follow-up, the date of censored data was recorded as the date of the patient’s last follow-up. The follow-up period ended on December 31, 2020.
Statistical methods
Statistical analyses were performed using SPSS 22.0 statistical software. The t-test was used to compare continuous variables between the two samples. Chi-square test was used to compare categorical variables. Survival analysis and survival curve plotting were performed using the Kaplan-Meier method for univariate survival analysis. The log-rank test was used to compare prognostic values, and multivariate analysis was performed using the Cox proportional hazards model. P<0.05 was considered statistically significant.
Results
Sixty patients were enrolled in the study, with 30 receiving a biliary stent combined with iodine-125 seed strand implantation (study group) and 30 receiving biliary stent implantation alone (control group). The clinical and follow-up data of the enrolled patients improved, and tumor staging was based on the 2017 8th edition of the American Joint Committee on Cancer (AJCC) staging criteria for biliary malignancies (5).
Among the 60 patients, there were 38 males and 22 females. The patients were 39–80 years old (mean age 63.3 years), including 28 patients ≥65 years old and 32 patients <65 years old. The primary diseases included intrahepatic cholangiocarcinoma (37 cases), cholangiocarcinoma in the porta hepatis (20 cases), and carcinoma of the gallbladder (3 cases). The tumors were divided according to stage, and included 1 stage II tumor, 26 stage III tumors, and 33 stage IV tumors. According to the location of the obstruction, tumors were divided into 29 cases of high obstruction and 31 cases of low obstruction. Based on the obstruction range, 10 cases were designated short-segment obstruction (<3 cm), and 50 cases were designated long-segment obstruction (≥3 cm). Patients were stratified according to preoperative Child-Pugh score, with 2 patients classified as grade A, 55 as grade B, and 3 as grade C. The baseline characteristics of the two groups of patients are presented in Table 1. The baseline differences between the two groups were not statistically significant (P>0.05).
Table 1
| Baseline data | Study group (n=30) | Control group (n=30) | P value |
|---|---|---|---|
| Sex | 0.284 | ||
| Male | 21 | 17 | |
| Female | 9 | 13 | |
| Age (years) | 0.605 | ||
| <65 | 17 | 15 | |
| ≥65 | 13 | 15 | |
| Causes of jaundice | 0.739 | ||
| Primary lesion | 25 | 24 | |
| Metastases | 5 | 6 | |
| Distant metastasis | 0.795 | ||
| None | 13 | 14 | |
| Yes | 17 | 16 | |
| Obstruction site | 0.438 | ||
| High obstruction | 16 | 13 | |
| Low obstruction | 14 | 17 | |
| Obstruction range (cm) | 0.488 | ||
| <3 | 6 | 4 | |
| ≥3 | 24 | 26 | |
| Preoperative Child-Pugh score | 0.405 | ||
| ≤7 | 8 | 11 | |
| >7 | 22 | 19 | |
General information
All 60 patients successfully underwent the operation, and the technical success rate of the operation was 100%. Five patients (5/60, 8.3%) received double stent implantation, and the remaining patients (55/60, 91.7%) received a single stent implantation. In the study group, the activity of the seeds used was 0.6–0.8 mCi, and the number was 6–40 seeds/patient, with an average of 17.7 seeds/patient.
Safety evaluation
Among the patients, 5 cases of intervention-related adverse events occurred, including 3 cases in the study group (3/30, 10%) and 2 cases in the control group (2/30, 6.7%). The adverse events included 2 cases of infection, 2 cases of bleeding, and 1 case of drainage tube detachment. Two cases of severe adverse reactions were reported: 1 patient in the study group (1/30, 3.3%) developed postoperative biliary tract infection and secondary pancreatitis that improved after transfer to the Department of Infectious Diseases, and 1 patient in the control group (1/30, 3.3%) experienced severe bleeding in the puncture tract that improved after interventional hemostasis.
Efficacy evaluation
Improvement in jaundice
The preoperative total bilirubin levels in the two groups of patients were 268.14±114.97 µmol/L in the study group and 228.89±162.04 µmol/L in the control group. No significant difference in the baseline level was observed between the two groups (P=0.284).
