
Efficacy and safety of intravenous paricalcitol treatment in Chinese hemodialysis patients: a real-world database analysis
Introduction
Chronic kidney disease (CKD) is a global health concern. The prevalence of CKD in Chinese adults is estimated to be 10.8% (1). Secondary hyperparathyroidism (SHPT) is a common chronic complication of CKD, presenting in most patients on dialysis (2-5). The pathogenesis of SHPT involves the reduced synthesis of calcitriol with phosphate retention due to renal impairment, eventually resulting in fluctuating parathyroid hormone (PTH) levels. As a uremic toxin, uncontrolled PTH contributes to abnormalities of bone turnover and mineralization and other serious complications beyond bone, decreased quality of life, and increased mortality risk. However, only a small proportion of hemodialysis (HD) patients with SHPT achieve their targets, and consistent failure to achieve the recommended targets strongly predicts mortality (6-8).
Vitamin D receptors (VDRs) are targets for pharmacological therapy of SHPT. The chronic kidney disease-mineral and bone disorder (CKD-MBD) guidelines from Kidney Disease Improving Global Outcomes (KDIGO), Kidney Disease Outcomes Quality Initiative (KDOQI), and China have emphasized the important role of vitamin D analogs in the treatment of SHPT in dialysis patients (9-12). Although calcitriol is a conventional therapeutic approach for SHPT, it can result in hypercalcemia and hyperphosphatemia (13). To identify a vitamin D receptor activator (VDRA) that would be more specific and selective, paricalcitol was developed by chemical modification of the side chain and the A-ring of the molecule (14). Paricalcitol, a selective vitamin D analogue with a higher affinity for the parathyroid glands than for the gastrointestinal tract, was introduced in China in 2013 (15,16). Previous studies have demonstrated that paricalcitol suppresses intact parathyroid hormone (iPTH) more rapidly with a lower rate of hypercalcemia and hyperphosphatemia than calcitriol (17-20). Additionally, the differences between calcitriol and paricalcitol were demonstrated in smooth muscle vascular calcification in preclinical studies in uremic animals (21). The use of paricalcitol was shown to result in improvements in the survival of HD patients (22,23). Although Xie et al. (24) published a meta-analysis in 2017 was insufficient to draw a conclusion regarding whether paricalcitol therapy has a comparative efficacy and safety over other VDRAs (calcitriol, alfacalcidol and maxacalcitol) for treating dialysis patients with SHPT, another two latest meta-analysis demonstrated that paricalcitol was crucial in reducing the iPTH and improving the overall survival than other VDRAs (calcitriol, alfacalcidol, maxacalcitol and doxercalciferol) in patients undergoing hemodialysis (25,26).
However, no information is available on the impact of paricalcitol on markers of SHPT in real-world clinical practice in China. To address this, an observational study including Chinese HD patients was designed to obtain information regarding paricalcitol treatment of SHPT in routine treatment for various observation periods (from 0.5 to 48 months). The results will provide abundant experience to improve SHPT management. We present the following article in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3966/rc).
Methods
Data source
To help more patients with SHPT, the China Primary Health Care Foundation (CPHCF) launched the Better Life for Future (BLFF) project in 2014. From January 2015 to May 2019, the BLFF project accumulated data from 1,744 patients (including Ca, P, iPTH, and dose).
The medical criteria for BLFF project were based on the Chinese package insert of paricalcitol injection (Zemplar®) and KDOQI guidelines. Patients with the following conditions were excluded: (I) hypersensitivity to vitamin D, Vitamin D-related compounds, or any ingredient in the paricalcitol injection solution; (II) vitamin D toxicity; (III) aluminum toxicity from recent long-term use of aluminum-containing or magnesium-containing preparations; and (IV) severe hepatic impairment. Paricalcitol was manufactured by AbbVie (Lake Bluff, IL, USA) under the trade name Zemplar® and was administered intravenously during dialysis. The decision to initiate treatment with intravenous paricalcitol was determined solely by the treating physician. The disease evaluation form filled out by the physician and the last original report of SHPT-related markers were required for each application for paricalcitol. These data were entered into the BLFF database using the patient’s medical records and evaluation form as source data. During the treatment, follow-up by telephone will be used to continuously to monitor adverse events to ensure the patient’s safety.
