
Clinical efficacy and safety of vitamin C in the treatment of septic shock patients: systematic review and meta-analysis
Introduction
Sepsis is a common and life-threatening medical emergency, characterized by systemic inflammation, extensive tissue damage, and organ dysfunction (1). The incidence of sepsis continues to rise in major hospitals and intensive care units (ICUs) worldwide, with about 31 million cases of sepsis per year and about 6 million deaths worldwide (2-5). Sepsis became the third leading cause of death in hospital, estimated to cost nearly 60 billion per year, and even surviving patients are at risk of poor physical condition, mood, and cognitive outcomes, and thus, decreased quality of life (6).
There is no treatment that directly targets the pathogenesis of sepsis, and the management protocol mainly relies on early active fluid resuscitation, early appropriate antibiotics, hemodynamic support with vasopressors, and identification and control of sites of infection (7-10). Given the characteristics of high morbidity, high mortality, and poor prognosis of sepsis, it is necessary to discover new treatments to reduce patient mortality and improve patient outcomes. Despite the exploration and practice of a large number of treatment methods, the mortality rate of sepsis has not been significantly reduced, and finding new adjuvant therapies to improve the prognosis of patients with sepsis has become a research hotspot.
Vitamin C, also known as ascorbate, is an important antioxidant and enzyme cofactor involved in many important biological reactions (11). Current meaning after editorial changes is that reactive oxygen species (ROS) cause massive damage to the mitochondria of endothelial cells (12). The role of vitamin C in severe sepsis and septic shock include its antioxidant and anti-inflammatory properties, cortisol retention effect, inhibitory effects on nitric oxide synthase and increased catecholamine synthesis in the brain and adrenal medulla (13). Ascorbic acid can increase vasopressor synthesis, reduce oxidative stress and inflammatory response; a previous randomized trial in 24 patients showed that high doses of vitamin C reduced sepsis-related organ failure in a dose-dependent manner, a retrospective single-center trial of 200 mg/12 h within 24 h of onset with improved lactate clearance and 28 day mortality rate compared with matched controls by Anand et al. (14); a combination of ascorbic acid (1,500 mg/6 h) and hydrocortisone (50 mg/6 h) was found to improve patient organ damage, duration of shock reversal, and mortality rate.
This study added more sample sizes on the basis of previous studies, and in addition to analyzing the risk correlation between vitamin C and sepsis, we also further explored the mechanism of serum immune factors related to the occurrence of sepsis (15). In addition, only ICU patients were included in this study, which could more effectively reduce selection bias and statistical errors of results. Sepsis is a systemic inflammatory syndrome caused by potential or known infectious factors, and its progression can lead to shock and multiple organ dysfunction, which is the main cause of death in ICU patients. Despite extensive research on molecular pathogenesis based on targeted therapies, survival rates for severe sepsis and septic shock have not improved significantly (16). In recent years. Host cell-mediated immunity is of great importance to understand the pathologic process of sepsis and its multiple organ injury complications. Studies have shown that innate immune cells such as neutrophils, macrophages, dendritic cells, T lymphocytes, regulatory T cells, and natural killer T cells (NKT) play a crucial role in maintaining internal environmental balance and regulating immune response during sepsis (17,18). Early in sepsis, infection caused a gradual amplification of a moderate host response, followed by dysregulation. Inflammatory response is partially mediated by innate immune cells to initiate or inhibit the host inflammatory response through the production of proinflammatory cytokines, high-mobility-family protein-1 (HMGB-1), or inflammatory suppressors such as IL-10. The efficacy and safety of vitamin C adjuvant therapy for septic shock are inconsistent in many studies, so it is very important to systematically evaluate the adjuvant effect of intravenous vitamin C in the treatment of septic shock.
In recent years, clinical trials have reported that intravenous vitamin C reduces the organ function damage caused by sepsis and improves survival (15), and it has been demonstrated that parenteral vitamin C administration reduces organ injury and improves the survival of septic mice (16). However, the precise role of vitamin C as a neoadjuvant in sepsis and septic shock therapy is more controversial (17). Therefore, we conducted a meta-analysis of the effect of vitamin C on the mortality rate of patients with sepsis and septic shock, to provide strong evidence-based medical advice. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-225/rc).