The total bilirubin level in the two groups decreased significantly within 1 week of operation. The total bilirubin level in the study group decreased to 103.22±84.19 µmol/L, and that in the control group decreased to 138.27±92.69 µmol/L. The difference in total bilirubin levels between the two groups was not statistically significant (P=0.131). At 1 month after operation, the total bilirubin level decreased to 54.00±80.78 µmol/L in the study group and 58.80±61.14 µmol/L in the control group. The difference in total bilirubin levels between the two groups was not statistically significant (P=0.796).
The definition of clinical success was a more than 20% decrease in the total bilirubin level at 4 weeks after stent implantation compared with the baseline level. In the present study, the clinical success rates of the two groups of patients were both 96.7%, and the difference was not statistically significant (P=0.228).
Changes in liver function
The average preoperative Child-Pugh scores of the two groups were 7.83±0.59 points in the study group and 7.93±1.08 points in the control group. A significant difference in the baseline score was not observed between the two groups (P=0.659).
The liver function of the two groups of patients improved within 1 week of operation. The Child-Pugh score of the study group decreased to 7.30±0.99 points and that of the control group decreased to 7.47±1.28 points. The difference between the two groups was not statistically significant (P=0.574). At 1 month after operation, the Child-Pugh score of the study group decreased to 6.20±1.03 points, while that of the control group decreased to 7.07±1.39 points, and the difference between the two groups was statistically significant (P=0.008).
Evaluation of the short-term efficacy of tumor treatment
Follow-up examination was performed 4 weeks after operation. The short-term efficacy of the tumor treatment was evaluated based on the results of enhanced CT/MRI combined with auxiliary examinations, such as the measurement of tumor markers. In the study group, no patients achieved complete remission (CR), 2 achieved partial remission (PR), 23 achieved stable disease (SD), and 5 experienced progressive disease (PD). The ORR was 6.7% (2/30) and the DCR was 83.3%. In the control group, no patients achieved CR, 1 achieved PR, 17 achieved SD, and 12 experienced PD. The ORR was 3.3% (1/30) and the DCR was 60% (18/30). The difference in the ORR between the two groups was not statistically significant (P=1), but the difference in the DCR was statistically significant (P=0.045).
Survival analysis
Among the 60 patients, the median stent patency time was 41.71±3.46 weeks in the study group and 29.00±5.81 weeks in the control group, and the difference was statistically significant (Figure 2, P=0.037). The median overall survival (OS) was 51.00±7.77 weeks in the study group and 36.43±9.44 weeks in the control group, but the difference was not statistically significant (Figure 3, P=0.11). A subsequent multivariate survival analysis showed that patients with a decrease of more than 1 point in the Child-Pugh score at 4 weeks after operation had an independent OS advantage [relative risk (RR) =0.401, 95% confidence interval (CI): 0.164–0.982, P=0.046], while OS was not significantly correlated with sex, age, disease stage, site and extent of obstruction, or treatment method (P>0.05).
Discussion
In China, cholangiocarcinoma accounts for 3.9% of the incidence of malignant tumors, and the incidence is increasing annually. With a 5-year survival rate of only 10%, the prognosis of patients with cholangiocarcinoma is extremely poor, which seriously endangers people’s health.
The difficulty encountered in treating biliary tract tumors is that the early symptoms are not obvious and are thus difficult to detect. At the time of diagnosis, the tumor is frequently at an advanced stage and MOJ has occurred. At this time, the opportunity for surgery is often lost (2). The difficulty in implementing effective bile drainage is an important risk factor for death (3). For these patients, the current consensus on treatment is to open the stenotic segment as soon as possible to preserve liver function and create conditions for tumor treatment. Currently, commonly used interventional methods for lowering jaundice include PTCD and PTIBS, which are technically mature and widely used by hospitals at all levels. However, many patients experience rapid tumor progression during the process of reducing jaundice, thus losing the opportunity for follow-up treatment. In addition, stents only physically expand the stenotic segment but do not inhibit tumor growth. Therefore, the occurrence of in-stent restenosis must not be ignored (6). If the tumor can be treated at the same time as jaundice, the efficacy of MOJ treatment will be substantially improved.