The frequency of measurements was also determined by the treating nephrologist. In general, iPTH was measured quarterly and additionally when medication or doses changed in China. No interventions were made based on treatment, patient discontinuation, or the follow-up decisions of the physicians.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (XHEC-D-2020-099). Each patient was required to sign informed consent before receiving treatment, agreeing to provide their health-related information and medical records (including outpatient, inpatient, emergency, follow-up, etc.) to CPHCF and the project hospitals for future research. The personal information of patients in the database was scrambled to protect privacy.
Study design and participants
This multicenter database included HD patients with SHPT who received intravenous paricalcitol from 170 dialysis centers in 60 cities in China between January 2015 and May 2019. The inclusion criteria were as follows: (I) at least 2 consecutive follow-up data points for each patient and (II) a follow-up interval of no more than 6 months. The exclusion criteria were as follows: (I) iPTH >2,500 pg/mL with no exact value; (II) iPTH >3,000 pg/mL; and (III) a follow-up interval more than 6 months (this group was excluded from the analyses because they were considered to have discontinued treatment). The number of cases in the database during the study period determined the sample size. Of 1,744 patients screened, 1,076 did not meet eligibility criteria for the abovementioned reasons. In total, 668 patients were finally enrolled into the study. The characteristics of patients and reasons for withdrawal are provided in Figure 1.
Analysis end points
Conventional PTH assays/second-generation immunometric PTH assays were employed. Further laboratory blood parameters included Ca and P. Baseline iPTH, Ca, and P were defined as the first values recorded. Endpoint iPTH, Ca, and P were defined as the final values recorded. It was estimated that the proportion of patients with ≥30% or even ≥60% decrease in iPTH after paricalcitol administration. The proportions of patients achieving the targets from KDIGO and the Chinese guideline of 120–600 pg/mL for iPTH were determined at baseline and final follow-up. We also recorded the total dose of paricalcitol given, and then the average weekly dose was estimated for each patient. Safety analysis was conducted by comparing the incidence of adverse events. Hypercalcemia and hyperphosphatemia were respectively defined as serum Ca >2.5 mmol/L, and serum P>1.78 mmol/L according to the Chinese guideline.
Statistical analyses
The analysis included all participants with at least 2 consecutive follow-up data points between January 2015 and May 2019. No imputation was used for missing endpoint data. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA). For numeric variables and continuous variables, descriptive statistics were expressed as the mean, median (Q1–Q3), and standard deviation. For categorical values, descriptive statistics were expressed as frequencies and percentages. Subsequent laboratory values were compared using the 2-sample t-test, Fisher’s exact test, and chi-square test. For correlation analysis, simple linear regression, multiple linear regression analysis, and logistic regression analysis were conducted. For significance of the decrease in iPTH among different groups, we utilized one-way analysis of variance (ANOVA). Survival analysis was considered for determine the adequate treatment period. Statistical significance was defined as P<0.05. No testing of statistical hypotheses was conducted.
Results
Patient characteristics
A total of 668 Chinese HD patients from 104 dialysis centers were included in the analysis set between January 2015 to May 2019. The mean age was 51±14 years, and 44.61% of patients were female. Initial iPTH levels were as follows: iPTH <1,000 pg/mL, 37.06% of patients (n=255); iPTH ≥1,000 pg/mL, and 60.03% of patients (n=413). Patients were divided into 5 groups based on the duration of follow-up: Month 0.5–3 (Day 14–90), Month 3–6 (Day 91–180), Month 6–12 (Day 181–360), Month 12–24 (Day 361–720), and Month 24–48 (Day 721–1,440) (Table 1).
Table 1
| Parameter | Month 0.5–3 | Month 3–6 | Month 6–12 | Month 12–24 | Month 24–48 | Cases (n) |
|---|---|---|---|---|---|---|
| n (%) | 203 (30.39) | 205 (30.69) | 150 (22.46) | 85 (12.72) | 25 (3.74) | 668 |
| Female, n (%) | 92 (45.32) | 85 (41.46) | 70 (46.67) | 40 (47.06) | 11 (44.00) | 298 (44.61) |
| Age, yearsa | 51±14 | 52±14 | 53±15 | 50±14 | 49±10 | 51±14 |
| Dialysis centers, n | 74 | 74 | 59 | 45 | 21 | 104 |
| iPTH <1,000 pg/mL, n | 65 | 90 | 67 | 29 | 4 | 255 |
| iPTH ≥1,000 pg/mL, n | 138 | 115 | 83 | 56 | 21 | 413 |
a, mean ± SD. n, number of patients/dialysis centers; iPTH, intact parathyroid hormone.
