
Analysis of influencing factors of community-acquired pneumonia with deep venous thrombosis in elderly communities
Introduction
Both deep venous thrombosis (DVT) and pulmonary thromboembolism (PTE) are forms of venous thromboembolism (VTE), with DVT the most common. DVT is the third most common type of cardiovascular disease, with an annual incidence of 1–2/1,000 in the general population. Among VTE patients, approximately 27–56% show symptoms of PTE, while 20–50% of PTE patients experience venous thrombotic syndrome, indicating PTE patients with venous thrombotic syndrome are greatly incapacitated and have a reduced quality of life.
The results of large-scale international clinical studies showed the incidence of VTE was 4.96–14.90%, on the condition that thrombosis precautions were not taken by internal medicine inpatients, and around 5% of patients were likely to have fatal PTE. The incidence of VTE is higher in critically ill patients, and the incidence of VTE in intensive care unit (ICU) patients is 28% to 33%. A domestic study indicated the incidence of VTE in ICU patients was 27%, and the incidence of VTE in internal medicine inpatients was 12% (1). The early stage of sepsis (within 1 week) is the peak period of DVT (2,3). In this study, a retrospective case-control study was conducted to analyze the clinical characteristics and related influencing factors of DVT in elderly patients with community-acquired pneumonia (CAP). To explore the early warning indicators before DVT in elderly patients with CAP, so as to achieve early detection and early prevention. We present the following article in accordance with the STARD reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-557/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Beijing Shijitan Hospital affiliated to Capital Medical University [No. sjtkyll-lx-2021(46)]. Individual consent for this retrospective analysis was waived.
General information
DVT group
A total of 505 patients (≥70 years old) who were hospitalized in the Department of Respiratory and Critical Care Medicine of Beijing Shijitan Hospital Affiliated to Capital Medical University from January 2017 to December 2019 and met the diagnostic criteria for CAP were selected (4). There were 334 males, with an average age of 82.98±6.6 years old, and 171 females, with an average age of 82.29±6.0 years old. Each patient was assessed and evaluated as follows: (I) local tenderness, (II) swelling, (III) pitting edema, and/or (IV) erythema of each upper and lower extremity. On the condition that a central venous catheter (CVC) was present, the upper extremity was also assessed. All patients underwent color ultrasound examination of the upper and lower limb veins, and the DVT diagnostic criteria were as follows: the deep vein of the lower limb shows partial or complete filling of the deep vein cavity with low echo; probe compression lumen cannot be flattened; and patients stayed at hospital less than 48 hours with superficial vein thrombosis excluded. Eventually, 133 patients were selected as the DVT group.
Clinical classification of DVT: (I) proximal DVT: acute thrombosis involving the popliteal fossa and/or more deep vein segments of the lower extremity or any deep vein segment of the upper extremity. (II) Distal DVT: acute thrombosis in any deep vein segment at the distal end of the popliteal vein (5).
Control group
Patients with pneumonia were confirmed to not have DVT by intravenous color ultrasound examination, and 372 patients were selected as the control group.
Severe pneumonia group
A total of 57 patients met the criteria for severe pneumonia according to the diagnostic criteria for severe pneumonia in the Guidelines for the Diagnosis and Treatment of Community-Acquired Pneumonia in Chinese Adults (2016 edition) (4).
Patients who meet the following 1 major criteria or more than 3 secondary criteria can be diagnosed. (I) Main criteria: (i) endotracheal intubation is needed for mechanical ventilation; (ii) septic shock still needs vasoactive drugs after active fluid resuscitation. (II) Secondary criteria: (i) respiratory rate ≥30 beats/min; (ii) oxygenation index ≤50 mmHg; (iii) multiple lobes infiltration; (iv) disturbance of consciousness and/or disorientation; (v) blood urea nitrogen ≥7.14 mmol/L; (vi) active fluid resuscitation is needed for systolic blood pressure <90 mmHg.
