
Efficacy and safety of microvascular decompression versus percutaneous balloon compression in the treatment of trigeminal neuralgia: a systematic review and meta-analysis
Introduction
Trigeminal neuralgia (TN) is a type of transient and recurrent paroxysmal severe pain confined to the trigeminal nerve region (1). Typical TN and atypical TN are the main types of TN. The onset of TN pain is spontaneous or triggered by talking, eating, or other non-noxious stimuli (2). TN is considered a rare disease, and reports on its incidence range from 4.3–27 new cases per 100,000 people per year (3). Studies on its prevalence indicate that the incidence of TN ranges from 0.03–0.3% in the general population (2,4). The initial treatment for TN is generally oral drugs (e.g., carbamazepine and obensiequine). However, such drugs may cause dizziness, nausea and lethargy (2,5). Moreover, after long-term use, the analgesic effect of these drugs of decrease significantly, and 50% of patients eventually need surgical management to relieve the pain (6).
Operative interventions for TN include MVD, PBC, percutaneous radiofrequency rhizotomy (PRR), percutaneous glycerol rhizotomy (PGR), and stereotactic radiosurgery (SRS)(7). Dandy’s hypothesis that vascular compression is the cause of TN is currently accepted. Based on this hypothesis and due to its excellent ability to control pain, microvascular decompression (MVD) has long been considered a common and reasonable method for the treatment of primary TN (8). Kaye et al. (8) reviewed the risks and benefits of various surgeries and reported that the rate of initial pain control ranges from 80.3–96% for MVD surgery. However, as vascular compression does not occur in all TN patients, radiofrequency thermocoagulation and percutaneous balloon compression (PBC) are also used to treat TN. Among the surgical options, MVD has been proven to be the most successful and durable surgical approach for treating TN, but the utility of MVD is restricted by the risk of craniotomy and complications, especially in elderly patients (2,9). It is essential to select feasible, fast, effective and safe surgical methods to improve the quality of life of elderly patients. Under the guidance of X-ray, PBC expands the balloon to compress the semilunar ganglion, so that the abnormal neurons related to TN are destroyed (10,11). According to one report, pain was relieved in 90% of patients following these PBC procedures, but up to 50% of patients were estimated to experience pain recurrence after 5 years (7,12). Traditionally, PBC has been reserved for older patients or patients who might not be able to endure the craniotomy necessary for MVD. Compared to MVD, PBC is simple, minimally invasive, and safe.
Results on the curative effect of MVD versus PBC for TN are inconsistent. A study has shown that patients treated with MVD had a better curative effect than those treated with PBC (13). While other studies have shown that the curative efficiency of MVD is equal to that of PMC (14,15). A recent meta-analysis revealed that MCD for elderly patients with TN could be undergone safely (16). Additionally, another meta-analysis suggested that for patients without specific surgical contraindications, MVD is a better option as the surgical treatment for TN (17). To date, little research has been conducted to compare MVD and PMC, and determine the best procedure; thus, well-designed studies are urgently needed. Moreover, previous studies included small sample sizes and had low reliability.
In this study, clinical studies related to MVD and PBC in TN were collected by searching the Chinese and English databases, and the efficacy and safety of MVD and PBC were compared using evidence-based methods to determine the best choice for clinical workers and patients. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3901/rc).
Methods
Search strategy
The study was conducted using Preferred Reporting Items for Systematic Reviews and MetaAnalyses statements (18). To find all the related articles, 2 investigators independently searched the PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Embase (http://www.embase.com), The Cochrane library (http://www.cochranelibrary.com), China National Knowledge Infrastructure(https://www.cnki.net), Wanfang (https://www.wanfangdata.com.cn), and Weipu (http://qikan.cqvip.com) databases for articles published from the dates of inception of the databases to October 9, 2021 using the following keywords: “trigeminal neuralgia”, “micro-vascularde compression”, “percutaneous micro balloon compression”, “PMC”, “PBC”, “microvascular decompression” and “MVD”. The reference lists of the retrieved articles were manually searched to identify additional eligible studies.