Control of iPTH
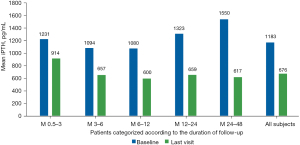
Overall, the median iPTH levels decreased from 1,183 pg/mL at baseline to 676 pg/mL at the final visit (P<0.0001), as shown in Table 2 and Figure 2.
Table 2
| Parameter | All cases | Baseline iPTH <1,000 | Baseline iPTH ≥1,000 | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Last measurement | Baseline | Last measurement | Baseline | Last measurement | |||
| Month 0.5–3 | ||||||||
| Median | 1,231 (880–1,850) | 914 (590–1,311) | 722 (610–854) | 624 (421–714) | 1,649 (1,228–2,012) | 1,107 (752–1,485) | ||
| Mean | 1,376±622 | 990±497 | 722±176 | 630±258 | 1,684±507 | 1,159±493 | ||
| Month 3–6 | ||||||||
| Median | 1,094 (797–1,609) | 657 (448–1,041) | 735 (512–840) | 492 (382–648) | 1,496 (1,217–1,913) | 956 (622–1,346) | ||
| Mean | 1210±599 | 816±475 | 689±205 | 546±226 | 1,618±477 | 1,027±511 | ||
| Month 6–12 | ||||||||
| Median | 1,080 (784–1,675) | 600 (434–860) | 739 (628–891) | 469 (404–574) | 1,578 (1,244–2,039) | 786 (599–1,134) | ||
| Mean | 1,276±619 | 721±400 | 748±171 | 501±137 | 1,702±511 | 899±452 | ||
| Month 12–24 | ||||||||
| Median | 1,323 (894–1,720) | 659 (512–887) | 766 (620–894) | 547 (417–684) | 1,574 (1,328–1,948) | 704 (556–1,102) | ||
| Mean | 1,359±611 | 736±374 | 750±183 | 541±165 | 1,675±506 | 837±412 | ||
| Month 24–48 | ||||||||
| Median | 1,550 (1,182–2,140) | 617 (507–926) | 694 (606–818) | 526 (449–548) | 1,652 (1,376–2,193) | 678 (507–940) | ||
| Mean | 1,576±600 | 675±282 | 712±159 | 498±84 | 1,741±501 | 708±295 | ||
| All subjects | ||||||||
| Median | 1,183 (828–1,713) | 676 (481–1,078) | 739 (590–877) | 511 (399–665) | 1,578 (1,240–2,013) | 941 (617–1,312) | ||
| Mean | 1,308±617 | 832±461 | 720±186 | 554±212 | 1,671±498 | 1,004±489 | ||
P<0.0001, significant decrease in mean iPTH from baseline to last measurement in all follow-up groups. iPTH, intact parathyroid hormone.
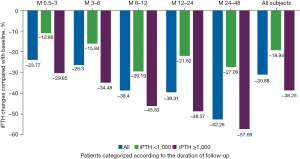
An iPTH decrease of 30.88% from baseline was seen in all participants. From Month 0.5–3 to 24–48, a gradual decrease in iPTH was noted. In the patients with a baseline iPTH ≥1,000 pg/mL, iPTH was reduced by 38.25% (Figure 3).
In all analysis sets, 56.14% of participants had a ≥30% decrease, and 29.34% had a ≥50% decrease in iPTH at the final measurement. As the duration of follow-up increased, the proportion of participants with a ≥30% or 50% decrease in iPTH gradually increased, as shown in Table 3 and Figure 4.