Sepsis and septic shock group
The diagnostic criteria of sepsis are the diagnostic criteria of sepsis jointly issued by the European Association of severe Medicine and the American Association of severe Medicine in 2016: (I) patient has infection; (II) Sequential Organ Failure Score (SOFA) is >2. Septic shock was defined as sepsis accompanied by persistent hypotension, and vasoactive drugs were still needed to maintain mean arterial pressure ≥65 mmHg and blood lactic acid level >2 mmol/L after full volume resuscitation (6,7). The diagnostic criteria of septic shock were met in 50 cases. Exclusion criteria: active malignant tumor, prior local or distal metastasis, and/or chemotherapy and radiation within the previous 6 months.
Study methods
DVT risk assessment
According to recommendations on the prevention, treatment, and management of intra-hospital VTE, patients were assessed for DVT risk with the Padua scale (8). Padua score included: active cancer, the patient has previously received local or distal metastasis and/or radiotherapy within 6 months, previous VTE has been immobilized, the patient has thrombotic tendency, antithrombin deficiency, protein C or S deficiency, Leiden V factor and prothrombin G20210A mutation, antiphospholipid antibody syndrome recent trauma or surgical, elderly aged more than 70 years, heart and/or respiratory failure, acute myocardial infarction and/or ischemic stroke, acute infection and/or rheumatic disease, obesity [body mass index (BMI) ≥30 kg/m2], undergoing hormone therapy. The risk of VTE is divided into low-risk (0–3 points) and high-risk (≥4 points).
Evaluation indexes
Coagulation function, blood routine examination and C-reactive protein (CRP) were collected from all patients.
Statistical methods
SPSS23.0 statistical software (IBM, USA) was used for statistical analysis. The K-s was used to test the normality of measurement data, and the measurement data of normal distribution is expressed as . T-test was used to compare the difference between the two groups. M (P25, P75) was used to represent the measurement data of non-normal distribution, and Mann-Whitney U test was used to compare the two groups. The count data is expressed as the number of cases (%), and the difference between the two groups was compared using χ2 test. Risk factors in the DVT group were analyzed by logistic regression, and the relative risk odds ratio (OR) and 95% confidence interval (CI) of thrombosis risk grade were calculated. Pearson test was used for correlation analysis. Two-sided test P<0.05 was considered as statistically significant.
Results
Demographic data of pneumonia patients
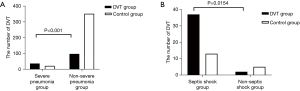
Among elderly patients with CAP, there were 334 males with an average age of (82.98±6.6) years, and 171 females with an average age of (82.29±6.0) years. The incidence of DVT was 26.3% (133/505). Among DVT patients, 25 (18.8%) were 70–79 years old, 89 (66.9%) were 80–89 years old, and 19 patients (14.3%) were over 90 years old. Among 133 cases of DVT, four cases showed symptomatic proximal DVT of the lower extremity, three showed asymptomatic proximal DVT of the lower extremity, 15 cases showed symptomatic distal DVT of the lower extremity, and 111 cases had asymptomatic distal DVT of the lower extremity. The number of CVC patients in the DVT group was higher than that in the control group (19.5/5.1), and the incidence of DVT was 15.6% (7/45) in 45 of the CVC patients. The incidence of distal DVT was 25.0% (126/505), and the incidence of asymptomatic distal DVT was 22.0% (111/505). Comparison for gender, age, and disease groups between groups of pneumonia patients, and the comparison of DVT incidence between groups of pneumonia patients, is shown in Figures 1,2.
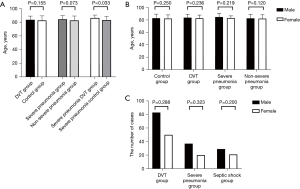
Padua score assessment
There were 316 cases of low-risk and 189 cases of high-risk clinically assessed by the Padua score, among which 50 cases and 83 cases were diagnosed with DVT, with diagnosis rates of 15.8% (50/316) and 43.9% (83/189), respectively. There was a statistically significant difference in the diagnosis rate of DVT between low-risk and high-risk patients (χ2=48.109; P<0.05).
Comparison of clinical indicators
The clinical indicators of DVT group and control group in pneumonia patients, as seen in Table 1.
