
Efficacy and safety of Corbrin Capsule on malnutrition, inflammation, and atherosclerosis syndrome in patients with uremia: systematic review and meta-analysis
Introduction
Uremia is an end-stage change in patients with chronic kidney disease (CKD), and maintenance hemodialysis treatment can remove toxic and side metabolites from the patient’s body and maintain the homeostasis of the body’s internal environment (1,2). However, patients undergoing maintenance hemodialysis for uremic disease develop a generalized microinflammatory state (3). In a long-term state of micro-inflammation, a series of changes in the heart structure and function of patients will also occur, which will eventually lead to the occurrence of cardiovascular disease (4). In addition, the release of inflammatory factors in the body of patients with uremia can reduce protein synthesis, further promote the decomposition and metabolism of muscle, and lead to malnutrition in patients (5). Malnutrition mainly refers to the lack of nutrients caused by protein or energy absorption disorders, insufficient intake levels, and a series of specific symptoms. It is therefore a common complication of end-stage renal disease (ESRD) and an important prognostic marker for patients with CKD (6-8). In uremic patients undergoing hemodialysis, inflammation affects the therapeutic effect and accelerates the progression of atherosclerosis, ultimately leading to cardiovascular disease (9). To this end, some scholars proposed the concept of malnutrition, inflammation, and atherosclerosis (MIA) syndrome, which emphasized the relationship between malnutrition, inflammation, and atherosclerotic cardiovascular disease in uremic patients (10,11).
Corbrin Capsule is mainly produced by low-temperature fermentation of Cordyceps sinensis strains. It has the effect of invigorating the lungs and kidneys and improving the essence and qi. It not only invigorates the lungs, kidneys, and spleen, but also regulates the metabolism of the organs, in turn promoting blood circulation and the excretion of toxins (12). Studies have confirmed that Corbrin Capsule is rich in Cordyceps polysaccharides, Cordyceps acid, nucleotides, and amino acids, which can improve the metabolic disorders of lipids and proteins in the body, and also have anti-inflammatory and antioxidant effects (13,14). Kai et al. showed that Corbrin Capsule could effectively reduce the levels of inflammatory factors and prevent the occurrence of contrast nephropathy when used for the protection of contrast nephropathy during coronary angiography in type 2 diabetic renal insufficiency (15). Zhao et al. studied the preventive effect of Corbrin Capsule on contrast agent nephropathy in patients with stable angina pectoris, and found that it can effectively prevent the damage of renal function caused by contrast agent (16). It can be concluded that most of the current research focuses on the prevention and treatment of contrast agent nephropathy, but there is no systematic evaluation of the clinical effect of Corbrin Capsule on MIA syndrome in uremia patients.
Therefore, in this study, we analyzed and explored Corbrin Capsule’s impact on MIA syndrome in uremic patients, and aimed to provide a reference for the future clinical treatment and prognosis of uremic MIA patients. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-291/rc).
Methods
Literature search
We performed a computer search of databases including PubMed, Web of Science, Embase, The Cochrane Library, and WanFang. The search time range was from January 2000 to September 2020. The search terms were set as “Bailing Capsule”, “uremia”, “Patients with end-stage renal disease”, “MIA syndrome”, “renal function”, “traditional Chinese medicine”.
Inclusion and exclusion criteria of literature
The inclusion and exclusion criteria were formulated according to the PICOS principle. Patients in the control group were given conventional treatment, while those in the treatment group were given Corbrin Capsule or Cordyceps sinensis on the basis of conventional treatment.
The inclusion criteria were as follows: (I) published literature exploring the effects of Corbrin Capsule on MIA syndrome in uremia patients; (II) direct or indirect evaluation of the indicators of MIA syndrome in patients with uremia; (III) at least 15 samples included in the study.
The exclusion criteria were as follows: (I) repeated publication of the same set of data; (II) review, conference report, experience lecture, case report, and research commentary; (III) research unrelated to the subject of this study; (IV) no control group set, or the samples between groups were not comparable; (V) studies where outcome indicators were not reported clearly and results data were incomplete.