Methods
Literature retrieval strategy
We performed a literature search of the English-language databases PubMed, EMBASE, The Cochrane Library, and Web of Science, as well as the Chinese databases Wanfang, China Biology Medicine (CBM), and China National Knowledge Infrastructure (CNKI), to August 2021, with no language restrictions. The search terms for all databases included ascorbic acid, vitamin C, sepsis, severe sepsis, septic shock, and randomized controlled trials (RCTs).
Inclusion and exclusion criteria
Inclusion criteria
- Study type: RCT or observational study, including cohort studies;
- Subjects investigated: patients with sepsis or septic shock, aged >18 years, met the 2020 diagnostic criteria for sepsis 3.0;
- Experimental group: routine anti-infective therapy + vitamin C; control group: routine anti-infective therapy;
- Outcomes: includes in-patient or ICU mortality rate;
- Relevant research literature was included strictly according to PICOS standards. PICOS: P is the subject of study. The target group or representative of the subject is relevant to the subject; I is for interventions. Therapeutic interventions or observational measures used in the study population; C is for comparison group. Indicators representing control groups and treatment measures or observations; O indicates end. Representative achievement indicators and related issues; S is for research, and that is what is a study design, cohort study, case control or cross-sectional study.
Exclusion criteria
- Subjects younger than 18 years;
- Incomplete or unavailable data;
- Study type: reviews, editorials, case reports, etc.;
- Literature not in Chinese or English.
Literature screening
Two researchers independently conducted the literature screening and data extraction according to the inclusion and exclusion criteria, and checked against each other. If there was a disagreement, they were screened again by a third researcher. Data extracted included article information (title, first author, date of publication, literature source, etc.); study information (number of subjects in the experimental and control groups, specific intervention methods); outcome index and correlation, outcome data (case fatality rate). If the data were incomplete or in doubt, the first author or corresponding author was contacted to obtain the relevant data.
Literature quality evaluation and data extraction
Study quality was evaluated by the Cochrane literature quality evaluation method, namely, randomized method, allocation concealment, implementation of blind method, lost visit bias, etc. Cohort studies were quality evaluated using the Newcastle-Ottawa Scale (NOS) scale (Table 1) and RCTs using a modified Jadad score scale (Table 2). The quality evaluation was conducted by two researchers, retaining only high-quality studies. Then the basic data of all studies were extracted, including the first author’s name, year of publication, study design and population, number of participants, and patient characteristics; and drugs in the trial group. Our primary outcome measure of the in-hospital mortality rate, and the secondary outcome measures of ICU mortality rate, duration of booster drug use and ICU stay, total hospitalization, and 72-h SOFA score were finally combined (Table 3).
Table 1
| Score list | NOS scale | |||
|---|---|---|---|---|
| >1 | >1 | 0 | 0 | |
| Representative of the exposure cohort | Good | Good | NA | NA |
| Selection of the non-exposed cohort | Same population | Different population | NA | NA |
| Subjects had no studied disease | Yes | No | NA | NA |
| Analysis of phase exposure and non-exposure cohorts | Most important factor | Secondary cause | NA | NA |
| Method of outcome determination | Independent assays | Reliable record | NA | NA |
| Whether the follow-up time was long enough | Yes | NA | No | NA |
| Integrity of follow-up | Follow-up rate =100% | Follow-up rate >90% | Follow-up rate <90% | NA |
NOS, Newcastle-Ottawa Scale; NA, not available.
Table 2
| Score list | Jadad score | |||
|---|---|---|---|---|
| 2 | 1 | 0 | 0 | |
| Random series | The computer produces random numbers or similar method | Randomized trial did not describe the method of randomized assignment | Alternate assignment method | NA |
| Randomization hidden | Clinical investigators and subjects were unable to predict the assignment sequence method | Only indicates the use of random number tables or any other allocation scheme | Alternate assignment, case numbers, Sundays, open random number tables, and any measures that do not prevent grouping predictability | NA |
| Blind method | A completely consistent placebo was used | Trial statements were blinded, and the methods were not described | The adoption of blindness was improper | NA |
| Remove and exit | NA | The number and reasons for the exits are described | The number and reasons for the exits are not described | NA |
NA, not available.