Table 1
| Content | DVT group (n=133) | Control group (n=372) | P value |
|---|---|---|---|
| BMI (kg/m2), | 27.7±5.5 | 26.8±3.9 | 0.173 |
| Central vein catheterization, n (%) | 26 (19.5)* | 19 (5.1) | 0.034 |
| Septic shock, n (%) | 37 (27.8)* | 13 (3.5) | 0.015 |
| PT (s), | 13.39±7.11 | 12.51±2.63 | 0.064 |
| APTT (s), | 31.94±6.74 | 32.12±4.73 | 0.055 |
| INR | 1.18±0.44 | 1.12±0.26 | 0.078 |
| WBC (×109/L), | 9.58±5.54 | 10.27±5.06 | 0.122 |
| N (%), | 76.08±14.19 | 77.57±11.44 | 0.106 |
| PLT (×109/L), | 194±110 | 206±87 | 0.067 |
Compared with the control group, *, P<0.05. DVT, deep venous thrombosis; BMI, body mass index; PT, prothrombin time; APTT, activated partial thromboplastin time; INR, international normalized ratio; WBC, white blood cell; N, neutrophils; PLT, platelet.
DVT risk factors and logistic regression analysis
The results demonstrated CVC, the D-dimer value, Padua score, and other factors were significantly correlated with thrombosis, as seen in Table 2.
Table 2
| Content | OR | 95% CI | P value |
|---|---|---|---|
| Gender (male, female) | 1.045 | 0.688 to 1.585 | 0.837 |
| Age | 1.000 | 0.966 to 1.034 | 0.981 |
| Stay in bed | 2.482 | 0.779 to 1.423 | 0.125 |
| Central vein catheterization | 3.390 | 1.064 to 1.059 | 0.042 |
| Padua score (points) | 1.356 | 1.221 to 1.507 | <0.001 |
| D-dimer (ng/mL) | 1.000 | 1.000 to 1.001 | 0.043 |
| CRP (mg/L) | 0.996 | 0.986 to 1.002 | 0.277 |
| APTT (s) | 0.921 | 0.825 to 1.023 | 0.128 |
| PT (s) | 0.921 | 0.715 to 1.221 | 0.521 |
| INR | 8.635 | 0.162 to 1.182 | 0.271 |
| WBC (×109/L) | 1.034 | 0.900 to 1.182 | 0.657 |
| N (%) | 0.982 | 0.931 to 1.034 | 0.445 |
| PLT (×109/L) | 0.994 | 0.988 to 1.003 | 0.166 |
DVT, deep venous thrombosis; OR, odds ratio; CI, confidence interval; CRP, C-reactive protein; APTT, activated partial thromboplastin time; PT, prothrombin time; INR, international normalized ratio; WBC, white blood cell; N, neutrophils; PLT, platelet.
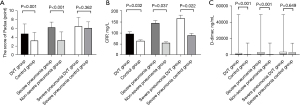
Comparison between Padua score, D-dimer, CRP
Padua score, CRP, D-dimer among groups. The results are shown in Figure 3.
Correlation analysis
The Padua score was positively correlated with the D-dimer (R=0.300; P<0.01),
Discussion
A review of a study in Western Europe, North America, Australia and southern Latin America found the incidence of VTE in the general population at 0.75–2.7 per 1,000 population (9). In both DVT and PTE, incidence rates are higher in men than women with a sex ratio of 1.2:1 (10,11). The incidence of DVT alone (45–117 per 100,000) is higher than that of PTE with or without DVT (29–78/100,000) (10). The incidence of DVT increases with age, with an annual incidence of 1.8/1,000 in 65–69 years old and 3.1/1,000 in 85–89 years old, although the incidence is higher in women of childbearing age. Up to 60% of VTE events occur in people over 70 years of age. The incidence of DVT per 1,000 person-years is 2.94 for age 75–79, and 4.73 for age ≥85 (11). Procoagulant factors such as coagulation factor VIII, coagulation factor VII, homocysteine and fibrinogen increase naturally with age (12). Inflammatory conditions associated with the development of concomitant diseases such as cancer and chronic diseases also contribute to the high incidence of VTE in the elderly population. A meta-analysis demonstrated that a history of VTE was the strongest predictive factor in the development of VTE. Older age, elevated CRP, D-dimer, and infections are all risk factors for the development of VTE in hospitalized patients (13). In this study, the incidence of DVT in patients with CAP was 26.3%, and the incidence of severe pneumonia was as high as 63.2%. However, there was no difference in the proportion of males and females with DVT (24.9/29.2). Qiu (14) found that VTE was detected in 6 (18%) patients with coronavirus disease 2019 (COVID-19) and 18 (35%) patients with CAP. The major type was distal DVT. Barba (15) found being over 70 years old was an independent risk factor for VTE and increased its risk by VTE by 8%, which may be associated with decreased muscle activity and tone and degenerative vascular lesions in the elderly.