Table 3
| Parameter | Month 0.5–3 | Month 3–6 | Month 6–12 | Month 12–24 | Month 24–48 | All cases |
|---|---|---|---|---|---|---|
| All cases | ||||||
| Absolute value of iPTH decrease (pg/mL) | 459 | 468 | 576 | 643 | 904 | 528 |
| Percentage of iPTH decrease (%) | –23.77 | –26.30 | –38.40 | –39.31 | –52.26 | –30.88 |
| Patients with ≥30% iPTH decrease (n) | 89 | 107 | 104 | 56 | 19 | 375 |
| Patients with ≥30% iPTH decrease (%) | 43.84 | 52.20 | 69.33 | 65.88 | 76.00 | 56.14 |
| Patients with ≥50% iPTH decrease (n) | 41 | 55 | 52 | 33 | 15 | 196 |
| Patients with ≥50% iPTH decrease (%) | 20.20 | 26.83 | 34.67 | 38.82 | 60.00 | 29.34 |
| Baseline iPTH <1,000 pg/mL | ||||||
| Percentage of iPTH decrease (%) | –10.86 | –15.84 | –29.19 | –21.82 | –27.06 | –18.94 |
| Patients with ≥30% iPTH decrease (n) | 18 | 40 | 40 | 12 | 2 | 112 |
| Patients with ≥30% iPTH decrease (%) | 27.69 | 44.44 | 59.70 | 41.38 | 50.00 | 43.92 |
| Patients with ≥50% iPTH decrease (n) | 5 | 13 | 15 | 6 | 0 | 39 |
| Patients with ≥50% iPTH decrease (%) | 7.69 | 14.44 | 22.39 | 20.69 | 0 | 15.29 |
| Baseline iPTH ≥1,000 pg/mL | ||||||
| Percentage of iPTH decrease (%) | –29.85 | –34.48 | –45.83 | –48.37 | –57.06 | –38.25 |
| Patients with ≥30% iPTH decrease (n) | 71 | 67 | 64 | 44 | 17 | 263 |
| Patients with ≥30% iPTH decrease (%) | 51.45 | 58.26 | 77.11 | 78.57 | 80.95 | 63.68 |
| Patients with ≥50% iPTH decrease (n) | 36 | 42 | 37 | 27 | 15 | 157 |
| Patients with ≥50% iPTH decrease (%) | 26.09 | 36.52 | 44.58 | 48.21 | 71.43 | 38.01 |
iPTH, intact parathyroid hormone.
Proportion of patients with iPTH within 2019 Chinese CKD-MBD Guideline target
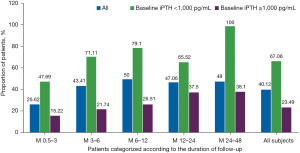
In all cases, the proportion of patients achieving the Chinese CKD-MBD Guideline target range (<600 pg/mL) increased from 9.88% to 40.12% at the final observation. In the analysis of different follow-up groups, a proportional increase in participants reaching target range was noted from Month 0.5–3 to 24–48. The proportion of cases who had final documented iPTH values <600 pg/mL was significantly greater in patients with lower baseline iPTH (iPTH <1,000 pg/mL, 67.06%) than in those in the higher baseline iPTH group (iPTH ≥1,000 pg/mL, 23.49%). Table 4 and Figure 5 show the Chinese Guideline target achievement rates stratified by baseline iPTH values. Only 1 patient developed iPTH <150 pg/mL at the final measurement.
Table 4
| Parameter | Month 0.5–3 | Month 3–6 | Month 6–12 | Month 12–24 | Month 24–48 | All cases |
|---|---|---|---|---|---|---|
| At baseline | ||||||
| N | 16 | 32 | 12 | 5 | 1 | 66 |
| Proportion (%) | 7.88 | 15.61 | 8.00 | 5.88 | 4.00 | 9.88 |
| At last measurement | ||||||
| All cases | ||||||
| N | 52 | 89 | 75 | 40 | 12 | 268 |
| Proportion (%) | 25.62 | 43.41 | 50.00 | 47.06 | 48.00 | 40.12 |
| Subgroups dependent on baseline iPTH <1,000 pg/mL | ||||||
| N | 31 | 64 | 53 | 19 | 4 | 171 |
| Proportion (%) | 47.69 | 71.11 | 79.10 | 65.52 | 100.00 | 67.06 |
| Subgroups dependent on baseline iPTH ≥1,000 pg/mL | ||||||
| N | 21 | 25 | 22 | 21 | 8 | 97 |
| Proportion (%) | 15.22 | 21.74 | 26.51 | 37.50 | 38.10 | 23.49 |
iPTH, intact parathyroid hormone.