Quality assessment of literature
The full texts of retrieved literature were read independently by two researchers, and they extracted relevant data. Disagreements between the two researchers were resolved through discussion, and if they failed to reach an agreement, a third researcher was invited to arbitrate. The Cochrane Reviewer’s Handbook 4.2.5 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) was used to evaluate the literature quality in terms of (I) randomized trial; (II) allocation concealment; (III) blind testing; (IV) complete result data; (V) selective reporting of results; and (VI) other bias. The specific evaluation methods of Cochrane Reviewer’s Handbook 4.2.5 were shown in Table 1.
Table 1
| Field | Item | Descriptions | ||
|---|---|---|---|---|
| High risk of bias | Low risk of bias | Unknown risk of bias | ||
| Selection bias | Generation of random sequences | Selection bias due to improper random sequence generation | Random sequence generation can generate comparison groups | Insufficient description details |
| Allocation hidden | Selection bias due to improper allocation concealment | The allocation of interventions cannot be predicted prior to enrolment | Insufficient description details | |
| Implementation bias | Investigators and implementers were blinded | Workers understand the intervention implementation of the study and lead to performance bias | Blinding may work | Insufficient description details |
| Measurement bias | Blind evaluation of study results | Workers understand the intervention implementation of the study and lead to detection bias | Blinding may work | Insufficient description details |
| Follow-up bias | Integrity of the resulting data | Attrition bias due to incomplete outcome data | Missing outcome data have been treated with low potential for bias | Insufficient reporting of loss to follow-up and exclusion, leading to inability to judge |
| Reporting bias | Selective reporting of research findings | Bias due to selective reporting of results | No bias was detected in relation to selective reporting of results | Not giving enough information to make a judgment |
| Other bias | Other sources of bias | Bias due to issues not covered above | No other bias was detected | Possible risk of bias, insufficient information to assess other important risk of bias |
Data extraction
The data extracted included the following: (I) first author, year of publication, and evaluation results of the included literature; (II) the evaluation results such as the number of participants, experimental design, measures, study time, and outcome indicators; (III) baseline data of patients; (IV) indicators of feedback research quality.
Statistical methods
The software Review Manager 5.3 (RevMan 5.3; The Cochrane Collaboration, Denmark) was used for data statistics and analysis. First, heterogeneity testing was carried out for the test results at α=0.05. The heterogeneity among literatures was analyzed using the Peto method. No heterogeneity was indicated when I2<50%, and the fixed effects model (FEM) was adopted. If I2>50%, heterogeneity was indicated, and the random effects model (REM) was used for analysis. Continuous variables can be meta-analyzed using mean difference (MD), weighted mean difference (WMD), or standard mean difference (SMD). Dichotomous variables can be meta-analyzed using odds ratio (OR), relative risk (RR), etc. All results were expressed with 95% confidence interval (CI). A funnel plot was drawn and the publication bias was evaluated by the concentration of literature to the midline. Sensitivity analysis was used to assess the reliability and stability of the results.
Results
Literature search results and profile analysis
A total of 579 records were retrieved from the database search, and 267 abstracts were obtained after duplicates had been deleted. A total of 85 articles meeting the requirements were selected preliminarily. After further reading of full texts, articles that were not randomized, were repeatedly published, and for which full texts were unavailable were excluded, and 6 articles that met the requirements were finally included in this study (17-22). The specific retrieval process is shown in Figure 1, and Table 2 shows the basic information of all literature.
Table 2
| First author | Publish year | Outcome indicators | MIA/treatment | Control |
|---|---|---|---|---|
| Turkmen K | 2013 | BMI and CRP | 79 | 20 |
| Bammens B | 2004 | BMI and CRP | 26 | 24 |
| Terrier N | 2005 | BMI and CRP | 57 | 120 |
| Mutluay R | 2019 | BMI and CRP | 79 | 78 |
| Zhang Z | 2011 | BUN and sCr | 122 | 109 |
| Wang W | 2013 | BUN and sCr | 80 | 100 |
BMI, body mass index; CRP, C-reactive protein; BUN, blood urea nitrogen; sCr, serum creatinine; MIA, malnutrition, inflammation, and atherosclerosis.
