
Efficacy and safety of dexmedetomidine-ropivacaine versus sufentanil-ropivacaine for epidural labor analgesia: a randomized controlled trial
Introduction
The pain associated with delivery can cause a series of neurophysiological changes, such as increased maternal stress hormones, elevated blood pressure, hyperventilation, decreased fetal oxygen transport, and psychological distress (1,2). Epidural analgesia is a central nerve block technique that is achieved by injecting local anesthetics near the nerve that transmits pain, and it is widely used for epidural labor analgesia because of its obvious effects, good safety, and convenience of operation (3,4). However, epidural labor analgesia may result in poor outcomes due to the use of anesthetics, such as motor block, maternal hypotension, prolonged second stage of labor, and urinary retention (3). Therefore, the selection of anesthetics is of great importance for epidural labor analgesia.
Ropivacaine is a long-acting amide local anesthetic, usually used in clinical practice for delivery, which has less toxic side effects to the central nervous system and no adverse effects on the fetus (5,6). At present, the combined use of local anesthetics and adjuvant drugs is common for epidural labor analgesia, which can reduce the dose of local anesthetics, improve the analgesic effect, avoid motor block, and reduce the incidence of related side effects (7). Sufentanil as an adjuvant to ropivacaine has been widely used for epidural labor analgesia, and can reduce the incidence of instrumental delivery, cesarean section, and postpartum hospitalization (8-10). However, sufentanil as an opioid may cause adverse effects such as respiratory depression, vomiting, headache, and urinary retention (11,12). Dexmedetomidine is an α2-adrenoceptor agonist that has been successfully used for epidural labor analgesia with fewer adverse effects (13,14). Several studies have shown that dexmedetomidine combined with ropivacaine had a better analgesic effect and shorter first-stage labor than sufentanil combined with ropivacaine (15,16). However, the efficacy and safety of dexmedetomidine combined with ropivacaine as a new type of epidural labor analgesia need to be further studied.
In this study, we hypothesized that dexmedetomidine-ropivacaine was superior to sufentanil-ropivacaine in epidural labor analgesia. The efficacy and safety of dexmedetomidine and sufentanil combined with ropivacaine for epidural labor analgesia were analyzed to explore a more effective and safe labor analgesia protocol in clinical practice. We present the following article in accordance with the CONSORT reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-264/rc).
Methods
Study design and participants
This triple-blinded, randomized controlled trial (RCT) was conducted between October 2020 and February 2021 at Chengdu Women’s and Children’s Central Hospital. A total of 160 parturient women were divided into the sufentanil combined with ropivacaine group (RS group) and the dexmedetomidine combined with ropivacaine group (RD group). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Chengdu Women’s and Children’s Central Hospital Ethics Committee [No. 2020(89)] and informed consent was taken from all the patients.
Women were eligible to participate if they met the following inclusion criteria: (I) age ≥18 years; (II) full-term primigravidae with singleton pregnancy (≥37 gestation weeks) and required labor analgesia in hospital; (III) met the criteria for American Society of Anesthesiology Physical Status I/II; (IV) were informed and willing to participate in the trial.
The exclusion criteria were as follows: (I) severe heart, lung, liver, and kidney diseases, hemorrhagic diseases, or other systemic diseases; (II) contraindications to the epidural anesthesia or allergic to the anesthetics used; (III) body temperature >37.5 °C before analgesia; (IV) cervical dilation >3 cm, non-cephalic pregnancy, cesarean section history, or labor induction history; (V) known genetic or congenital fetal malformations, fetal growth restriction, or oligohydramnios; (VI) malignant tumors or severe preeclampsia; (VII) lower abdominal surgery or urological surgery history; (VIII) spinal deformity or previous spinal surgery.