In our study, the proportion of pneumonia patients complicated with DVT was highest at the age of 80–89. Research has shown the incidence of distal DVT is 4.7–37% (16), and usually the symptoms are asymptomatic or mild (17). When pulmonary embolism, does occur, 7–10% are isolated distal DVT (DVT limited below the popliteal vein of the lower limb), and 4–15% are isolated DVT which account for 31–56% of all diagnosed DVT of the lower limb (18). A study by Ciuti et al. (18) found that asymptomatic isolated distal DVT had a high incidence (16.2%) in a cohort of patients with medical emergencies. In our study, the incidence of distal DVT was 25% and the incidence of asymptomatic distal DVT was 22%, which is consistent with the literature.
Inflammatory diseases, especially acute infectious diseases, are the most common underlying diseases in DVT patients, and inflammation is an essential risk factor for the formation of DVT. Smeeth et al. (19) observed 7,278 cases of DVT patients with acute infection in the community and found 3,375 cases (46.4%) of DVT patients had one or more respiratory tract infections, and 2,258 cases (31.0%) had one or more urinary tract infections, while among PTE patients, 1,042 (2.7%) had one or more urinary tract infections. The results of that study illustrate acute infection is associated with a transient increase in the risk of DVT and PTE in a community setting, suggesting acute infection plays a role in triggering such events. In the infection-induced systemic inflammatory response syndrome, both deranged endothelial cells and activated monocytes produce pro-inflammatory cytokines that promote clotting. Inflammation of the blood vessel wall leads to the formation of intact venous thrombosis, where the inflammatory and clotting system are coupled through a common activation pathway. The first event of thrombosis might be the activation of endothelial cells, PLTs, and white blood cells (WBCs), the initiation of inflammation, and the formation of particles that trigger the clotting system through the induction of tissue factors. Therefore, the key event initiating venous thrombosis is likely to be venous wall inflammation. However, the contribution of specific immunomodulators has not been elucidated. Recently, studies have shown that VTE might be associated with other inflammatory markers such as CRP, interleukin (IL)-6, IL-8, and tumor necrosis factor-α (TNF-α) (20,21). These proinflammatory cytokines play an important role in VTE and promote the procoagulant state mainly by inducing the expression of tissue factors. In recent years, pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) in the early stage of sepsis have been shown to triggers the expression of monocyte tissue factor and the release of neutrophil extracellular trap (NET) in neutrophil, promoting immune thrombosis (22).
In our study, we found the incidence of DVT in severe pneumonia was higher than that in non-severe pneumonia (63.2/21.7), and severe pneumonia with septic shock was higher than in non-septic shock (70.0/28.6). Sepsis, especially when combined with hypotension and shock, is considered as a risk factor for VTE, including DVT and PTE in upper and lower limbs (23,24). Kaplan et al. (25) found a higher incidence of VTE (37.2%) in sepsis patients, although these patients utilized common and guideline recommended anticoagulants. Most patients (88%) had clinically significant VTE (symptomatic PTE, proximal DVT, or symptomatic distal DVT), and this phenomenon was significantly associated with how long they stayed in the ICU. The incidence of VTE in critically ill sepsis patients was significantly higher than in ICU patients without sepsis. In a prospective study of 261 critically ill patients (26), the majority (94.6%) admitted with a diagnosis other than sepsis had only a 9.6% incidence of DVT. In another retrospective study of 600 critically ill patients (27), only 3.0% developed VTE during ICU hospitalization, and this was mainly found in neurosurgical patients. The result of Kaplan support the observation that the incidence of VTE in sepsis patients is significantly higher (3–10 times) than ICU patients hospitalized for reasons other than sepsis.