Bias risk assessment of included literature
The literature quality was assessed by referring to the specific criteria in the Cochrane Handbook for Systematic Reviews of Interventions (The Cochrane Collaboration, Denmark), and the results are shown in Figures 2,3. None of these 6 studies had random sequence generation (selection bias), incomplete outcome data (selection bias), and selective reporting (reporting bias). Moreover, the overall risk of articles included in the study was low. As displayed in Table 2, all the 6 literatures included in the study had a low risk of bias, which met the requirements for subsequent analysis.
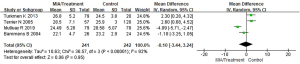
Meta-analysis of body mass index (BMI) of patients with MIA syndrome
Of the included articles, 4 studies evaluated the BMI scores of patients with MIA syndrome in the uremic patient population in detail. The BMI between the patients with MIA syndrome and the control group in the uremia patient group were compared, and the results are shown in Figure 4. The BMI scores of patients in the MIA group and the control group were heterogeneous (I2=92%; P<0.00001). The REM analysis results showed that the total BMI scores of patients with MIA syndrome group and control group are analyzed as MD (95% CI): −0.10 (−3.44 to 3.24) with Z=0.06, P=0.95. This suggested that the BMI scores of the two groups of patients were not significantly different (P>0.05).
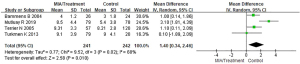
Meta-analysis of CRP levels in patients with MIA syndrome
Of the included literatures, 4 studies evaluated the CRP levels of patients with MIA syndrome in uremic patients in detail. The CRP index between the MIA syndrome patient group and the control group in the uremic patient group was compared, and the results are shown in Figure 5. The CRP index data of patients in the MIA group and the control group were heterogeneous (I2=68%; P=0.02). The REM analysis showed the total CRP level of patients with MIA syndrome group and control group were MD (95% CI): 1.40 (0.34 to 2.46) with Z=2.58 and P=0.010. The results showed that the CRP index data of the two groups of patients were significantly different (P<0.05).
Meta-analysis of the influence of Corbrin Capsule on blood urea nitrogen (BUN) of MIA syndrome in patients with uremia
Of the included literatures, 2 studies evaluated the effect of Corbrin Capsule on MIA syndrome in patients with uremia in detail. Although other articles had simple descriptions, they did not evaluate in detail the impact of Corbrin Capsule on the specific physiological indicators of uremia patients with MIA syndrome. The BUN between the Corbrin Capsule treatment group and the control group of patients with MIA syndrome was compared, and the results are shown in Figure 6. The BUN of the treatment group and the control group were heterogeneous (I2=82%; P=0.02). The REM analysis revealed that BUN of the treatment group and the control group were MD (95% CI): −1.15 (−3.05 to 0.75), Z=1.18, P=0.24. This showed that the BUN scores of the two groups were not significantly different (P>0.05).
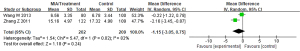
Meta-analysis of the influence of Corbrin Capsule on serum creatinine (sCr) of MIA syndrome in patients with uremia
Of the included articles, 2 studies evaluated the effect of Corbrin Capsule on MIA syndrome in patients with uremia in detail. Although other articles had simple descriptions, they did not evaluate in detail the impact of Corbrin Capsule on the specific physiological indicators of uremia patients with MIA syndrome. The sCr between the Corbrin Capsule treatment group and the control group of patients with MIA syndrome was compared, and the results are shown in Figure 7. The sCr of patients in the treatment group and the control group were heterogeneous (I2=100%; P<0.00001). The REM analysis results of sCr of patients in the treatment group and the control group were as follows: MD (95% CI): −72.82 (−202.16 to 56.52), Z=1.10, P=0.27. This showed that the sCr scores of the two groups of patients were not significantly different (P>0.05).