Table 3
| Study | Study type | Age (years, SD) | Sex | SOFA score (SD) | APACHE II (SD) | Interventions | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | 8.3–8.7 | 22.1–22 | Experimental group | Control group | ||||||
| Marik et al. 2017 | Cohort | 58.3 | 50 | 44 | NA | 95–96 | Vitamin C | Standard treatment | |||
| Sadaka et al. 2020 | Cohort | 67 | 32 | 30 | 9.7–10.6 | 21–21.5 | Vitamin C | Standard treatment | |||
| Litwak et al. 2019 | Cohort | 58.2 | 57 | 37 | 6–6.9 | NA | Vitamin C | Standard treatment | |||
| Mitchell et al. 2020 | Cohort | 68 | 73 | 3 | NA | 100–107 | Vitamin C | Standard treatment | |||
| Grady et al. 2019 | Cohort | 63 | 24 | 20 | NA | NA | Vitamin C | Standard treatment | |||
| Long et al. 2020 | Cohort | 64.4 | NA | NA | NA | NA | Vitamin C | Standard treatment | |||
| Chang et al. 2020 | RCT | 59.5 | 43 | 27 | 9.6–10.1 | 22.1–23.8 | Vitamin C | Standard treatment | |||
| Mohamed et al. 2020 | RCT | 58.69 | 63 | 25 | 10.89–11.22 | NA | Vitamin C | Standard treatment | |||
| Moskowitz et al. 2020 | RCT | 68.9 | 111 | 89 | NA | NA | Vitamin C | Standard treatment | |||
| Wani et al. 2020 | RCT | 65 | 59 | 51 | 9.22–9.36 | NA | Vitamin C | Standard treatment | |||
| Iglesias et al. 2020 | RCT | 70 | 59 | 78 | 7.9–8.3 | 24–24.9 | Vitamin C | Standard treatment | |||
| Sevransky et al. 2021 | RCT | 62 | 274 | 228 | NA | NA | Vitamin C | Standard treatment | |||
| Fujii et al. 2020 | RCT | 61.9 | 133 | 78 | 8.4–8.6 | 77.4–83.3 | Vitamin C | Standard treatment | |||
SD, standard deviation; SOFA, Sequential Organ Failure Assessment; APACHE II, Acute Physiology and Chronic Health Evaluation II; NA, not available; RCT, randomized controlled trial.
Statistical analysis
Heterogeneity test and treatment
After sorting out the relevant literature data according to the requirements for a meta-analysis, statistical analysis was conducted on the data using Stata 12.0 software. I2 quantitatively judged the size of the heterogeneity of each study. The value range of I2 was set to between 0% and 100%, and the greater the I2 value, the greater the heterogeneity. The heterogeneity of I2 is acceptable as long as it is not greater than 50%, with three degrees of high (75%), medium (50%), and low (25%) classified by Guinot et al. (17). If the study results show I2>50%, P<0.05, with heterogeneity, the meta-analysis used a random-effect model; if I2<50%, P>0.05, with no statistical heterogeneity, the fixed-effect model was used. If heterogeneity exists, a sensitivity analysis was performed by successively deleting single trials to check whether deletion of the single trials affected the results.
Selection of effect sizes and combined effect sizes
For dichotomous variables, we used the odds ratio (OR) of the 95% confidence interval (CI). For continuous variables, we used the SD mean difference (SMD) and its 95% CI. For studies with missing data, we contacted the corresponding author, who reported continuous data for the median and interquartile range (IQR), requiring data for the mean and standard deviation (SD). If the mean and SD values were not obtained or no data were available, we derived the data from the reported median and IQR.
Bias test
Funnel plots were made to assess publication bias in the included studies, and if large, it was further assessed using Begg’s plots and Egger’s test.
Results
Literature screening
A total of 320 documents were initially retrieved by computer search and after strict screening according to the inclusion and exclusion criteria, 13 studies were finally included with a total of 1,423 participants (18-30). The literature screening flowchart is shown in Figure 1.
Basic characteristics and quality evaluation of the studies
A total of 13 studies included 6 cohort studies, and 7 RCTs with Jadad score >4 points. Basic characteristics of the included studies are shown in Table 3.