Safety profile-changes in Ca and P
We also analyzed levels of serum Ca, P, and their changes of level during study. Serum Ca levels showed a significant increase in Month 12–24 (P=0.0479). Serum P levels remained stable from baseline to the end of observation in all follow-up groups (P=0.2648), as Table 5.
Table 5
| Parameter | Month 0.5–3 | Month 3–6 | Month 6–12 | Month 12–24 | Month 24–48 | All cases |
|---|---|---|---|---|---|---|
| At baseline | ||||||
| Calcium | 2.26±0.22 | 2.28±0.21 | 2.28±0.17 | 2.27±0.18 | 2.29±0.19 | 2.27±0.20 |
| Phosphate | 1.64±0.37 | 1.64±0.38 | 1.61±0.34 | 1.56±0.36 | 1.54±0.39 | 1.62±0.37 |
| At last measurement | ||||||
| Calcium | 2.29±0.16 | 2.29±0.18 | 2.29±0.15 | 2.31±0.15* | 2.31±0.17 | 2.29±0.16 |
| Phosphate | 1.59±0.39 | 1.64±0.33 | 1.58±0.33 | 1.62±0.33 | 1.48±0.33 | 1.60±0.35 |
*P=0.0479, there was only a statistically significant increase in serum Ca levels in the group of Month 12–24 (P<0.05). There were no significant differences in the changes from baseline to last measurement in other follow-up groups (P=0.2648). iPTH, intact parathyroid hormone; Ca, calcium; P, phosphorus.
Serum Ca levels were >2.5 mmol/L before starting treatment with paricalcitol in 58 cases (8.68%). Only 126 participants developed hypercalcemia (18.86%). In all groups, the incidence of triple consecutive hypercalcemia was significantly lower than that for at least once or twice consecutive hypercalcemia (Table 6).
Table 6
| Parameter | Month 0.5–3 | Month 3–6 | Month 6–12 | Month 12–24 | Month 24–48 | All cases |
|---|---|---|---|---|---|---|
| Patients with hypercalcemia at baseline (n, %) | 14 (6.90) | 24 (11.71) | 13 (8.67) | 4 (4.71) | 3 (12.00) | 58 (8.68) |
| Patients with hypercalcemia at last visit (n, %) | 15 (7.39) | 23 (11.22) | 10 (6.67) | 4 (4.71) | 3 (12.00) | 55 (8.23) |
| Documented events of elevated serum calcium (n) | 30 | 54 | 54 | 45 | 28 | 211 |
| Hypercalcemia at least once during treatment (n, %) | 22 (10.84) | 42 (20.49) | 28 (18.67) | 21 (24.71) | 13 (52.00) | 126 (18.86) |
| Hypercalcemia for 2 consecutive blood draws (n, %) | 6 (2.96) | 10 (4.88) | 12 (8.00) | 7 (8.24) | 5 (20.00) | 40 (5.99) |
| Hypercalcemia for 3 consecutive blood draws (n, %) | 1 (0.49) | 1 (0.49) | 6 (4.00) | 5 (5.88) | 1 (4.00) | 14 (2.10) |
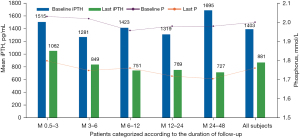
Subgroup analyses of 221 patients with serum P>1.78 mmol/L at baseline showed a P decrease of 2.00±0.20 mmol/L (baseline) to 1.76±0.34 mmol/L at the final visit (P<0.0001), representing an 11.64% decrease in P levels. A significant decrease in P levels was observed in just 0.5–3 months of treatment with paricalcitol. In this hyperphosphatemia subgroup, mean iPTH at baseline was 1,403±619 pg/mL, decreasing progressively to 881±493 pg/mL at the final measurement, indicating a significant and progressive decrease in iPTH levels (−32.79%, P<0.0001) (Table 7 and Figure 6).