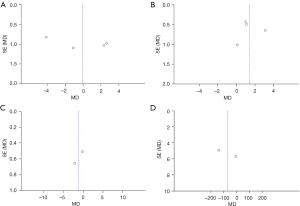
Publication bias analysis
A total of 4 evaluation indicators of patients with uremic MIA syndrome were analyzed, including BMI, CRP, BUN, and sCr, and the publication bias results are shown in Figure 8. The funnel charts of BMI, CRP, and BUN were basically symmetrical, and the data were also relatively concentrated. As shown in Figure 8D, there were individual samples in the funnel of sCr that did not fall into the funnel. This revealed that there was no big publication bias in the 4 functional indicators included in the literature of this study.
Discussion
As the terminal stage of chronic kidney failure, ESRD can be confirmed when the glomerular filtration rate (GFR) is lower than the specified level (15 mL/min/1.73 m2) (23). Stage V CKD can be considered ESRD. After continual accumulation, toxins can cause uremia, and for this, hemodialysis must be performed for patients to maintain their normal life (24). In addition, if suitable kidney sources are found for transplant replacement, treatment can be carried out. However, patients with such diseases need to undergo prolonged hemodialysis to maintain normal physical indicators (25,26). Moreover, long-term hemodialysis often brings other types of complications to patients.
Patients with renal diseases such as uremia treated by dialysis have problems of poor quality of life and high mortality. Cardiovascular disease is the main cause of death in dialysis patients, and 30% to 60% of patients with ESRD suffer from malnutrition (27). In recent years, a large number of studies have confirmed that there is a causal relationship between malnutrition, inflammation and atherosclerosis (28,29). Due to the accumulation of toxins in the body, patients with uremia will experience problems such as anorexia, nausea and vomiting, and cause insufficient protein and calorie intake. Due to the above problems, patients with uremia will have problems such as abnormal metabolism and abnormal hormone levels, which will cause malnutrition in patients. MIA syndrome is prevalent in patients with renal insufficiency and affects the prognosis of patients (30).
According to relevant literature data, the incidence of MIA syndrome in patients with ESRD during perinatal hemodialysis treatment is up to 80%, and it has become the leading killer of kidney disease patients (31). Researchers have suggested that inflammation is a key issue in the 3 complications of MIA syndrome. Inflammation can lead to further accelerated kidney failure in patients with ESRD (32). In related studies, Corbrin Capsule has been shown to significantly reduce inflammation (33,34). The data showed that Corbrin Capsule has an ideal effect on reducing CRP levels in patients with inflammation (35). Corbrin Capsule is a product processed and extracted from cordyceps sinensis. It can effectively improve the inflammatory response of the human body, restore metabolic capacity, and have a good effect in accelerating the protein synthesis of the human body.
In short, the effects of Corbrin Capsule on MIA syndrome in patients with uremia were analyzed and compared. The results showed that patients with uremia syndrome and patients with MIA syndrome have significant differences in these indicators, which had effectively reduced BUN and sCr. However, due to the conditions for systematic review and meta-analysis, the analysis of evaluation indicators was still few, which should be further increased to more indicators analysis.