Meta-analysis
Main outcome index: in-hospital mortality rate
As shown in Figure 2, 6 cohort studies and 5 RCTs were analyzed, with intravenous vitamin C not significant in sepsis (OR =0.91, 95% CI: 0.76–1.08, P=0.27), and acceptable heterogeneity between studies (I2=0%, Z=1.54).
Secondary outcome indicators
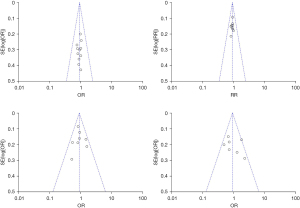
- ICU mortality: Figure 3 shows the analysis of 5 cohort studies and 4 RCTs that reported ICU mortality rates, and intravenous vitamin C was not statistically significant in reducing the ICU mortality rates in sepsis patients (OR =0.84, 95% CI: 0.69–1.01, P=0.07), with acceptable heterogeneity between studies (I2=0%, Z=1.82).
- ICU stay: Figure 4 shows the analysis of - studies and 4 RCTs that reported ICU stay (OR =0.88, 95% CI: 0.72–1.08, P=0.23), indicating that intravenous vitamin C was not statistically significant for shortening ICU stay in sepsis patients, with greater heterogeneity between studies (I2=1%, Z=1.19).
- Total length of stay:
Figure 5 shows the analysis of the 3 cohort studies and 4 RCTs that reported total length of stay (OR =0.91, 95% CI: 0.68–1.21, P=0.51), and vitamin C were not statistically significant in the outcome for sepsis patients, with no heterogeneity observed between studies (I2=45%, Z=0.66). - 72-h sequential organ failure assessment (72-h SOFA) score:
3 cohort studies and 5 randomized controlled experiments reported 72-h SOFA score (OR =0.95, 95% CI: 0.77–1.18, P=0.66), indicating that intravenous thiamine, ascorbate and glucocorticoid triple were not statistically significant for improved 72-h SOFA score changes, with greater heterogeneity between studies (I2=24%, Z=0.44) (Figure 6).
Heterogeneity test and sensitivity analyses
If the three outcome indicators, namely in-patient mortality rate, ICU mortality rate <50%, had acceptable heterogeneity, a fixed-effect model was used; if I2>50% heterogeneous for ICU stay and 72-h SOFA score, a random-effect model, and sensitivity analysis, respectively (Figures 7,8). As all the studies included in the meta-analysis have differences, we call various variations among different studies in the meta-analysis heterogeneity. These variations are mainly in subjects, study designs, interventions and outcome measurements.
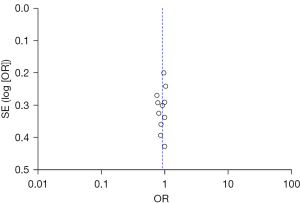

Publication bias analysis
Publication bias in the included studies for assessment of in-hospital mortality rate was assessed by a funnel plot (Figure 9), which was essentially symmetrical, and no publication bias was found.
Literature quality assessment
According to the Cochrane literature quality evaluation method, 7 of the trials had mild bias risk, which was grade A, 4 had moderate bias risk, which was grade B, and 2 had severe bias risk, which was grade C (Figure 10).
Discussion
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection and is the major global cause of morbidity and mortality. Because there is no direct treatment for the pathogenesis of sepsis, clinical management relies on early identification and rapid administration of antibiotics, intravenous fluids and booster when appropriate (31). Over the past 30 years, more than 100 clinical trials have been conducted to evaluate various newly discovered drug and therapeutic interventions to improve the outcomes in patients with sepsis, but none to date has improved sepsis outcomes (32).