Interventions
Parturient women were randomly divided into 2 groups: (I) participants in the RD group received 10 mL 0.5 μg/mL dexmedetomidine combined with 0.1% ropivacaine; (II) participants in the RS group received 10 mL 0.5 μg/mL sufentanil combined with 0.1% ropivacaine. The mixed solutions of the 2 groups were infused continuously by the patient-controlled analgesia pump at a rate of 6 mL/h. Participants in both groups received the same treatments and obstetric care.
Outcomes
The primary outcomes were the pain relief of parturient women and the physical status of newborns. The duration of labor stages and adverse reactions of parturient women and newborns were secondary outcomes. Pain relief was quantified by visual analog scale (VAS) scores measured at 0, 15, and 120 min. The physical status of newborns was assessed through Apgar and neonatal behavioral neurological assessment (NBNA) scores. Apgar scores were measured at 1 and 5 min after delivery, and NBNA scores were measured 3 days later.
Sample size
According to the previous study (17), by setting the VAS score after epidural administration as the primary variable. The sample size was calculated by PASS software (two independent means), and 80 participants were assigned to each group with an α-error of 0.05 and a power of 0.9 (two-sided).
Randomization and blinding
Eligible participants were randomly assigned in a 1:1 ratio to 2 groups before the start of labor. Randomization was computer-generated, and the allocation was concealed through opaque serially numbered sealed envelopes. The computer randomly generated numbers from 0 to 160, and each participant was randomly assigned a number. The epidural labor analgesia plan corresponding to each number was executed by a designated person. Study physicians and outcome evaluators were blinded to the analgesic plan. Participants were blinded to the sufentanil or dexmedetomidine groups throughout the trial. Data collectors and statisticians were also unaware of group assignments.
Data collection
The demographic and baseline measurements including age, gestational age, body mass index (BMI), cervical dilation before analgesia, systolic blood pressure (SBP), diastolic blood pressure (DBP), respiratory rate, temperature, mean arterial pressure (MAP), and heart rate were recorded. Outcome indicators included VAS scores, Ramsay Sedation Scale (RSS) scores, blood loss, duration of labor stages (first and second stages), onset time of analgesia, the dose of analgesics, Apgar scores, NBNA scores, and adverse reactions (hypotension, tremble, nausea and vomiting, motor nerve block, bradycardia, and respiratory depression). The VAS score and RSS score were utilized to measure pain intensity and sedation level of parturient women, respectively. The total score of VAS is 10, with higher scores indicating greater pain intensity (0= no pain; 10= extreme pain). The RSS value is divided into 6 levels, with RSS value ≥2 representing better sedation (1= anxiety, restless; 2= patients cooperative and tranquil; 3= responsive to the commands; 4= asleep, brisk response to stimulus; 5= asleep, sluggish response to stimulus; 6= asleep, no response). The total score of Apgar score is 10, which is based on five signs of neonatal activity, pulse, grimace, appearance, and respiration (10= normal newborn; <7= mild asphyxia; <4= severe asphyxia). The NBNA score is a 20-item assessment of neonatal behavioral nerves with a total score of 40. A neonatal NBNA score >37 within 1 week is considered normal, and the neonatal NBNA score within 2 weeks cannot exceed 37, and long-term follow-up is required.
Procedures
After entering the delivery room, the parturient women’s venous channels were opened, and they were provided with cannulas for oxygen inhalation at 1–2 L/min. The vital signs of parturient women such as heart rate, respiratory rate, pulse oxygen saturation, and non-invasive blood pressure were monitored, and fetal heart rate was monitored by a Doppler fetal heart monitor. When cervical dilation was about 2 cm, epidural analgesia was performed. Parturient women were placed in the left decubitus position, with an 18-gauge epidural needle used for epidural puncture in the L2–3 interspace, and the head of the epidural catheter was inserted 3–4 cm into the epidural space. When the blood and cerebrospinal fluid aspiration test was negative, a test dose of 3 mL 1% lidocaine was given and observed for 5 min. If no adverse reactions were observed, parturient women received 10 mL 0.5 µg/mL dexmedetomidine or 0.5 µg/mL sufentanil combined with 0.1% ropivacaine as a loading dose, which was infused continuously by the patient-controlled analgesia pump at a rate of 6 mL/h. When the VAS scores were ≥5, a rapid bolus injection of 6 mL (lockout for 20 min) was given by a pump. The patient-controlled analgesia pump stopped during full cervical dilation. Local anesthetic solutions for epidural labor analgesia were prepared by another anesthetist, and the investigators were blinded to these solutions. The progress of cervical dilation was assessed by skilled midwives every 2 h during the incubation period and every hour during the active period.