Inclusion and exclusion criteria
To be included in the meta-analysis, the studies had to meet the following criteria: (I) be a published clinical study examining the use of MVD and PMC in the treatment of TN; (II) include patients with primary TN or secondary recurrence after surgical treatment; (III) include patients who had received the MVD or PBC intervention measures; and (IV) include outcomes on the postoperative overall effective rate, recurrence rate, and rate of adverse reactions. Studies were excluded from the meta-analysis if they met any of the following exclusion criteria: (I) were not a clinical study (e.g., were reviews, case reports, or conference reports); (II) included patients with secondary TN; (III) included patients with organic lesions in the brain tissue; and (IV) examined non-human patients.
Data extraction and risk of bias assessment
According to a pre-designed standardized form, 2 researchers independently extracted the relevant data from the eligible studies, including data on the first author, publication year, mean age of participants, number of study patients, and TN disease type. The methodological quality of the articles was independently evaluated by 2 researchers. Any disagreement were resolved through discussion between the 2 reviewers or by consulting a third party. Based on the Cochrane risk of bias tool for assessing the risk of bias (19), the following 7 items were used to evaluate the studies: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessments, incomplete outcome data, selective outcome reporting, and other biases. The articles were classified as having a “low risk”, “high risk”, or “unclear risk”.
Statistical analysis
The meta-analysis was conducted using RevMan5.2 software. The measures of effect for dichotomous data are expressed as odds ratios (ORs) with 95% confidence intervals (CIs). If the I2>50% or the P value <0.05, a random-effects model was used; otherwise, a fixed-effects model was used (20). A P value <0.05 was considered statistically significant.
Results
Literature review and study characteristics
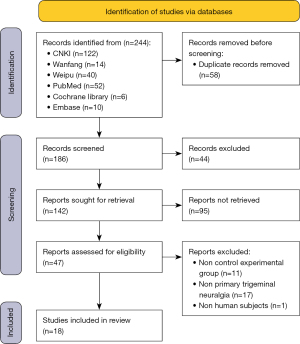
The search in this meta-analysis retrieved 244 articles from 2 types of language databases (Chinese databases =176, and English databases =68). After duplication check and initial exclusion, only 142 potentially suitable articles were further evaluated. Ultimately, 18 studies (13-15,21-35) met the selection criteria, and were included in the meta-analysis. A flow diagram of the meta-analysis is presented in Figure 1.
The basic information of the studies included in this meta-analysis is summarized in Table 1. The included studies were published in 2 different countries from 2013 to 2021, and most of them (n=16) were published in China. All patients suffered from primary TN. The sample size of the included studies were 12–100 male and 12–132 female patients after PBC treatment, and 7–98 male and 11–134 female patients after MVD treatment. The age of patients ranged from 47.32±8.08 to 71.13±11.42 years in the PBC group, and 48.12±7.92 to 78.9±7.8 years in the MVD group. The TN outcomes for all patients included the overall response rate, recurrence rate, and incidence of adverse reactions (see Table 1).