Vitamin C can increase vasopressin synthesis, reduce oxidative stress and inflammatory responses, enhance immune cell function, improve vascular endothelial cell function, and epigenetic immune modification (33). This meta-analysis showed that vitamin C use significantly reduced sepsis-induced death. Coopersmith et al. showed that for septic shock patients, thiamine treatment within 24 h of admission was associated with increased lactate clearance and reduced 28-day mortality rate compared with a matched cohort of patients not given thiamine (34). Glucocorticoids have organ-protective effects by reducing mitochondrial damage, inhibiting pro-apoptotic proteins, and reducing cytokine release. Vitamin C, a free radical scavenging antioxidant, has been shown to increase sensitivity to the glucocorticoid receptor and increase cellular uptake of glucocorticoids (hydrocortisone) (35). In turn, hydrocortisone can increase sodium–vitamin C expression of the transport protein 2 receptor, which thereby increases vitamin C absorption. However, taking a high dose of vitamin C results in calcium oxalate nephropathy, which worsens renal function, and thiamine can prevent this response. Triple therapy of thiamine, ascorbic acid and glucocorticoid has biological rationality in the treatment of sepsis. It has been shown to improve prognosis and reduce the mortality rate in patients with sepsis (36). Subsequent cohort studies and RCTs have reported (32-35) reduced patient or ICU mortality rates, and identified potential benefits such as shorter duration of vasopressor therapy, improved lactate clearance, reduced procalcitonin, and reduced SOFA scores. Thus, vitamin C is considered a potential treatment option (37).
The results of our meta-analysis showed that vitamin C did not reduce patient mortality rates, either in-hospital or in the ICU, did not reduce total length of stay or ICU stay, or improve 72-h SOFA scores, compared with the control group. For in-hospital and ICU mortality rates, some retrospective studies (37-39) have shown a reduction among patients treated with vitamin C. Although the studies were retrospectively designed, there were no significant differences in baseline characteristics or disease severity between groups. In addition, patients included in those studies had similar severity of disease as measured by acute analysis and Chronic Health Assessment II (APACHE II) or SOFA scores compared with the patients in our selected RCTs. There may be confounding variables; the 5 of the RCTs included in our study showed that vitamin C therapy did not reduce either the in-patient or ICU mortality rate. In our included studies, intravenous vitamin C did not significantly improve survival over 7 days or shorten the time to administration of vasopressors or resolve septic shock faster than hydrocortisone alone. However, Bughrara et al. showed that vitamin C therapy accelerated reversal of shock (38). The 72-h SOFA score would indicate whether organ injury had improved, but our results showed that vitamin C therapy did not improve the 72-h SOFA score in patients with sepsis (39).
IL-6 is produced by a variety of immune cells such as B and T lymphocytes, monocytes/macrophages, with functions in stimulating T and B lymphocytes as well as participating in cell proliferation and differentiation, and enhancing their function. The expression level of IL-6 is closely associated with the severity of sepsis patients, and there is high value in predicting the disease development and efficacy of sepsis with IL-6 levels alone or in combination with other infection indicators (40). IL-18, on the other hand, has the ability of the chemokines MIP-1a, MIP-1b, and MCP-1 that induce infection in monocytes and macrophages, thereby triggering the inflammatory process.
Our meta-analysis is the most comprehensive study to date of vitamin C treatment because we focused on five study indicators: total in-hospital mortality rate, ICU mortality rate, total length of stay, ICU stay, and 72-h SOFA score.
Study limitations