Table 7
| Parameter | Patients (n) | Phosphate (mmol/L) | iPTH (pg/mL) | |||
|---|---|---|---|---|---|---|
| Baseline | Last measurement | Baseline | Last measurement | |||
| Month 0.5–3 | 68 | 2.03±0.25 | 1.80±0.35*** | 1,515±608 | 1,062±516*** | |
| Month 3–6 | 75 | 2.02±0.18 | 1.75±0.34*** | 1,281±624 | 849±467*** | |
| Month 6–12 | 48 | 1.96±0.15 | 1.76±0.34** | 1,423±600 | 751±446*** | |
| Month 12–24 | 22 | 1.98±0.20 | 1.72±0.29** | 1,319±612 | 769±528*** | |
| Month 24–48 | 8 | 1.98±0.12 | 1.71±0.29* | 1,696±670* | 727±316* | |
| All subjects | 221 | 2.00±0.20 | 1.76±0.34*** | 1,403±619 | 881±493*** | |
*P<0.05, **P<0.001, ***P<0.0001, significant decrease in mean iPTH and P from baseline to last measurement in the all subgroups of hyperphosphatemia. iPTH, intact parathyroid hormone; P, phosphorus.
Therapy duration and dose
As shown in Table 8, 62.72% of patients (n=419) experienced a 30% decrease in iPTH within a median time of 16.86 weeks [95% confidence interval (CI): 15.57 to 17.86 weeks], and 38.17% of patients (n=255) experienced a 50% decrease in iPTH within a median time of 21.29 weeks (95% CI: 19.86 to 23.14 weeks). The average weekly dose of paricalcitol was 20±9 µg/week. The greatest average weekly dose was observed in the group of Month 0.5–3 (26±10 µg/week). With increased duration of follow-up, the average weekly dose of paricalcitol gradually decreased (Table 9). With the average weekly dose increases, a gradual decrease in iPTH was noted (P<0.0001) (Table 10). Overall, the average weekly dose was gradually reduced with prolonged treatment of paricalcitol (P<0.0001), especially in the patients with higher baseline iPTH (≥1,000 pg/mL) (Table 11).
Table 8
| FAS | Experienced a 30% decrease in iPTH | Experienced a 50% decrease in iPTH |
|---|---|---|
| Total | 668 | 668 |
| No. of event | 419 | 255 |
| No. of censored | 249 | 413 |
| 25th percentile (95% CI) | 10.14–12.00 | 12.86–15.43 |
| Median (95% CI) | 15.57–17.86 | 19.86–23.14 |
| 75th percentile (95% CI) | 23.00–26.43 | 28.71–38.14 |
| Q1 | 11.14 | 14.43 |
| Median | 16.86 | 21.29 |
| Q3 | 24.43 | 33.43 |
| Mean ± SD | 21.61±1.38 | 26.85±1.27 |
| Min–Max | 0.50–474.71 | 0.50–164.86 |
iPTH, intact parathyroid hormone; FAS, full analysis set; CI, confidence interval.
Table 9
| Parameter | Month 0.5–3 | Month 3–6 | Month 6–12 | Month 12–24 | Month 24–48 | All cases |
|---|---|---|---|---|---|---|
| All (n=668) | 26±10 | 17±8 | 18±6 | 18±6 | 18±6 | 20±9 |
| iPTH <1,000 pg/mL | 21±8 | 13±5 | 17±6 | 17±5 | 15±6 | 17±7 |
| iPTH ≥1,000 pg/mL | 28±11 | 19±9 | 18±7 | 19±6 | 19±6 | 22±10 |
iPTH, intact parathyroid hormone.
Table 10
| Average weekly dose | Absolute values of iPTH decrease (pg/mL) | Percentage of iPTH decrease (%) |
|---|---|---|
| ≤15 µg (n=256) | 414 | −26.36 |
| 15 µg< dose ≤30 µg (n=334) | 564 | −33.43 |
| >30 µg (n=78) | 752 | −34.78 |
There was a significant difference in the decrease of PTH between different weekly dose groups (P<0.0001). iPTH, intact parathyroid hormone; PTH, parathyroid hormone.
Table 11
| Parameter | OR | P value |
|---|---|---|
| All | −0.0649 | <0.0001 |
| iPTH <1,000 (pg/mL) | −0.0359 | 0.0685 |
| iPTH ≥1,000 (pg/mL) | −0.0806 | <0.0001 |
iPTH, intact parathyroid hormone; OR, odds ratio.