Statistical analysis
Statistical analysis was performed with SPSS software (version 26.0; IBM Corp., Armonk, NY, USA). Quantitative variables were expressed as mean ± standard deviation (SD) and the groups were to be compared by using t-test, or presented as a median and interquartile range [M (Q25, Q75)] and the groups were to be compared by using Mann-Whitney U-test. Categorical variables were displayed as number and percentage [n (%)] and the comparison between groups was performed by Chi-square test (χ2 test). Because of the low incidence of adverse reactions in the parturient women and newborns, more than 20% of the expected values were less than 5 when the χ2 test was performed; therefore, Fisher’s exact test was used instead. Statistical analyses were two-sided tests and P<0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 160 parturient women were included between October 2020 and February 2021. Of these women, 80 were in the RD group and the other 80 were in group RS (Figure 1). Parturient women had a mean age of 29.0±3.3 years, and the range was 22 to 39 years. Comparing the baseline data of the 2 groups, the heart rate of parturient women in group RS was higher than that of group RD (86.4±11.58 vs. 82.6±8.0, P=0.016), while other demographic characteristics and vital signs had no significant differences (all P>0.05). There were also no significant differences between the 2 groups in terms of duration of labor stages, blood loss, onset time, and analgesic dose (P>0.05). The physical status of newborns assessed by NABA and Apgar scores showed no differences between the 2 groups (P>0.05). More detailed characteristics of parturient women and newborns are shown in Table 1.
Table 1
| Characteristics | Group RD (n=80) | Group RS (n=80) | P |
|---|---|---|---|
| Parturient women | |||
| Demographic characteristics | |||
| Age (years) | 28.9±3.5 | 29.2±3.2 | 0.669a |
| BMI (kg/m2) | 26.2±2.8 | 26.4±2.8 | 0.545a |
| Gestational age (weeks) | 39.4±0.9 | 39.2±0.9 | 0.754a |
| Vital signs | |||
| SBP (mmHg) | 112.9±10.0 | 114±10.4 | 0.359a |
| DBP (mmHg) | 73.2±7.8 | 73.4±8.1 | 0.921a |
| Respiratory rate (beats/min) | 19.8±0.7 | 19.6±1.1 | 0.152a |
| Heart rate (beats/min) | 82.6±8.0 | 86.4±11.5 | 0.016a |
| Body temperature (°C) | 36.6±0.2 | 36.6±0.2 | 0.747a |
| Cervical dilation before analgesia (cm) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | 0.811b |
| Effects | |||
| Duration of the first labor (min) | 323.8±118.6 | 311.8±103.1 | 0.493a |
| Duration of the second labor (min) | 132.0±38.4 | 138.1±36.9 | 0.312a |
| Number of women with blood loss, n (%) | 69 (86.30) | 69 (86.30) | 1.000c |
| Blood loss (mL) | 175.4±55.0 | 176.5±51.9 | 0.899a |
| Onset time of analgesia (min) | 10.9±4.7 | 10.5±1.5 | 0.433a |
| Dose of analgesics (mL) | 78.3±28.4 | 74.7±26.2 | 0.408a |
| Newborns | |||
| Demographic characteristics | |||
| Male, n (%) | 43 (53.80) | 43 (53.80) | 1.000c |
| Body weight (g) | 3084.3±275.0 | 3147.5±356.8 | 0.211a |
| Effects | |||
| NBNA scores | 39.9±0.4 | 39.8±0.5 | 0.368a |
| Apgar scores in 1 min | 9.8±0.7 | 9.7±0.8 | 0.424a |
| Apgar score in 5 min | 9.9±0.4 | 9.9±0.4 | 0.837a |
Group RD received 10 mL 0.5 μg/mL dexmedetomidine combined with 0.1% ropivacaine for epidural labor analgesia, and group RS received 10 mL 0.5 μg/mL sufentanil combined with 0.1% ropivacaine for epidural labor analgesia. The statistical methods used: a, Student’s t-test; b, Mann-Whitney U test; c, Chi square test. RD, dexmedetomidine-ropivacaine; RS, sufentanil-ropivacaine; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; NBNA, neonatal behavioral neurological assessment.