Conclusions
The objective of this study was to analyze the effects of Corbrin Capsule on MIA syndrome in uremia patients. A total of 6 appropriate references were selected, including 1,103 patients. Then, RevMan 5.3 was used to conduct meta-analysis of the experimental data. According to the results of meta-analysis, there were significant differences in BUN and CRP in patients after treatment. The effects of Corbrin Capsule on MIA syndrome in uremia patients were analyzed and compared, and it was found that patients with uremia syndrome and patients with MIA syndrome have significant differences in these indicators, which had effectively reduced BUN and sCr. In summary, this study provides a further theoretical basis for subsequent studies on the efficacy of Corbrin Capsule in the treatment of MIA syndrome in uremia patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-291/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-291/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Girndt M, Trojanowicz B, Ulrich C. Monocytes in Uremia. Toxins (Basel) 2020;12:340. [Crossref] [PubMed]
- Puri I, Shirazi NM, Yap E, et al. Intestinal dialysis for conservative management of Uremia. Curr Opin Nephrol Hypertens 2020;29:64-70. [Crossref] [PubMed]
- Martinez Cantarin MP, Whitaker-Menezes D, Lin Z, et al. Uremia induces adipose tissue inflammation and muscle mitochondrial dysfunction. Nephrol Dial Transplant 2017;32:943-51. [Crossref] [PubMed]
- Azevedo CAB, da Cunha RS, Junho CVC, et al. Extracellular Vesicles and Their Relationship with the Heart-Kidney Axis, Uremia and Peritoneal Dialysis. Toxins (Basel) 2021;13:778. [Crossref] [PubMed]
- Sahathevan S, Khor BH, Ng HM, et al. Understanding Development of Malnutrition in Hemodialysis Patients: A Narrative Review. Nutrients 2020;12:3147. [Crossref] [PubMed]
- Zhang H, Lin C, Yuan S, et al. Application of Holistic Nursing in Uremic Patients with Hematodialysis Related Malnutrition. Iran J Public Health 2017;46:500-5. [PubMed]
- Larson-Nath C, Goday P. Malnutrition in Children With Chronic Disease. Nutr Clin Pract 2019;34:349-58. [Crossref] [PubMed]
- Maraj M, Kuśnierz-Cabala B, Dumnicka P, et al. Malnutrition, Inflammation, Atherosclerosis Syndrome (MIA) and Diet Recommendations among End-Stage Renal Disease Patients Treated with Maintenance Hemodialysis. Nutrients 2018;10:69. [Crossref] [PubMed]
- Christoffersen C, Bartels ED, Aarup A, et al. ApoB and apoM - New aspects of lipoprotein biology in uremia-induced atherosclerosis. Eur J Pharmacol 2017;816:154-60. [Crossref] [PubMed]
- Huang Z, Fang J, Song A, et al. The association between self-management ability and malnutrition-inflammation-atherosclerosis syndrome in peritoneal dialysis patients: a cross-sectional study. BMC Nephrol 2021;22:13. [Crossref] [PubMed]
- Kamijo Y, Kanda E, Ishibashi Y, et al. Sarcopenia and Frailty in PD: Impact on Mortality, Malnutrition, and Inflammation. Perit Dial Int 2018;38:447-54. [Crossref] [PubMed]
- Zhu J, Yan L, Ji JN, et al. Fluvastatin combined with corbrin capsule can improve depression and anxiety in the treatment of chronic obstructive pulmonary disease. Am J Transl Res 2021;13:10501-8. [PubMed]
- He T, Zhao R, Lu Y, et al. Dual-Directional Immunomodulatory Effects of Corbrin Capsule on Autoimmune Thyroid Diseases. Evid Based Complement Alternat Med 2016;2016:1360386. [Crossref] [PubMed]
- Wu J, Yan W, Wu X, et al. Protective effects of Corbrin Capsule against permanent cerebral ischemia in mice. Biomed Pharmacother 2020;121:109646. [Crossref] [PubMed]
- Kai Z, Yongjian L, Sheng G, et al. Effect of Dongchongxiacao (Cordyceps) therapy on contrast-induced nephropathy in patients with type 2 diabetes and renal insufficiency undergoing coronary angiography. J Tradit Chin Med 2015;35:422-7. [Crossref] [PubMed]
- Zhao K, Li Y, Zhang H. Role of dongchongxiacao (Cordyceps) in prevention of contrast-induced nephropathy in patients with stable angina pectoris. J Tradit Chin Med 2013;33:283-6. [Crossref] [PubMed]