In recent years, the intratumoral radiotherapy technique represented by iodine-125 seed implantation has become increasingly advanced and has been widely used to treat a variety of malignant tumors (4,7-9). Iodine-125 seeds release 80% of the energy in the range of 1 cm, and the implantation of iodine-125 seeds inside the tumor substantially increases the therapeutic dose administered to the tumor tissue, while the damage to the surrounding tissue is significantly reduced. Meanwhile, the half-life is approximately 60 days, which achieves uninterrupted continuous irradiation and kills or inhibits the growth of tumor cells at almost any stage (10-12). On these grounds, it has been proposed that in the treatment of biliary tumors with MOJ, biliary iodine-125 seed implantation be performed during the intervention for reducing jaundice. In recent years, researchers have conducted a series of relevant studies and obtained promising clinical results (4,13,14).
In animal experiments, irradiation with iodine-125 seeds at close range caused epithelial cell necrosis and inflammatory cell infiltration in biliary tract tissues. However, repair of the damaged epithelium was completed in approximately 60 days, and no significant radiological damage to the surrounding tissue was detected (15). Therefore, biliary intraparticle radiotherapy is safe and feasible, and many clinical studies have also confirmed its safety (6,8,14,16,17). Common adverse reactions of biliary seed implantation include biliary tract infection, pancreatitis, puncture tract bleeding, bile leakage, ascites leakage, and seed strand displacement, but few serious life-threatening complications occur. Five cases of adverse reactions occurred in the 60 patients in the present study. Among the 5 adverse reactions, there was 1 serious adverse event documented in the study group and 1 in the control group. The serious adverse events were associated with bile duct puncture and stent implantation, while no significant correlation with the implantation of the seed strand was observed. No radiological damage to normal organs was observed in the study. Therefore, we postulated that treatment with iodine-125 seed strand implantation in the bile duct was safe and reliable.
In general, after an intervention to reduce jaundice, if the bile drainage is smooth, the patient’s jaundice symptoms will quickly subside. As shown in the present study, the serum total bilirubin level decreased significantly in most patients after biliary drainage and stent implantation. The total bilirubin level in the study group decreased by an average of 49.4% within 1 week of operation compared with that before operation, while the average decrease in the control group was 31.2%. Within 1 month of operation, the total bilirubin level decreased by 79.8% in the study group, and the mean decrease was 71.9% in the control group, indicating that the efficiency of the treatment in ameliorating jaundice in the two groups of patients was comparable. With the clinical success criterion of a greater than 20% decrease in the total bilirubin level within 4 weeks of stent implantation compared with the baseline level, the clinical success rates of the two groups were both 96.7%. Therefore, regardless of whether the biliary stent was combined with iodine-125 seeds, the effect of reducing jaundice was equally reliable.
For patients with biliary tract tumors combined with MOJ, the primary goal of interventional therapy is to preserve liver function to the greatest extent such that tumor treatment can be started as soon as possible. In the present study, the liver function of most patients improved after treatment. At 1 week after operation, the degree of improvement in liver function was comparable between the two groups. At 1 month after operation, the reduction in Child-Pugh score in the study group was significantly greater than that in the control group (1.63±1.07 vs. 0.90±1.51 points, respectively; P=0.034). Therefore, stents combined with iodine-125 seed implantation in the biliary tract exerted a definite protective effect on liver function. A potential explanation is that when the seeds were continuously irradiating in the biliary tract for 1 month, the effect of local radiotherapy was observed. In the evaluation of antitumor efficacy, although a significant difference in the ORR was not observed between the two groups (P=1), the difference in the DCR was statistically significant (P=0.045). Therefore, local control of the tumors may have promoted the effective recovery of liver function.
After traditional biliary stent implantation, in-stent restenosis caused by tumor progression is a difficult problem to avoid. One study reported that the recurrence rate of biliary obstruction at 3 months, 6 months, and 12 months after MOJ simple biliary stent implantation was 14.3%, 21.0%, and 27.7%, respectively (18). Stents combined with biliary seed implantation have been shown to effectively reduce the risk of jaundice recurrence and significantly extend the patency time of the stent (6,14). In the current study, analysis of follow-up data showed that the median stent patency time of the study group was up to 41.71±3.46 weeks, which was significantly better than that of the control group (29.00±5.81 weeks). Based on these results, biliary seed implantation effectively inhibited tumor growth and prevented MOJ recurrence.