Table 1
| First author, year | Type | PBC | MVD | Age | Outcome indicators | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | PBC (mean ± SD) | MVD (mean ± SD) | |||||
| Ni et al., 2020 (24) | PTN | 12 | 18 | 13 | 17 | 71.13±11.42 | 71.13±11.42 | ①②③ | ||
| Chen et al., 2018 (21) | PTN | 15 | 27 | 23 | 45 | 63.4a | 59.3a | ①②③ | ||
| Bao, 2013 (29) | PTN | 15 | 12 | 7 | 11 | 59.7±7.9 | 51.6±11 | ①②③ | ||
| Dong, 2019 (30) | PTN | 13 | 16 | 18 | 23 | 47.32±8.08 | 48.12±7.92 | ①③ | ||
| Du, 2020 (13) | PTN | 100 | 132 | 98 | 134 | 60.23±5.94 | 59.78±6.61 | ①③ | ||
| Fu et al., 2020 (35) | PTN | 14 | 24 | 18 | 24 | 79.1±7.2 | 78.9±7.8 | ①②③ | ||
| Hu et al., 2018 (32) | PTN | 35 | 32 | 13 | 12 | 52.5±10.6 | 50.7±11.1 | ①②③ | ||
| Li et al., 2021(26) | PTN | 27 | 31 | 31 | 23 | 68.23±6.24 | 67.35±5.36 | ①③ | ||
| Li et al., 2016 (22) | PTN | 22 | 40 | 20 | 42 | 66.5±2.9 | 66.8±2.7 | ①②③ | ||
| Shang et al., 2020 (34) | PTN | 17 | 19 | 17 | 23 | 53.5±4.8 | 50.5±6.0 | ①②③ | ||
| Wang et al., 2014 (28) | PTN | – | – | – | – | – | – | ①②③ | ||
| Wang et al., 2021 (27) | PTN | 10 | 28 | 11 | 27 | 55.47±13.02 | 54.68±10.44 | ①②③ | ||
| Xing et al., 2020 (33) | PTN | 23 | 23 | 20 | 26 | 59.82±4.75 | 58.51±3.13 | ①②③ | ||
| Xu, 2021 (25) | PTN | 13 | 19 | 12 | 20 | 53.94±21.75 | 54.32±21.17 | ① | ||
| Yao, 2015 (31) | PTN | 73 | 51 | 12 | 17 | 58a | 52a | ①②③ | ||
| Ye et al., 2019 (23) | PTN | 19 | 31 | 20 | 30 | 53.23±10.56 | 53.68±10.19 | ②③ | ||
| Zhang et al., 2020 (15) | PTN | 27 | 18 | 25 | 20 | 54.5±6.1 | 53.6±7.2 | ① | ||
| Zhu et al., 2021 (14) | PTN | 16 | 17 | 14 | 17 | 58.14±6.72 | 57.09±6.53 | ①③ | ||
a, The study did not report the standard deviation of patient age; ① total effective rate; ② recurrence rate; ③ incidence of adverse reactions; –, this value is not reported in the article. MVD, microvascular decompression; PBC, percutaneous balloon compression; PTN, primary trigeminal neuralgia.
Meta-analysis of the outcomes
Overall effective rates after treatment with PBC versus MVD
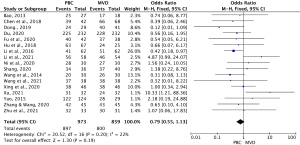
Among the 18 studies, 17 (13-15,21,22,24-35) comprising 1,832 cases (863 MVD cases and 969 PBC cases) examined the overall effective rate of the treatments. As the heterogeneity test results were statistically insignificant (I2=22%, P=0.20), the fixed-effects model was used to pool the data for the meta-analysis. The OR for the overall effective rate for PBC versus MVD treatment was 0.79 (95% CI: 0.55–1.13, P=0.19), and was not statistically significant. Thus, the overall effective rate of MVD treatment was similar to that of PBC treatment (see Figure 2).
Recurrence rates after treatment with PBC versus MVD
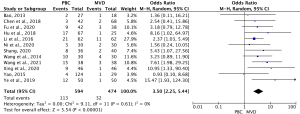
Twelve studies (14,21-24,27-29,31-33,35) comprising 1,068 cases (590 PBC cases, and 478 MVD cases) reported the recurrence rate of TN after treatment. The heterogeneity results were I2=0% (P=0.61); thus, the OR for PBC versus MVD treatment was 3.50 (95% CI: 2.25–5.44; P<0.00001) using the fixed-effects model. The results showed that the MVD treatment level was statistically lower than the PMC treatment level (see Figure 3).