Pain relief during labor
The vital signs of parturient women were monitored during labor. The results showed that the body temperature, DBP, SBP, MAP, and heart rate of parturient women in group RD had no differences compared with those of group RS (all P>0.05). There were no significant differences in VAS scores between the 2 groups at the times of 0, 15, and 120 min (P>0.05). Regarding the VAS scores at 0 min, the pain relief at 15 min was obvious in both groups. The mean VAS scores at 0 min were 7.0 in the RD group and 7.2 in the RS group, and 15 min after epidural labor analgesia, the mean VAS scores were both 2.8. The mean RSS values in both groups were 1.8 at 0 min and 2.0 at both 15 and 120 min, with no significant difference between the 2 groups (P>0.05) (Figure 2; Table 2).
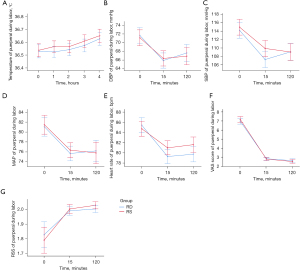
Table 2
| Indicators | Group RD (n=80) | Group RS (n=80) | P |
|---|---|---|---|
| Body temperature (°C) | |||
| 0 h | 36.50±0.22 | 36.54±0.24 | 0.811 |
| 1 h | 36.52±0.20 | 36.57±0.21 | 0.197 |
| 2 h | 36.54±0.23 | 36.57±0.19 | 0.478 |
| 3 h | 36.57±0.21 | 36.61±0.21 | 0.348 |
| 4 h | 36.63±0.22 | 36.65±0.24 | 0.519 |
| DBP (mmHg) | |||
| 0 min | 71.6±8.7 | 71.1±7.8 | 0.687 |
| 15 min | 65.9±8.0 | 66.3±7.8 | 0.697 |
| 120 min | 67.6±9.1 | 66.9±8.7 | 0.594 |
| SBP (mmHg) | |||
| 0 min | 114.1±7.8 | 114.9±9.3 | 0.569 |
| 15 min | 107.2±9.4 | 109.9±7.8 | 0.055 |
| 120 min | 109.0±9.2 | 109.0±8.7 | 0.979 |
| MAP (mmHg) | |||
| 0 min | 81.0±8.6 | 81.4±8.7 | 0.762 |
| 15 min | 75.6±6.6 | 76.3±7.0 | 0.521 |
| 120 min | 76.2±11.3 | 75.7±8.0 | 0.790 |
| Heart rate (beats/min) | |||
| 0 min | 85.4±6.0 | 84.7±7.2 | 0.510 |
| 15 min | 79.2±6.2 | 80.9±6.3 | 0.086 |
| 120 min | 79.7 ±5.7 | 81.6±7.8 | 0.840 |
| VAS scores | |||
| 0 min | 7.0±1.5 | 7.2±1.4 | 0.380 |
| 15 min | 2.8±0.8 | 2.8±0.8 | 0.766 |
| 120 min | 2.6±1.0 | 2.5±0.8 | 0.489 |
| RSS values | |||
| 0 min | 1.8±0.4 | 1.8±0.4 | 0.551 |
| 15 min | 2.0±0.1 | 2.0±0.2 | 0.566 |
| 120 min | 2.0±0.0 | 2.0±0.2 | 0.157 |
The statistical method used for the data of this table was Student’s t-test. RD, dexmedetomidine-ropivacaine; RS, sufentanil-ropivacaine; DBP, diastolic blood pressure; SBP, systolic blood pressure; MAP, mean arterial pressure; VAS, visual analog scale; RSS, Ramsay Sedation Scale.