Hemostasis and coagulation dysfunction in severe sepsis and septic shock differ from other non-infectious critical diseases. Although the exact mechanisms are not fully understood, sepsis induces a distinct pro-inflammatory and pre-thrombotic systemic environment. These abnormal reactions include cellular activation, deposition of intravascular PLT-fibrin thrombus, and the development of disseminated intravascular coagulation. These and other factors lead to a hypercoagulable state and may partly explain the high incidence of VTE in sepsis patients. CVC is closely associated with VTE in severe sepsis and septic shock, and studies have shown that the risk of DVT in an ipsilateral site after femoral vein catheterization in ICU patients increased by 6 times (28). In the study of Chen et al. (29), patients with CVC, especially femoral vein catheterization, were more likely to develop DVT. In our study, the CVC catheterization rate in the DVT group was higher than that in the control group, and 15.6% of patients developed DVT. Regression analysis demonstrated CVC was closely related to thrombosis. Obstacles in the veins cause changes in blood flow, creating turbulence in some areas and stasis in others. In addition, damage to the endothelium at the site of catheter insertion can cause venous thrombosis. At the same time, the results showed the incidence of septic shock in the DVT group was higher than in the control group (27.8/3.5). The results also suggest the risk of sepsis should be assessed in a timely manner in patients with pneumonia complicated with DVT, and observations for the occurrence of septic shock should be vigilant. Furthermore, CVC should be removed when no longer required to avoid the occurrence of DVT.
In terms of inflammatory indicators, this study found that CRP levels increased in the DVT group compared with the control group, and in the DVT group with severe pneumonia compared with the control group. CRP is a non-specific acute phase protein synthesized by liver, which can activate the complement system, promote phagocytosis, and regulate immunity. A common pathway and interaction between the inflammatory response and clotting pathways also exists. During inflammation, the body can induce clotting tissue factors to activate clotting through proinflammatory cytokines, influence inflammatory cells and endothelial cell receptors, and intensify inflammatory response; the inflammation and clotting interacting to form a cascade amplification effect. Studies have shown inflammation plays an important role in the pathophysiology of thrombosis, and elevated CRP levels increase the risk of DVT. A study of 246 patients with VTE showed the CRP level in a VTE group was significantly higher than that in a control group (30). Several prospective studies have described an association between CRP levels and the risk of developing VTE, including cancer and obesity, but there was no association between CRP and a higher risk of VTE. However, another prospective study (31) suggested CRP levels could be used as a diagnostic biomarker in suspected patients. The conclusion of this study is consistent with the above research.
D-dimer is produced during the decomposition of fibrin and serves as a marker of fibrinolytic activity. Elevated D-dimer levels have been reported in studies of patients with CAP (32,33). Relationships between proinflammatory cytokines and markers of clotting (including D-dimer) cascade activation have been demonstrated in critically ill patients and sepsis patients (34,35). Under inflammatory conditions, the hemostatic balance of alveoli is altered and prethrombotic activity predominates. In addition, pro-inflammatory cytokines may be involved in endothelial injury and may activate coagulation and inhibit fibrinolysis in patients with severe sepsis. The more severe the injury caused by infection, the more disordered the coagulation-fibrinolysis system and the higher the D-dimer value. This study showed the value of D-dimer of the severe pneumonia group was higher than that of non-severe pneumonia, and that of septic shock was higher than that of non-septic shock. Regression analysis illustrated the B-dimer value was correlated with the occurrence of DVT. Yu (36) found the D-dimer value was positively correlated with the pneumonia severity index score, and the D-dimer value was better than other coagulation indexes, such as prothrombin time (PT) and activated partial thromboplastin time (APTT), in predicting VTE, and the plasma D-dimer value was a high-risk factor for hospitalized death of elderly patients with severe pneumonia.