Adverse reaction rates after treatment with PBC versus MVD
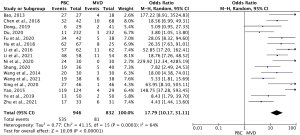
Sixteen studies (13,21-35) compared the adverse reaction rates between MVD and PBC cases. The heterogeneity results were I2=64% (P=0.0003); thus, the rate of adverse reaction for PBC versus MVD treatment was 17.79 (95% CI: 10.17–31.11; P<0.00001) using the random-effects model. The results showed that the rate of adverse reaction was significantly lower in MVD cases than PMC cases (see Figure 4).
Quality evaluation of the included studies
As Figure 5 shows, the random-number table method was mentioned in 4 studies (13,22,23,27), and the free-combination method was mentioned in 2 studies (11,15), which indicated a low risk of bias; the remaining studies failed to mention any method. None of the studies mentioned distribution concealment or implementation of blindness. The data of 4 studies (13,15,23,25) were insufficient, but not enough to affect the effect value. As for the selective reporting result, 2 studies (15,25) did not report on the number of adverse reactions, and 5 studies (13,15,23,25,30) did not report on the number of recurrences, and thus could not be included in the meta-analysis. No other source of bias was found in any of the studies.
Discussion
A primary risk factor for TN is age, and the incidence of TN gradually increases with age (1). TN is most common in middle-aged and elderly people, and the average age of onset is between 45 and 65 years (36). With a male to female ratio of 3:2, the disease is more common in women than men (6). The main cause for TN is the compression of the 5th cranial nerve, which contains 3 major branches that control the motor functions of the face (37). However, the pathogenesis of TN is not completely clear (38,39). Due to the severe pain, TN often has a catastrophic effect on patients, and seriously affects their quality of life (40). In this meta-analysis, we compared the efficacies of MVD and PBC in the treatment of TN. The main finding of the present study is that there was no significant difference in the total response rate between the PBC and MVD groups (OR =0.79, 95% CI: 0.55–1.13, P=0.19); however, the recurrence rate of the PBC group was significantly higher than that of the MVD group (OR =3.89, 95% CI: 2.53–5.98; P<0.00001), and the incidence of adverse reactions in the PBC group was significantly higher than that of the MVD group (OR =17.79, 95% CI: 10.17–31.11; P<0.00001).
In relation to the overall effective rates, Zhang et al. found that there was no significant difference between MVD (93.3%) and PBC groups (95.6%) (P>0.05) (15). Similarly, Zhu et al. reported overall effective rates of 96.97% and 96.77% for PBC and MVD, respectively, and found no statistically significant difference between the 2 surgical methods (P>0.05) (14). Thus, the 2 surgical methods have similar treatment efficiency. Both MVD and PBC surgery obviously relieve the pain symptoms of TN patients. The accurate localization and correct treatment of the responsible vessels are key to the success of MVD, which is an effective method with a good curative effect. MVD and PBC use different principles to treat TN, but the concepts and technologies behind both MVD and PBC have been greatly improved after years of development. Notably, Xu et al. found that PBS had significantly better effective rates than MVD in the treatment of TN (P<0.05) (25), while the effective rates of MVD was better than PBS (MVD 97.56%: PBS 82.75%; P<0.05). However, Xu et al.’s results may be ascribed to the small sample size of the study, different follow-up time, and different patient selection criteria.
Recurrence and complications following treatments for TN, specifically those following PBC, remain problems. A previous study indicated that the recurrence rate of TN following MVD was as high as 10.00%, and often occurs 2 years after operation (41). Similarly, another study reported that the recurrence rate of TN following PBC was 10.70% (42). Patients in the PBC group were more likely to experience recurrent TN than those in the MVD group (21). These results are consisted with the finding of the present study. The data also suggested that patients treated with MVD had a lower recurrence rate for TN than those treated with PBC. MVD requires a craniotomy, but provides direct vision of the vascular loops that lead to compression and thus is better able to treat lesions. Conversely, while PBC can be used to physically damage the trigeminal semilunar ganglion in the patient's focal area, it cannot be used to thoroughly treat the cause (43,44). We also confirmed that the incidence of numbness and complications in the MVD group was significantly lower than that in the PBC group.