Pain relief after delivery
After delivery, the analgesia was changed to local anesthesia and wounds were sutured. Prior to the application of anesthesia, the mean VAS scores of the RD group and RS group at 0 min were 4.0 and 3.9, respectively, and 15 min later the mean VAS scores of the 2 groups were both 2.8. The mean RSS values of both groups were maintained at 2.0. However, the mean VAS scores and other characteristics of parturient women had no differences between the RD and RS groups (all P>0.05). Detailed indicators and values are shown in Figure 3 and Table 3.
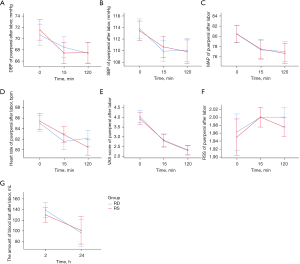
Table 3
| Indicators | Group RD (n=80) | Group RS (n=80) | P |
|---|---|---|---|
| DBP (mmHg) | |||
| 0 min | 70.6±8.0 | 71.5±8.3 | 0.494a |
| 15 min | 68.4±7.9 | 67.4±8.9 | 0.444a |
| 120 min | 67.4±8.3 | 67.5±8.5 | 0.947a |
| SBP (mmHg) | |||
| 0 min | 113.8±8.0 | 113.4±7.3 | 0.766a |
| 15 min | 109.9±8.4 | 110.6±8.4 | 0.572a |
| 120 min | 110.1±8.2 | 109.8±10.0 | 0.863a |
| MAP (mmHg) | |||
| 0 min | 80.4±7.2 | 80.4±8.3 | 0.976a |
| 15 min | 77.3±8.1 | 77.5±8.8 | 0.904a |
| 120 min | 77.0±8.7 | 76.6±9.0 | 0.783a |
| Heart rate (beats/min) | |||
| 0 min | 85.0±6.7 | 85.3±5.9 | 0.727a |
| 15 min | 81.5±6.6 | 82.8±7.5 | 0.250a |
| 120 min | 82.1±7.6 | 80.4±5.9 | 0.115a |
| VAS scores | |||
| 0 min | 4.0±1.4 | 3.9±1.7 | 0.614a |
| 15 min | 2.8±1.4 | 2.8±1.5 | 0.871a |
| 120 min | 2.3±1.1 | 2.3±1.1 | 0.885a |
| RSS values | |||
| 0 min | 2.0±0.2 | 1.9±0.2 | 0.701a |
| 15 min | 2.0±0.0 | 2.0±0.2 | 1.000a |
| 120 min | 2.0±0.0 | 2.0±0.2 | 0.159a |
| Blood loss (mL) | |||
| 2 h | 150.0 (100.0, 172.5) | 150.0 (57.5, 150.0) | 0.369b |
| 24 h | 50.0 (50.0, 70.0) | 50.0 (50.0, 73.8) | 0.982b |
The statistical methods used: a, Student’s t-test; b, Mann-Whitney U test. RD, dexmedetomidine-ropivacaine; RS, sufentanil-ropivacaine; DBP, diastolic blood pressure; SBP, systolic blood pressure; MAP, mean arterial pressure; VAS, visual analog scale; RSS, Ramsay Sedation Scale.