At present, seed implantation methods used by different centers vary. Ma et al. (19) used the stent combined with seed strand implantation method and observed a median stent patency period of 278 days and a median survival period of 394 days. Li et al. (20) achieved a median OS of 11 months by placing iodine-125 seeds into the biliary drainage tube, which was significantly better than the median OS of 9 months recorded after simple biliary drainage. Chen et al. (17) used the integrated iodine-125 seed stent and found that the median time of stent patency and OS time were significantly better than those of patients treated with a metal stent (8.1 vs. 3.9 months and 298 vs. 139 days, respectively). A multicenter randomized controlled study (21) found that seed stents achieved a lower stent restenosis rate than bare stents (90 days: 9% vs. 15%; 180 days: 16% vs. 27%; 360 days: 21% vs. 33%, respectively) and a longer median survival time (202 vs. 140 days, respectively). A consensus on which seed implantation method is better has not been reached. In the present study, inserting a drainage tube or a stent with a seed strand were both used and the 2 methods were equally effective.
In our study, the safety was satisfactory, regardless of the level of seed activity or stent type used. Comparison between single and double stents showed that jaundice reduction efficiency and median stent patency time of the latter were not as good as those of the former. After analyzing the potential reasons, we proposed that the parallel double-stent system is likely to cause obstruction of bile drainage, which is not conducive to rapid jaundice reduction. In the long term, excess stent mesh may become a site for adherence of sticky bile and necrotic tissues, thus increasing the susceptibility to biliary reobstruction. Therefore, for patients with complex biliary obstruction, we recommend the implantation of double stents in a nested manner or the implantation of a single stent combined with double drainage tubes to improve the efficiency of bile drainage.
Generally, biliary seed implantation is considered only a local treatment that does not significantly prolong OS. However, this study found a potential survival benefit. Among the 60 patients, the median OS of the study group was 51.00±7.77 weeks, while that of the control group was only 36.43±9.44 weeks, although this difference was not statistically significant (P=0.11). However, multivariate survival analysis showed that patients with a decrease of more than 1 point in the Child-Pugh score at 4 weeks after operation had an independent OS advantage (P=0.046). In combination with the information described above, a stent combined with iodine-125 seed implantation in the biliary tract exerted a definite protective effect on liver function. Therefore, we postulated that this treatment method rapidly and continuously relieved biliary obstruction, improved liver function, and improved the sensitivity and tolerance of patients to subsequent treatments, thereby prolonging the survival of patients. We hope that future in-depth studies will confirm this hypothesis.
In summary, biliary stents combined with seed implantation helped improve the liver function of patients with biliary tract tumors combined with MOJ, reduced the incidence of in-stent restenosis, and improved the overall treatment effect. However, the subjects of this study were not randomly enrolled, and the sample size was small, which may have affected the accuracy of the results. In the future, we hope to conduct in-depth prospective randomized controlled studies with large samples to provide references for clinical work.
Acknowledgments
Funding: This study was supported by the Medicine and Health Science Project of Zhejiang Province (No. 2020KY483) and the Major Medical and Health Science and Technology Project of Zhejiang Province (No. WKJ-ZJ-2002).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-676/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-676/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-676/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics committee of Zhejiang Cancer Hospital (No. IRB-2020-6), and signed informed consent was taken from all the patients. The Second Affiliated Hospital of Wenzhou Medical University was informed and agreed the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Satiya J, Schwartz I, Tabibian JH, et al. Ablative therapies for hepatic and biliary tumors: endohepatology coming of age. Transl Gastroenterol Hepatol 2020;5:15. [Crossref] [PubMed]
- Barkay O, Mosler P, Schmitt CM, et al. Effect of endoscopic stenting of malignant bile duct obstruction on quality of life. J Clin Gastroenterol 2013;47:526-31. [Crossref] [PubMed]
- Kurniawan J, Hasan I, Gani RA, et al. Mortality-related Factors in Patients with Malignant Obstructive Jaundice. Acta Med Indones 2016;48:282-8. [PubMed]
- Pang Q, Zhou L, Hu XS, et al. Biliary stenting alone versus biliary stenting combined with 125I particles intracavitary irradiation for the treatment of advanced cholangiocarcinoma. Sci Rep 2019;9:11348. [Crossref] [PubMed]
- Doescher J, Veit JA, Hoffmann TK. The 8th edition of the AJCC Cancer Staging Manual: Updates in otorhinolaryngology, head and neck surgery. HNO 2017;65:956-61.