In a previous study, 3 patients (10.0%) and 2 patients (6.67%) had recurrent TN in the PBC and MVD groups, respectively, during the 6-month follow-up period, and facial numbness was the most common postoperative complication. In another study, 24 of 30 (80%) patients experienced facial numbness after PBC (24). In Li et al.’s study, complications in the PMC group (82.67%) were higher than those in the MVD group (20.37%) (26). Complications of MVD include facial palsy or weakness, loss of hearing, cerebellum injury, infection, death, and spinal fluid leakage (45). Postoperative facial numbness is related to excessive intraoperative traction, so trigeminal nerve should be handled gently to avoid excessive traction during surgery. PBC is a kind of neuro-destructive surgery, which may lead to sequential histological changes of nerve ganglia, and postoperative complications, such as facial numbness and masseter weakness (46). Most patients (90%) experienced postoperative facial numbness when the balloon was pressed for 1–1.5 minutes (47) A previous study found that facial numbness was the most common complication after PBC (50.0%) (27). This may be due to the surgical approach (i.e., balloon compression causes a compression injury to the nerve tissue, and can cause the same partial or total loss of trigeminal nerve function as radiofrequency ablation) (48). These results were consistent with the findings of our study, and suggested that MVD is superior to PBC in terms of adverse reactions, such as facial numbness and masseter weakness, in the treatment of TN patients. Operative interventions for TN include MVD, PBC, percutaneous radiofrequency rhizotomy (PRR), percutaneous glycerol rhizotomy (PGR), and stereotactic radiosurgery (SRS) (7). MVD is generally considered to have the best long-term efficacy, highest first postoperative remission rate with lowest incidence of sensory loss complication. and the lowest recurrence rate (7). In addition, PBC operation is completely fast. Due to the operation under general anesthesia, so the patient pain is small and high safety, especially suitable for the elderly patients who could not tolerate craniotomy (49,50).
The effective rate of long-term postoperative pain relief was 94.37% in MVD group and 91.67% in PMC group, with no statistical significance between the two groups (P>0.05), suggesting that both surgical methods could relieve TN for a long time and stably. The pain relief rate was 90.00% and 86.67% after PBC and MVD surgery, respectively (P>0.05). The levels of IL-1b, TNF-a, and IL-6 were significantly decreased at post-operative 3 days and 5 days compared with pre-operation in the 2 groups (P<0.05) (24).
With regard to treatment, carbamazepine is the first choice for TN drug. And baclofen, clonazepam and ocazepam could be used. A study by Stomal-Słowińska et al. (51) suggests that oxazepine has a low incidence of adverse reactions and a better therapeutic effect. In addition, studies have shown that combination medication is safer and more reasonable than single medication(52), which can not only improve the degree of pain relief, but also reduce the side effects of medication. However, at present, many patients are still treated with single drug(53). Therefore, it is suggested to use combined drugs as far as possible in clinical practice. With the development and progress of endoscopy technology, endoscopic MVD will be widely used in the future due to its smaller trauma, shorter operation time and lower recurrence rate. The etiology and pathogenesis of PTN are still unclear, so it is necessary to develop new drugs to treat TN in the future.
This study had several advantages. First, the statistical heterogeneity of the included studies was small. Second, the proposed methodologies in the literature were high quality. Third, there was no significant publication bias in any of the outcome indicators, and the combined results had high credibility. However, this study also had a number of limitations. First, most of the included studies were published by Chinese scholars, and the lack of high-quality clinical studies in foreign countries may lead to selection bias. Second, the included studies were retrospective or non-randomized controlled trials with a low level of evidence. Third, the corresponding follow-up time was inconsistent and the failure to perform subgroup analyses across different age groups may have resulted in biased reporting.