Adverse reactions
The constituent ratio of adverse reactions in parturient women and newborns is shown in Table 4. The incidence of adverse reactions was low in both groups. There were no significant differences in the incidence of hypotension, trembling, nausea and vomiting, motor nerve block, bradycardia, and respiratory depression between the 2 groups (all P>0.05).
Table 4
| Adverse reactions | Group RD (n=80) | Group RS (n=80) | P |
|---|---|---|---|
| Parturient women, n (%) | |||
| Hypotension | 0 | 1 (1.30) | 1.000 |
| Trembling | 0 | 2 (2.50) | 0.497 |
| Nausea and vomiting | 1 (1.30) | 1 (1.30) | 1.000 |
| Motor nerve block | 1 (1.30) | 2 (2.50) | 1.000 |
| Newborns, n (%) | |||
| Bradycardia | 0 | 1 (1.30) | 1.000 |
| Respiratory depression | 0 | 1 (1.30) | 1.000 |
The statistical method used for the data of this table was Fisher’s exact test. RD, dexmedetomidine-ropivacaine; RS, sufentanil-ropivacaine.
Discussion
This RCT showed that dexmedetomidine or sufentanil combined with ropivacaine had good analgesia effects for epidural labor analgesia and can effectively relieve the pain associated with delivery. Our results found that the heart rate of group RS was higher than that of group RD, but there were no significant differences between the 2 groups in terms of demographic characteristics, vital signs, VAS scores, RSS values, blood loss, duration of labor stages (first and second stages), onset time of analgesia, and dose of analgesics, and there were also no differences in newborns’ Apgar scores and NBNA scores. The incidence of adverse reactions in parturient women and newborns such as hypotension, trembling, nausea and vomiting, motor nerve block, bradycardia, and respiratory depression was low in both groups.
The mechanism of epidural labor analgesia is that anesthetics inhibit nerve conduction by blocking sodium channels in nerve membranes, thereby preventing the propagation of nerve impulses along these fibers. Blocking the impulse of pain through the nerve in the epidural space results in analgesia, which usually occurs within 10 to 20 min after delivery of anesthesia (3). Sufentanil is an opioid anesthetic adjuvant, and its combination with ropivacaine can reduce the incidence of instrumental delivery and cesarean section and the length of postpartum hospital stay (18). In recent studies, dexmedetomidine has also been successfully used for epidural labor analgesia (13,14). In our study, the efficacy of sufentanil combined with ropivacaine and dexmedetomidine combined with ropivacaine for epidural labor analgesia was compared. We found that after injection of analgesics, the VAS scores of both groups were lower, and the pain was relieved quickly. This was consistent with previous studies demonstrating that dexmedetomidine and sufentanil combined with ropivacaine had good analgesic effects on parturient women (15,16). However, there were no significant differences in VAS scores between the 2 groups. Previous studies showed that compared with group RS, group RD had lower VAS scores (15,16). The study of Li et al. also found that the combination of ropivacaine, dexmedetomidine, and sufentanil resulted in lower VAS scores than ropivacaine combined with dexmedetomidine or sufentanil (17). This finding may be due to the fact that VAS scores were the significant indicator for evaluating the pain of parturient women, and the VAS is based only on patient self-reports and may be affected by culture, age, and situational factors (19,20). In addition, some limitations of the VAS in paper format should be considered, and Escalona-Marfil et al.’s study suggested that some limitations can be mitigated with the introduction of an electronic VAS version (21).
Ropivacaine combined with anesthesia adjuvant has been widely used for epidural labor analgesia (22). However, previous studies concluded that the use of analgesia may prolong the duration of first and second labor stages (23,24). In our study, the duration of the first and second stages was similar in the 2 groups, which was supported by the study conducted by Cheng et al. (16). However, the study of Zhang et al. showed that the duration of the first labor stage was shorter in group RD compared with that in group RS (15). One possible explanation was that dexmedetomidine may cause uterus smooth muscle contractions and shorten the duration of the first stage of labor (15). Furthermore, one study found that women who received sufentanil were more likely to suffer from adverse reactions such as vomiting, pruritus, urinary retention, and respiratory depression (25). It is known that adverse reactions play an important role in drug safety. The findings of our study demonstrated that the incidence of adverse reactions of parturient women and newborns was low in both groups, and there was no difference between the 2 groups. In addition, our results showed that the NBNA and Apgar scores were higher than normal standard in both groups.