In 2010, Barbar et al. (8) designed and developed a Padua scoring model to evaluate the risk of VTE in internal medicine inpatients. In our study, the Padua score in patients with pneumonia combined with the DVT group was higher than that in the control group, and the score and prevalence of DVT in severe pneumonia patients was higher than those in non-severe pneumonia patients. Moreover, there was a statistically significant difference in the diagnostic rate of VTE between high-risk and low-risk patients clinically assessed by the Padua score. Regression analysis showed the Padua score was significantly correlated with DVT, and the risk of DVT was further increased as the disease progressed to septic shock. Elderly patients with pneumonia are often complicated with multiple diseases. Padua scores include respiratory failure, rheumatic diseases, obesity, current hormone therapy, acute myocardial infarction and/or ischemic stroke, and other risk factors for VTE, making the score more effective in diagnosing VTE in patients with acute infection. Liu et al. (37) suggested in a retrospective study that the Padua risk assessment model could effectively quantify the risk of VTE in hospitalized respiratory patients based on individual risk factors. On the other hand, in our study, although there was no significant difference in the Padua score between DVT patients with severe pneumonia and the control group, the scores of both groups were ≥4. Considering most elderly patients with severe pneumonia are bedridden and complicated with sepsis, and the risk of DVT is significantly increased, the Padua score is performed after admission to screen out highly suspected VTE patients from high-risk patients, and dynamic follow-up observation is conducted for patients with a high Padua score but negative DVT. This allows for timely and reasonably improved imaging examinations, improves the accuracy of VTE diagnosis, suggests drugs or machinery to prevent DVT, and further reduces the occurrence of PTE.
A deficiency of this study is that there is no further observation on the prevention of high-risk cases of DVT in pneumonia patients and the results of anticoagulant therapy combined with DVT. More attention should be paid to this in future prospective studies. In our retrospective study of elderly patients with CAP complicated with DVT, severe pneumonia, septic shock, and central venous catheterization were found to be risk factors for DVT. Padua scores combined with D-dimer detection can improve the diagnostic efficiency of DVT, and the prevention of DVT in patients with pneumonia could reduce the occurrence of VTE. At the same time, medical workers should pay increased attention to VTE and improve its prevention and treatment in hospitalized patients.
Acknowledgments
Funding: This work was supported by the National Key Research and Development Program (No. 2020YFC2005404).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-557/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-557/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-557/coif). All authors report that this work was supported by the National Key Research and Development Program (No. 2020YFC2005404). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Beijing Shijitan Hospital affiliated to Capital Medical University [No. sjtkyll-lx-2021(46)]. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; et al. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006;25:101-61.
- Wang P, Wang X, Zhang LJ, et al. The effect of low molecular heparin on the prognosis of severe pneumonia in elderly patients. Chinese Critical Care Medicine 2013;25:734-7. [PubMed]
- Cohoon KP, Ashrani AA, Crusan DJ, et al. Is Infection an Independent Risk Factor for Venous Thromboembolism? A Population-Based, Case-Control Study. Am J Med 2018;131:307-16.e2. [Crossref] [PubMed]
- Chinese Society of Respiratory Medicine. Guidelines for the Diagnosis and Treatment of Community-acquired Pneumonia in Chinese Adults (2016 edition). Chinese Journal of Tuberculosis and Respiratory Diseases 2016;39:253-79.
- Cook D, Meade M, Guyatt G, et al. Clinically important deep vein thrombosis in the intensive care unit: a survey of intensivists. Crit Care 2004;8:R145-52. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013;41:580-637. [Crossref] [PubMed]
- Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost 2010;8:2450-7. [Crossref] [PubMed]
- Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol 2014;34:2363-71. [Crossref] [PubMed]
- Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis 2016;41:3-14. [Crossref] [PubMed]
- Naess IA, Christiansen SC, Romundstad P, et al. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 2007;5:692-9. [Crossref] [PubMed]
- Mari D, Ogliari G, Castaldi D, et al. Hemostasis and ageing. Immun Ageing 2008;5:12. [Crossref] [PubMed]
- Darzi AJ, Karam SG, Charide R, et al. Prognostic factors for VTE and bleeding in hospitalized medical patients: a systematic review and meta-analysis. Blood 2020;135:1788-810. [Crossref] [PubMed]
- Qiu C, Li T, Wei G, et al. Hemorrhage and venous thromboembolism in critically ill patients with COVID-19. SAGE Open Med 2021;9:20503121211020167. [Crossref] [PubMed]
- Barba R, Zapatero A, Losa JE, et al. Venous thromboembolism in acutely ill hospitalized medical patients. Thromb Res 2010;126:276-9. [Crossref] [PubMed]
- Galanaud JP, Sevestre MA, Genty C, et al. Comparison of the clinical history of symptomatic isolated muscular calf vein thrombosis versus deep calf vein thrombosis. J Vasc Surg 2010;52:932-8, 938.e1-2.