Conclusions
Our meta-analysis demonstrated that MVD and PBC are both viable alternatives in the treatment of TN. Notably, MVD resulted in lower recurrence rate and a lower rate of adverse reactions in the treatment of TN than PBC. The purpose of generating high-quality evidence is to provide guidance on the application of MVD or PBC in clinical practice. Thus, further randomized controlled trials with large samples sizes, scientific designs, and strict implementations need to be conducted to study the efficacy and safety of MVD or PBC in the treatment of TN.
Acknowledgments
Funding: The study was supported by Hainan Province Medical and Health Research (Project No. 18A200176), and Hainan Province Medical and Health Research (Project No. 19A200131).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3901/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3901/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smith CA, Paskhover B, Mammis A. Molecular mechanisms of trigeminal neuralgia: A systematic review. Clin Neurol Neurosurg 2021;200:106397. [Crossref] [PubMed]
- Araya EI, Claudino RF, Piovesan EJ, et al. Trigeminal Neuralgia: Basic and Clinical Aspects. Curr Neuropharmacol 2020;18:109-19. [Crossref] [PubMed]
- Cheshire WP. Trigeminal neuralgia: diagnosis and treatment. Curr Neurol Neurosci Rep 2005;5:79-85. [Crossref] [PubMed]
- De Toledo IP, Conti Réus J, Fernandes M, et al. Prevalence of trigeminal neuralgia: A systematic review. J Am Dent Assoc 2016;147:570-576.e2. [Crossref] [PubMed]
- Bendtsen L, Zakrzewska JM, Heinskou TB, et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol 2020;19:784-96. [Crossref] [PubMed]
- Khan M, Nishi SE, Hassan SN, et al. Trigeminal Neuralgia, Glossopharyngeal Neuralgia, and Myofascial Pain Dysfunction Syndrome: An Update. Pain Res Manag 2017;2017:7438326. [Crossref] [PubMed]
- Bick SKB, Eskandar EN. Surgical Treatment of Trigeminal Neuralgia. Neurosurg Clin N Am 2017;28:429-38. [Crossref] [PubMed]
- Kaye AH. Trigeminal neuralgia: vascular compression theory. Clin Neurosurg 2000;46:499-506. [PubMed]
- Thomas KL, Vilensky JA. The anatomy of vascular compression in trigeminal neuralgia. Clin Anat 2014;27:89-93. [Crossref] [PubMed]
- Du Y, Yang D, Dong X, et al. Percutaneous balloon compression (PBC) of trigeminal ganglion for recurrent trigeminal neuralgia after microvascular decompression (MVD). Ir J Med Sci 2015;184:745-51. [Crossref] [PubMed]
- Noorani I, Lodge A, Durnford A, et al. Comparison of first-time microvascular decompression with percutaneous surgery for trigeminal neuralgia: long-term outcomes and prognostic factors. Acta Neurochir (Wien) 2021;163:1623-34. [Crossref] [PubMed]
- Obermann M. Recent advances in understanding/managing trigeminal neuralgia. F1000Res 2019;8:eF1000 Faculty Rev-505.
- Du GX. Analysis of short-term clinical efficacy of microvascular decompression in the treatment of primary trigeminal neuralgia. China Modern Drug Application 2020;14:108-10.
- Zhu DD, Shi ZS, Zhao P, et al. Effect of percutaneous semilunar ganglion balloon compression and microvascular decompression in the treatment of trigeminal neuralgia. Medical Information 2021;34:119-21.
- Zhang Q, Wang XY. To compare the clinical efficacy of microvascular decompression (MVD) and percutaneous microcapsule compression (PMC) in the treatment of primary trigeminal neuralgia. Health Care Guide 2020;4:34-5.