- Turkmen K, Tonbul HZ, Erdur FM, et al. Peri-aortic fat tissue and malnutrition-inflammation-atherosclerosis/calcification syndrome in end-stage renal disease patients. Int Urol Nephrol 2013;45:857-67. [Crossref] [PubMed]
- Bammens B, Evenepoel P, Verbeke K, et al. Impairment of small intestinal protein assimilation in patients with end-stage renal disease: extending the malnutrition-inflammation-atherosclerosis concept. Am J Clin Nutr 2004;80:1536-43. [Crossref] [PubMed]
- Terrier N, Senécal L, Dupuy AM, et al. Association between novel indices of malnutrition-inflammation complex syndrome and cardiovascular disease in hemodialysis patients. Hemodial Int 2005;9:159-68. [Crossref] [PubMed]
- Mutluay R, Konca Değertekin C, Işıktaş Sayılar E, et al. Serum fetuin-A is associated with the components of MIAC(malnutrition, inflammation, atherosclerosis, calcification) syndrome in different stages of chronic kidney disease Turk J Med Sci 2019;49:327-35. [Crossref] [PubMed]
- Zhang Z, Wang X, Zhang Y, et al. Effect of Cordyceps sinensis on renal function of patients with chronic allograft nephropathy. Urol Int 2011;86:298-301. [Crossref] [PubMed]
- Wang W, Zhang XN, Yin H, et al. Effects of Bailing capsules for renal transplant recipients: a retrospective clinical study. Chin Med J (Engl) 2013;126:1895-9. [PubMed]
- Lai AC, Bienstock SW, Sharma R, et al. A Personalized Approach to Chronic Kidney Disease and Cardiovascular Disease: JACC Review Topic of the Week. J Am Coll Cardiol 2021;77:1470-9. [Crossref] [PubMed]
- Covino M, Vitiello R, De Matteis G, et al. Hip Fracture Risk in Elderly With Non-End-Stage Chronic Kidney Disease: A Fall Related Analysis. Am J Med Sci 2022;363:48-54. [Crossref] [PubMed]
- Ahmadmehrabi S, Tang WHW. Hemodialysis-induced cardiovascular disease. Semin Dial 2018;31:258-67. [Crossref] [PubMed]
- Reeves PB, Mc Causland FR. Mechanisms, Clinical Implications, and Treatment of Intradialytic Hypotension. Clin J Am Soc Nephrol 2018;13:1297-303. [Crossref] [PubMed]
- Yang X, Zhang H, Shi Y, et al. Association of serum angiopoietin-2 with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients: a prospective cohort study. J Transl Med 2018;16:312. [Crossref] [PubMed]
- Kirushnan BB, Rao BS, Annigeri R, et al. Impact of Malnutrition, Inflammation, and Atherosclerosis on the Outcome in Hemodialysis Patients. Indian J Nephrol 2017;27:277-83. [Crossref] [PubMed]
- Perunicić-Peković G, Rasić-Milutinović Z, Pljesa S. Predictors of mortality in dialysis patients--association between malnutrition, inflammation and atherosclerosis (MIA syndrome)]. Med Pregl 2004;57:149-52. [Crossref] [PubMed]
- Sueta D, Hokimoto S, Sakamoto K, et al. Validation of the high mortality rate of Malnutrition-Inflammation-Atherosclerosis syndrome: -Community-based observational study. Int J Cardiol 2017;230:97-102. [Crossref] [PubMed]
- Trifonova EA, Mustafin ZS, Lashin SA, et al. Abnormal mTOR Activity in Pediatric Autoimmune Neuropsychiatric and MIA-Associated Autism Spectrum Disorders. Int J Mol Sci 2022;23:967. [Crossref] [PubMed]
- Allawi AAD. Malnutrition, inflamation and atherosclerosis (MIA syndrome) in patients with end stage renal disease on maintenance hemodialysis (a single centre experience). Diabetes Metab Syndr 2018;12:91-7. [Crossref] [PubMed]
- Chen Y, Fu L, Han M, et al. The Prophylactic and Therapeutic Effects of Fermented Cordyceps sinensis Powder, Cs-C-Q80, on Subcortical Ischemic Vascular Dementia in Mice. Evid Based Complement Alternat Med 2018;2018:4362715. [Crossref] [PubMed]
- Zhao K, Lin Y, Li YJ, et al. Efficacy of short-term cordyceps sinensis for prevention of contrast-induced nephropathy in patients with acute coronary syndrome undergoing elective percutaneous coronary intervention. Int J Clin Exp Med 2014;7:5758-64. [PubMed]
- Hu R, Fu XC, Shen LM, et al. Corbrin shugan capsule for treatment of alcoholic hepatic fibrosis in rats. Zhejiang Da Xue Xue Bao Yi Xue Ban 2012;41:564-8. [PubMed]
(English Language Editor: J. Jones)