- Palareti G, Schellong S. Isolated distal deep vein thrombosis: what we know and what we are doing. J Thromb Haemost 2012;10:11-9. [Crossref] [PubMed]
- Ciuti G, Grifoni E, Pavellini A, et al. Incidence and characteristics of asymptomatic distal deep vein thrombosis unexpectedly found at admission in an Internal Medicine setting. Thromb Res 2012;130:591-5. [Crossref] [PubMed]
- Smeeth L, Cook C, Thomas S, et al. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006;367:1075-9. [Crossref] [PubMed]
- Folsom AR, Lutsey PL, Astor BC, et al. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb Haemost 2009;102:615-9. [Crossref] [PubMed]
- Gao Q, Zhang P, Wang W, et al. The correlation analysis of tumor necrosis factor-alpha-308G/A polymorphism and venous thromboembolism risk: A meta-analysis. Phlebology 2016;31:625-31. [Crossref] [PubMed]
- Ito T. PAMPs and DAMPs as triggers for DIC. J Intensive Care 2014;2:67. [Crossref] [PubMed]
- Marik PE, Andrews L, Maini B. The incidence of deep venous thrombosis in ICU patients. Chest 1997;111:661-4. [Crossref] [PubMed]
- Attia J, Ray JG, Cook DJ, et al. Deep vein thrombosis and its prevention in critically ill adults. Arch Intern Med 2001;161:1268-79. [Crossref] [PubMed]
- Kaplan D, Casper TC, Elliott CG, et al. VTE Incidence and Risk Factors in Patients With Severe Sepsis and Septic Shock. Chest 2015;148:1224-30. [Crossref] [PubMed]
- Cook D, Crowther M, Meade M, et al. Deep venous thrombosis in medical-surgical critically ill patients: prevalence, incidence, and risk factors. Crit Care Med 2005;33:1565-71. [Crossref] [PubMed]
- Muscedere JG, Heyland DK, Cook D. Venous thromboembolism in critical illness in a community intensive care unit. J Crit Care 2007;22:285-9. [Crossref] [PubMed]
- Shorr AF, Williams MD. Venous thromboembolism in critically ill patients. Observations from a randomized trial in sepsis. Thromb Haemost 2009;101:139-44. [Crossref] [PubMed]
- Chen XL, Wang Y, Pan L. Validity of caprini risk assessment scale for assessing risk of deep venous thrombosis in elderly patients with severe pneumonia. Beijing Medical Journal 2016;38:989-93.
- Ming Y, Yan MS, Liu QX, et al. Correlation between serum PCT, inflammatory factors and APACHE II, SOFA Scores in patients with sepsis. Modern Medical Journal 2020;48:357-61.
- Yuan P, Zhou XY, Zhou SZ, et al. Significance of Level of Inflammatory Cytokines in Patients with Acute Thrombosis. Chinese Journal of Clinical Medicine 2009;16:20-1.
- Nordenholz KE, Mitchell AM, Kline JA. Direct comparison of the diagnostic accuracy of fifty protein biological markers of pulmonary embolism for use in the emergency department. Acad Emerg Med 2008;15:795-9. [Crossref] [PubMed]
- Snijders D, Schoorl M, Schoorl M, et al. D-dimer levels in assessing severity and clinical outcome in patients with community-acquired pneumonia. A secondary analysis of a randomised clinical trial. Eur J Intern Med 2012;23:436-41. [Crossref] [PubMed]
- Salluh JIF, Rabello LSCF, Rosolem MM, et al. The impact of coagulation parameters on the outcomes of patients with severe community-acquired pneumonia requiring intensive care unit admission. J Crit Care 2011;26:496-501. [Crossref] [PubMed]
- Shorr AF, Thomas SJ, Alkins SA, et al. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest 2002;121:1262-8. [Crossref] [PubMed]
- Yu XZ. Plasma D-dimer Level and APACHE II Score and Prognosis in Elderly Patients with Severe Pneumonia. Modern Practical Medicine 2015;27:1592-4.
- Liu H, Liu CL, Qing SM, et al. Validity of Padua risk assessment model in identifying high venous thromboembolism risk patients among hospitalized patients in department of respiratory medicine. International Journal of Respiration 2018;38:590-3.
(English Language Editor: B. Draper)