- Sekula RF, Marchan EM, Fletcher LH, et al. Microvascular decompression for trigeminal neuralgia in elderly patients. J Neurosurg 2008;108:689-91. [Crossref] [PubMed]
- Li Y, Yang L, Ni J, et al. Microvascular decompression and radiofrequency for the treatment of trigeminal neuralgia: a meta-analysis. J Pain Res 2019;12:1937-45. [Crossref] [PubMed]
- Schünemann HJ, Oxman AD, Fellow JPHSSV, et al. Presenting Results and 'Summary of Findings' Tables. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series 2008;30:34-6.
- Higgins JP, Green S: Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Blackwell 2008;41:313-6.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Chen JN, Yu WH, Du HG, et al. Prospective Comparison of Redo Microvascular Decompression and Percutaneous Balloon Compression as Primary Surgery for Recurrent Trigeminal Neuralgia. J Korean Neurosurg Soc 2018;61:747-52. [Crossref] [PubMed]
- Li GP, Gan WH, Tang SH, et al. Clinical observation of microvascular decompression and percutaneous balloon compression in the treatment of primary trigeminal neuralgia. Advances in Modern Biomedicine 2016;16:2138-2140 + 2171.
- Ye YZ, Wang ZX, Liu XY, et al. Effect of microvascular decompression on primary trigeminal neuralgia. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine 2019;29:99-101.
- Ni H, Wang Y, Chen X, et al. Outcomes of Treatment for Elderly Patients With Trigeminal Neuralgia: Percutaneous Balloon Compression Versus Microvascular Decompression. J Craniofac Surg 2020;31:e685-8. [Crossref] [PubMed]
- Xu PC. Effect of percutaneous balloon compression of semilunar ganglion on inflammatory factors in patients with recurrent trigeminal neuralgia after microvascular decompression. Electronic Journal of Modern Medicine and Health Research 2021;5:75-7.
- Li GY, Liu Z, Wu CS, et al. Application of percutaneous microsphere compression in elderly patients with primary trigeminal neuralgia. Journal of Clinical Surgery 2021;29:488-91.
- Wang WL, Li Y, Qian T Comparison of microvascular decompression assisted by three-dimensional imaging and balloon compression in the treatment of primary trigeminal neuralgia. Medical Theory and Practice 2021;34:904-906 + 922.
- Wang H, Yu WH, Liu QJ, et al. Prospective cohort study on the clinical efficacy of microvascular decompression and percutaneous balloon compression in the treatment of primary trigeminal neuralgia. Journal of Practical Medicine 2014;30:3388-91.
- Bao L, Li X. Comparison of microvascular decompression (MVD) and microsphere capsule compression (PMC) in the treatment of primary trigeminal neuralgia. Journal of Liaoning Medical College 2013;37:85-7.
- Dong T. Clinical analysis of microvascular decompression versus balloon compression in the treatment of primary trigeminal neuralgia. Chinese Medical Guidelines 2019;17:138-9.
- Yao CC. Effect analysis of microvascular decompression and balloon compression in the treatment of primary trigeminal neuralgia. Dalian Medical University, 2015.
- Hu Q, Yu WH, Du Q, et al. Comparison of microvascular decompression and percutaneous balloon compression in the treatment of recurrent trigeminal neuralgia. Journal of Clinical Neurosurgery 2018;15:26-30 + 33.
- Xing JL, Xu GD, Wu LN, et al. Microvascular decompression and percutaneous balloon compression in the treatment of trigeminal neuralgia. Journal of Changzhi Medical College 2020;34:126-9.
- Shang YC. Comparison of microvascular decompression and percutaneous balloon compression in the treatment of primary trigeminal neuralgia . Bengbu Medical College, 2020.
- Fu XH, Zhu J, Chen HY. Clinical observation of percutaneous balloon compression in the treatment of elderly patients with trigeminal neuralgia. Geriatrics and Health Care, 2020;26:1030-1032 + 1041.