(I) We included some retrospective studies. Although there were good baseline characteristics for the treatment and control groups, there would be confounding factors that may affect the experimental results. (II) In a large study of 1,144 patients (38) with septic shock, the combination of vitamin C and thiamine did not result in a significant reduction in in-hospital or 28-day mortality rates. However, in a subgroup analysis, treatment was associated with a lower in-hospital mortality rate among patients with low albumin (<3.0 mg/dL) and SOFA score >10. (III) The current sample size was small, comprising only 1,423 patients. (IV) Some of the included studies used hydrocortisone in the control group. (V) Data of continuous variables are reported using median and IQR, which were used to calculate mean and standard deviation in some studies. (VI) Twenty-seven patients (57.4%) in Litwak et al. had inadequate treatment duration (20), which may have reduced the overall benefit in the treatment group. The VICTAS trial (21) terminated their RCT early for management reasons, which may also be one of the reasons for the difference in results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-225/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-225/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Waele E, Malbrain MLNG, Spapen H. Nutrition in Sepsis: A Bench-to-Bedside Review. Nutrients 2020;12:395. [Crossref] [PubMed]
- Belsky JB, Wira CR, Jacob V, et al. A review of micronutrients in sepsis: the role of thiamine, l-carnitine, vitamin C, selenium and vitamin D. Nutr Res Rev 2018;31:281-90. [Crossref] [PubMed]
- Moss SR, Prescott HC. Current Controversies in Sepsis Management. Semin Respir Crit Care Med 2019;40:594-603. [Crossref] [PubMed]
- Kashiouris MG, L'Heureux M, Cable CA, et al. The Emerging Role of Vitamin C as a Treatment for Sepsis. Nutrients 2020;12:292. [Crossref] [PubMed]
- Marik PE. Vitamin C for the treatment of sepsis: The scientific rationale. Pharmacol Ther 2018;189:63-70. [Crossref] [PubMed]
- Marik PE. Hydrocortisone, Ascorbic Acid and Thiamine (HAT Therapy) for the Treatment of Sepsis. Focus on Ascorbic Acid. Nutrients 2018;10:1762. [Crossref] [PubMed]
- De Backer D, Cecconi M, Lipman J, et al. Challenges in the management of septic shock: a narrative review. Intensive Care Med 2019;45:420-33. [Crossref] [PubMed]
- Teng J, Pourmand A, Mazer-Amirshahi M, Vitamin C. The next step in sepsis management? J Crit Care 2018;43:230-4. [Crossref] [PubMed]
- Busse LW, Barker N, Petersen C. Vasoplegic syndrome following cardiothoracic surgery-review of pathophysiology and update of treatment options. Crit Care 2020;24:36. [Crossref] [PubMed]
- Guirguis E, Grace Y, Maarsingh H, et al. Vitamin C, Thiamine, and Steroids in the Sepsis Conquest: Replete to Defeat. J Pharm Pract 2020;33:682-95. [Crossref] [PubMed]
- Obi J, Pastores SM, Ramanathan LV, et al. Treating sepsis with vitamin C, thiamine, and hydrocortisone: Exploring the quest for the magic elixir. J Crit Care 2020;57:231-9. [Crossref] [PubMed]
- Moskowitz A, Andersen LW, Huang DT, et al. Ascorbic acid, corticosteroids, and thiamine in sepsis: a review of the biologic rationale and the present state of clinical evaluation. Crit Care 2018;22:283. [Crossref] [PubMed]
- Badeaux JE, Martin JB. Emerging Adjunctive Approach for the Treatment of Sepsis: Vitamin C and Thiamine. Crit Care Nurs Clin North Am 2018;30:343-51. [Crossref] [PubMed]
- Anand T, Roller LK, Jurkovich GJ. Vitamin C in surgical sepsis. Curr Opin Crit Care 2019;25:712-6. [Crossref] [PubMed]
- Li T, Zeng J, Li DH, et al. Efficacy of intravenous vitamin C intervention for septic patients: A systematic review and meta-analysis based on randomized controlled trials. Am J Emerg Med 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Li YR, Zhu H. Vitamin C for sepsis intervention: from redox biochemistry to clinical medicine. Mol Cell Biochem 2021;476:4449-60. [Crossref] [PubMed]
- Guinot PG, Martin A, Berthoud V, et al. Vasopressor-Sparing Strategies in Patients with Shock: A Scoping-Review and an Evidence-Based Strategy Proposition. J Clin Med 2021;10:3164. [Crossref] [PubMed]
- Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017;151:1229-38. [Crossref] [PubMed]
- Sadaka F, Grady J, Organti N, et al. Ascorbic Acid, Thiamine, and Steroids in Septic Shock: Propensity Matched Analysis. J Intensive Care Med 2020;35:1302-6. [Crossref] [PubMed]
- Litwak JJ, Cho N, Nguyen HB, et al. Vitamin C, Hydrocortisone, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Analysis of Real-World Application. J Clin Med 2019;8:478. [Crossref] [PubMed]
- Mitchell AB, Ryan TE, Gillion AR, et al. Vitamin C and Thiamine for Sepsis and Septic Shock. Am J Med 2020;133:635-8. [Crossref] [PubMed]
- Grady J, Organti N, Donepudi B, et al. Vitamin C in patients with septic shock: A propensity matched analysis. Critical Care Medicine 2019;47:610.
- Long MT, Frommelt MA, Ries MP, et al. Early hydrocortisone, ascorbate and thiamine therapy for severe septic shock. Critical Care and Shock 2020;23:23-34.