However, there were several limitations of our study. First, although dexmedetomidine has been widely used in epidural labor analgesia, it has not yet received international consensus (26). Second, this study only assessed the effectiveness and safety of 0.1% ropivacaine combined with 0.5 µg/mL dexmedetomidine and 0.5 µg/mL sufentanil, and further studies should evaluate the use of different doses.
Conclusions
A randomized, triple-blinded, controlled trial was performed for epidural labor analgesia. This study assessed the efficacy of dexmedetomidine and sufentanil combined with ropivacaine for epidural labor analgesia, and evaluated their influence on parturient women and newborns. Patients who received dexmedetomidine combined with ropivacaine had similar analgesic effects to those who received sufentanil combined with ropivacaine. In addition, the incidence of adverse reactions in parturient women and newborns was low in both groups. Therefore, dexmedetomidine or sufentanil combined with ropivacaine for epidural labor analgesia had similar analgesic effects in clinical practice.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-264/rc
Trial Protocol: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-264/tp
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-264/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-264/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Chengdu Women’s and Children’s Central Hospital Ethics Committee [No. 2020(89)] and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hawkins JL. Epidural analgesia for labor and delivery. N Engl J Med 2010;362:1503-10. [Crossref] [PubMed]
- Yildiz PD, Ayers S, Phillips L. The prevalence of posttraumatic stress disorder in pregnancy and after birth: A systematic review and meta-analysis. J Affect Disord 2017;208:634-45. [Crossref] [PubMed]
- Anim-Somuah M, Smyth RM, Cyna AM, et al. Epidural versus non-epidural or no analgesia for pain management in labour. Cochrane Database Syst Rev 2018;5:CD000331. [Crossref] [PubMed]
- Ojo OA, Mehdiratta JE, Gamez BH, et al. Comparison of Programmed Intermittent Epidural Boluses With Continuous Epidural Infusion for the Maintenance of Labor Analgesia: A Randomized, Controlled, Double-Blind Study. Anesth Analg 2020;130:426-35. [Crossref] [PubMed]
- Ngan Kee WD, Ng FF, Khaw KS, et al. Dose-Response Curves for Intrathecal Bupivacaine, Levobupivacaine, and Ropivacaine Given for Labor Analgesia in Nulliparous Women. Reg Anesth Pain Med 2017;42:788-92. [Crossref] [PubMed]
- Zhou X, Li J, Deng S, et al. Ropivacaine at different concentrations on intrapartum fever, IL-6 and TNF-α in parturient with epidural labor analgesia. Exp Ther Med 2019;17:1631-6. [PubMed]
- Sng BL, Kwok SC, Sia AT. Modern neuraxial labour analgesia. Curr Opin Anaesthesiol 2015;28:285-9. [Crossref] [PubMed]
- Roelants F, Lavand'homme P. Clonidine versus sufentanil as an adjuvant to ropivacaine in patient-controlled epidural labour analgesia: A randomised double-blind trial. Eur J Anaesthesiol 2015;32:805-11. [Crossref] [PubMed]
- Cai S, Zheng J, Meng Q, et al. Investigation of the Minimum Local Analgesic Concentration of Epidural Sufentanil Combined With Ropivacaine for Labor Analgesia. Clin Ther 2020;42:210-9. [Crossref] [PubMed]