- Jiao D, Zhou X, Li Z, et al. A newly designed biliary brachytherapy drainage catheter for patients with malignant biliary obstruction: A pilot study. J Cancer Res Ther 2020;16:286-91. [Crossref] [PubMed]
- Wang Y, Lu J, Guo JH, et al. A Novel Tracheobronchial Stent Loaded with 125I Seeds in Patients with Malignant Airway Obstruction Compared to a Conventional Stent: A Prospective Randomized Controlled Study. EBioMedicine 2018;33:269-75. [Crossref] [PubMed]
- Chi Z, Chen L, Huang J, et al. A novel combination of percutaneous stenting with iodine-125 seed implantation and chemotherapy for the treatment of pancreatic head cancer with obstructive jaundice. Brachytherapy 2021;20:218-25. [Crossref] [PubMed]
- Li S, Li L, Li B, et al. Safety and efficacy of endovascular implantation of a portal vein stent combined with iodine-125 seed-strips followed by transcatheter arterial chemoembolization with sorafenib for the treatment of hepatocellular carcinoma with portal vein tumor thrombosis. Br J Radiol 2020;93:20190279. [Crossref] [PubMed]
- He Y, Li L, Liu J, et al. Iodine-125 seed brachytherapy inhibits non-small cell lung cancer by suppressing epithelial-mesenchymal transition. Brachytherapy 2018;17:696-701. [Crossref] [PubMed]
- Lu Z, Dong TH, Si PR, et al. Continuous Low-dose-rate Irradiation of Iodine-125 Seeds Inhibiting Perineural Invasion in Pancreatic Cancer. Chin Med J (Engl) 2016;129:2460-8. [Crossref] [PubMed]
- Takabayashi K, Kashiwagi K, Kawata T, et al. Continuous low-dose irradiation by I-125 seeds induces apoptosis of gastric cancer cells regardless of histological origin. Cancer Biol Ther 2014;15:81-8. [Crossref] [PubMed]
- Hu X, Pang Q, Liu H, et al. Inflammation-based prognostic scores in patients with extrahepatic bile duct lesions treated by percutaneous transhepatic biliary stenting combined with 125I seeds intracavitary irradiation. Clin Transl Oncol 2019;21:665-73. [Crossref] [PubMed]
- Pan T, Li MA, Mu LW, et al. Stent placement with iodine-125 seeds strand effectively extends the duration of stent patency and survival in patients with unresectable malignant obstructive jaundice. Scand J Gastroenterol 2020;55:123-8. [Crossref] [PubMed]
- Chen Y, Wang XL, Yan ZP, et al. Damage to pig bile duct caused by intraluminal brachytherapy using a (125)I ribbon. Acta Radiol 2013;54:272-7. [Crossref] [PubMed]
- Cao J, Wang Z, Cai H, et al. Effect of PTCD-based biliary stent placement combined with 125I particle intracavitary irradiation in treating pancreatic head cancer. J BUON 2020;25:1056-62. [PubMed]
- Chen W, Fang XM, Wang X, et al. Preliminary clinical application of integrated 125I seeds stents in the therapy of malignant lower biliary tract obstruction. J Xray Sci Technol 2018;26:865-75. [Crossref] [PubMed]
- Zhang JX, Wang B, Liu S, et al. Predictors of Recurrent Biliary Obstruction Following Percutaneous Uncovered Metal Stent Insertion in Patients with Distal Malignant Biliary Obstruction: An Analysis Using a Competing Risk Model. Cardiovasc Intervent Radiol 2019;42:276-82. [Crossref] [PubMed]
- Ma J, Luo J, Gu J, et al. Malignant obstructive jaundice treated with intraluminal placement of Iodine-125 seed strands and metal stents: An analysis of long-term outcomes and prognostic features. Brachytherapy 2018;17:689-95. [Crossref] [PubMed]
- Li S, He X, Dang L, et al. Efficacy of 125I Versus Non-125I Combined with Transcatheter Arterial Chemoembolization for the Treatment of Unresectable Hepatocellular Carcinoma with Obstructive Jaundice. Dig Dis Sci 2018;63:321-8. [Crossref] [PubMed]
- Zhu HD, Guo JH, Huang M, et al. Irradiation stents vs. conventional metal stents for unresectable malignant biliary obstruction: A multicenter trial. J Hepatol 2018;68:970-7. [Crossref] [PubMed]
(English Language Editor: A. Muijlwijk)