- Chang P, Liao Y, Guan J, et al. Combined Treatment With Hydrocortisone, Vitamin C, and Thiamine for Sepsis and Septic Shock: A Randomized Controlled Trial. Chest 2020;158:174-82. [Crossref] [PubMed]
- Mohamed ZU, Prasannan P, Moni M, et al. Vitamin C Therapy for Routine Care in Septic Shock (ViCTOR) Trial: Effect of Intravenous Vitamin C, Thiamine, and Hydrocortisone Administration on Inpatient Mortality among Patients with Septic Shock. Indian J Crit Care Med 2020;24:653-61. [Crossref] [PubMed]
- Moskowitz A, Huang DT, Hou PC, et al. Effect of Ascorbic Acid, Corticosteroids, and Thiamine on Organ Injury in Septic Shock: The ACTS Randomized Clinical Trial. JAMA 2020;324:642-50. [Crossref] [PubMed]
- Wani SJ, Mufti SA, Jan RA, et al. Combination of vitamin C, thiamine and hydrocortisone added to standard treatment in the management of sepsis: results from an open label randomised controlled clinical trial and a review of the literature. Infect Dis (Lond) 2020;52:271-8. [Crossref] [PubMed]
- Iglesias J, Vassallo AV, Patel VV, et al. Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis: The ORANGES Trial. Chest 2020;158:164-73. [Crossref] [PubMed]
- Sevransky JE, Rothman RE, Hager DN, et al. Effect of Vitamin C, Thiamine, and Hydrocortisone on Ventilator- and Vasopressor-Free Days in Patients With Sepsis: The VICTAS Randomized Clinical Trial. JAMA 2021;325:742-50. [Crossref] [PubMed]
- Fujii T, Luethi N, Young PJ, et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock The VITAMINS Randomized Clinical Trial. JAMA 2020;323:423-31. [Crossref] [PubMed]
- Wang Y, Lin H, Lin BW, et al. Effects of different ascorbic acid doses on the mortality of critically ill patients: a meta-analysis. Ann Intensive Care 2019;9:58. [Crossref] [PubMed]
- Font MD, Thyagarajan B, Khanna AK. Sepsis and Septic Shock - Basics of diagnosis, pathophysiology and clinical decision making. Med Clin North Am 2020;104:573-85. [Crossref] [PubMed]
- Esposito S, De Simone G, Boccia G, et al. Sepsis and septic shock: New definitions, new diagnostic and therapeutic approaches. J Glob Antimicrob Resist 2017;10:204-12. [Crossref] [PubMed]
- Coopersmith CM, De Backer D, Deutschman CS, et al. Surviving sepsis campaign: research priorities for sepsis and septic shock. Intensive Care Med 2018;44:1400-26. [Crossref] [PubMed]
- Sacha GL, Bauer SR, Lat I. Vasoactive Agent Use in Septic Shock: Beyond First-Line Recommendations. Pharmacotherapy 2019;39:369-81. [Crossref] [PubMed]
- Moskowitz A, Donnino MW. Thiamine (vitamin B1) in septic shock: a targeted therapy. J Thorac Dis 2020;12:S78-83. [Crossref] [PubMed]
- Dubin A, Lattanzio B, Gatti L. The spectrum of cardiovascular effects of dobutamine - from healthy subjects to septic shock patients. Rev Bras Ter Intensiva 2017;29:490-8. [Crossref] [PubMed]
- Bughrara N, Cha S, Safa R, et al. Perioperative Management of Patients with Sepsis and Septic Shock, Part I: Systematic Approach. Anesthesiol Clin 2020;38:107-22. [Crossref] [PubMed]
- Hilarius KWE, Skippen PW, Kissoon N. Early Recognition and Emergency Treatment of Sepsis and Septic Shock in Children. Pediatr Emerg Care 2020;36:101-6. [Crossref] [PubMed]
- Pandompatam G, Kashani K, Vallabhajosyula S. The role of natriuretic peptides in the management, outcomes and prognosis of sepsis and septic shock. Rev Bras Ter Intensiva 2019;31:368-78. [Crossref] [PubMed]
(English Language Editor: K. Brown)