- Shen X, Li Y, Xu S, et al. Epidural Analgesia During the Second Stage of Labor: A Randomized Controlled Trial. Obstet Gynecol 2017;130:1097-103. [Crossref] [PubMed]
- Henderson F, May WJ, Gruber RB, et al. Role of central and peripheral opiate receptors in the effects of fentanyl on analgesia, ventilation and arterial blood-gas chemistry in conscious rats. Respir Physiol Neurobiol 2014;191:95-105. [Crossref] [PubMed]
- Carey CM, Jena AB, Barnett ML. Patterns of Potential Opioid Misuse and Subsequent Adverse Outcomes in Medicare, 2008 to 2012. Ann Intern Med 2018;168:837-45. [Crossref] [PubMed]
- Zhao Y, Xin Y, Liu Y, et al. Effect of Epidural Dexmedetomidine Combined With Ropivacaine in Labor Analgesia: A Randomized Double-Blinded Controlled Study. Clin J Pain 2017;33:319-24. [Crossref] [PubMed]
- Wangping Z, Ming R. Optimal Dose of Epidural Dexmedetomidine Added to Ropivacaine for Epidural Labor Analgesia: A Pilot Study. Evid Based Complement Alternat Med 2017;2017:7924148. [Crossref] [PubMed]
- Zhang T, Yu Y, Zhang W, et al. Comparison of dexmedetomidine and sufentanil as adjuvants to local anesthetic for epidural labor analgesia: a randomized controlled trial. Drug Des Devel Ther 2019;13:1171-5. [Crossref] [PubMed]
- Cheng Q, Bi X, Zhang W, et al. Dexmedetomidine versus sufentanil with high- or low-concentration ropivacaine for labor epidural analgesia: A randomized trial. J Obstet Gynaecol Res 2019;45:2193-201. [Crossref] [PubMed]
- Li G, Xiao Y, Qi X, et al. Combination of sufentanil, dexmedetomidine and ropivacaine to improve epidural labor analgesia effect: A randomized controlled trial. Exp Ther Med 2020;20:454-60. [Crossref] [PubMed]
- Amer-Wåhlin I, Christoffersson M, Dahlgren N, et al. Epidural analgesia with sufentanil during labor and operative delivery. Acta Obstet Gynecol Scand 2000;79:538-42. [PubMed]
- Breivik EK, Björnsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain 2000;16:22-8. [Crossref] [PubMed]
- Winkelman C, Norman D, Maloni JA, et al. Pain measurement during labor: comparing the visual analog scale with dermatome assessment. Appl Nurs Res 2008;21:104-9. [Crossref] [PubMed]
- Escalona-Marfil C, Coda A, Ruiz-Moreno J, et al. Validation of an Electronic Visual Analog Scale mHealth Tool for Acute Pain Assessment: Prospective Cross-Sectional Study. J Med Internet Res 2020;22:e13468. [Crossref] [PubMed]
- Boulier V, Gomis P, Lautner C, et al. Minimum local analgesic concentrations of ropivacaine and levobupivacaine with sufentanil for epidural analgesia in labour. Int J Obstet Anesth 2009;18:226-30. [Crossref] [PubMed]
- Zeng H, Guo F, Lin B, et al. The effects of epidural analgesia using low-concentration local anesthetic during the entire labor on maternal and neonatal outcomes: a prospective group study. Arch Gynecol Obstet 2020;301:1153-8. [Crossref] [PubMed]
- Shmueli A, Salman L, Orbach-Zinger S, et al. The impact of epidural analgesia on the duration of the second stage of labor. Birth 2018;45:377-84. [Crossref] [PubMed]
- Wang T, Lu Y, Zhou P, et al. A Randomized Controlled Comparison of Epidural Analgesia Onset Time and Adverse Reactions During Labor With Different Dose Combinations of Bupivacaine and Sufentanil. Clin J Pain 2020;36:612-7. [Crossref] [PubMed]
- Konakci S, Adanir T, Yilmaz G, et al. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol 2008;25:403-9. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)