- Koopman JS, Dieleman JP, Huygen FJ, et al. Incidence of facial pain in the general population. Pain 2009;147:122-7. [Crossref] [PubMed]
- Campos WK, Linhares MN. A prospective study of 39 patients with trigeminal neuralgia treated with percutaneous balloon compression. Arq Neuropsiquiatr 2011;69:221-6. [Crossref] [PubMed]
- Sabalys G, Juodzbalys G, Wang HL. Aetiology and pathogenesis of trigeminal neuralgia: a comprehensive review. J Oral Maxillofac Res 2013;3:e2. [PubMed]
- Silva M, Ouanounou A. Trigeminal Neuralgia: Etiology, Diagnosis, and Treatment. SN Comprehensive Clinical Medicine 2020;118:561-6.
- McMillan R. Trigeminal Neuralgia - A Debilitating Facial Pain. Rev Pain 2011;5:26-34. [Crossref] [PubMed]
- Régis J, Tuleasca C, Resseguier N, et al. The Very Long-Term Outcome of Radiosurgery for Classical Trigeminal Neuralgia. Stereotact Funct Neurosurg 2016;94:24-32. [Crossref] [PubMed]
- Xu W, Jiang C, Yu C, et al. Percutaneous balloon compression for persistent or recurrent trigeminal neuralgia after microvascular decompression: personal experience of 28 patients. Acta Neurol Belg 2018;118:561-6. [Crossref] [PubMed]
- Broggi G, Ferroli P, Franzini A, et al. Operative findings and outcomes of microvascular decompression for trigeminal neuralgia in 35 patients affected by multiple sclerosis. Neurosurgery 2004;55:830-8; discussion 838-9. [Crossref] [PubMed]
- Chen GQ, Wang XS, Wang L, et al. Arterial compression of nerve is the primary cause of trigeminal neuralgia. Neurol Sci 2014;35:61-6. [Crossref] [PubMed]
- Zagzoog N, Attar A, Takroni R, et al. Endoscopic versus open microvascular decompression for trigeminal neuralgia: a systematic review and comparative meta-analysis. J Neurosurg 2018; [Epub ahead of print]. [PubMed]
- Zhu XL, Li XL, Rui H, et al. Technical specification for the treatment of trigeminal neuralgia by percutaneous puncture of semilunar ganglion microballoon expansion and compression. Chinese Journal of Pain 2021;4:15-8.
- Peng SP, Liu JX, Wang F, et al. Study on the relationship between intraoperative balloon pressure and postoperative complications and recurrence in the treatment of trigeminal neuralgia with PBC. Chinese Journal of Neuromedicine 2022;21:152-6.
- Huang B, Liu YQ, Yao M, et al. Comparison of common minimally invasive treatment methods for trigeminal neuralgia. Chinese Journal of Pain 2020;20-23.
- Heinskou TB, Rochat P, Maarbjerg S, et al. Prognostic factors for outcome of microvascular decompression in trigeminal neuralgia: A prospective systematic study using independent assessors. Cephalalgia 2019;39:197-208. [Crossref] [PubMed]
- Montano N, Papacci F, Cioni B, et al. The role of percutaneous balloon compression in the treatment of trigeminal neuralgia recurring after other surgical procedures. Acta Neurol Belg 2014;114:59-64. [Crossref] [PubMed]
- Stomal-Słowińska M, Słowiński J, Lee TK, et al. Correlation of clinical findings and results of percutaneous balloon compression for patients with trigeminal neuralgia. Clin Neurol Neurosurg 2011;113:14-21. [Crossref] [PubMed]
- Jones MR, Urits I, Ehrhardt KP, et al. A Comprehensive Review of Trigeminal Neuralgia. Curr Pain Headache Rep 2019;23:74. [Crossref] [PubMed]
- Wang CH, Zhao R, Ran DW. The Progress in Diagnosis and Treatment of Trigeminal neuralgia. Journal of Clinical Neurology 2019;3:7-12.
(English Language Editor: L. Huleatt)