Logistic regression for PTH and P decrease
Factors predicting PTH decrease (iPTH change from baseline) were analyzed using multiple regression. The following factors were introduced as independent variables: age, average weekly dose, follow-up duration, baseline serum P and Ca, baseline iPTH level, and total paricalcitol dose. Total paricalcitol dose [odds ratio (OR): −0.2825] and baseline iPTH (OR: −0.5456) were negatively correlated with the decrease in iPTH (Table 12).
Table 12
| Parameter | OR | P value |
|---|---|---|
| Age | −1.8902 | 0.0589 |
| Average weekly dose | 3.0850 | 0.1594 |
| Follow-up duration | 1.3514 | 0.3719 |
| Baseline Phosphate | −20.4016 | 0.7433 |
| Baseline Calcium | 5.3153 | 0.9633 |
| Baseline iPTH | −0.5456 | <0.0001* |
| Total paricalcitol dose | −0.2825 | 0.0002* |
*P<0.001. PTH, parathyroid hormone; iPTH, intact parathyroid hormone; OR, odds ratio.
Discussion
The present database observational study provides insights into the routine management of SHPT in Chinese HD patients, with a focus on paricalcitol therapy. Although no information regarding the use of phosphate binders and/or calcimimetics was provided by the data source, calcium-free phosphate binders and calcimimetics are rarely used in Chinese patients in clinical practice. The majority of the HD patients were treated with calcium-based phosphate binders (58.1%), followed by lanthanum (4.8%), and sevelamer (0.9%) (27). The percentage of cinacalcet use was only 0.9% for HD patients. The results from this database study can be applied more generally to the HD population and can reflect daily clinical practice in China. On average, the study showed a decrease of approximately 30.88% in iPTH levels depending on baseline value. Following the initiation of paricalcitol treatment, beneficial effects were observed for PTH in as little as 0.5–3 months (−23.77%, P<0.0001). After 24–48 months of treatment, iPTH levels in that group decreased from 1,183 to 676 pg/mL (−52.26%, P<0.0001). The proportion of patients with ≥30% or 50% decreases in iPTH increased with the duration of follow-up. The greatest decrease was observed in the longest follow-up group (Month 24–48). As with our study, other studies also observed these changes (28-30). Our data support the conclusion that, in the real-world, stable results can be obtained within 2 to 3 months of treatment and can be maintained for 48 months.
In general, the proportion of patients achieving the Chinese Guideline target (<600 pg/mL) increased from 9.88% to 40.12% at the final observation, which was slightly lower than that reported in IMPACT study (57.7%) (31) including patients with moderate SHPT (526±153 pg/mL), whereas we recruited patients over an extended iPTH range (1,308±617 pg/mL). Among the patients with lower initial iPTH (<1,000 pg/mL), 67.06% achieved the Chinese guideline target range (<600 pg/mL), whereas only 23.49% of patients achieved the target with higher initial iPTH (≥1,000 pg/mL). This suggests that patients with lower initial PTH have higher target achievement rates, which means early treatment is critical for SHPT management. Only 1 case developed iPTH levels lower than 150 pg/mL, suggesting a smaller risk of dynamic bone disease during the treatment when paricalcitol.
PTH elevations frequently (32) in CKD G3-4 (40% in CKD G3 and 80% in CKD G4) , and increased levels of PTH before HD start predicted a sustained increased PTH level at 1 year (33). Targeted iPTH control before dialysis may decrease the subsequent risk of parathyroid hyperplasia and uncontrolled SHPT on dialysis (33). Modern strategies to prevent SHPT in CKD patients give great relevance to vitamin D replacement therapy. However, lacking of testing, urgency to treat, consensus, and concrete guidelines, were all contribute to subpar management of SHPT for Non-Dialysis Patients with CKD (34). In China, there are three activated vitamin D/analogue (calcitriol capsule/alfacalcidol capsule/paricalcitol injection) available, and paricalcitol is different from the other two on its selectivity and formulation, which is approved for adult HD patients. It is convenient with good compliance to administer paricalcitol intravenously through a hemodialysis vascular access at any time during dialysis. Nevertheless, physicians’ concern on its influence on Ca/P is the biggest challenge for its widely use in clinical practice, as paricalcitol belongs to vitamin D.
Hypercalcemia and hyperphosphatemia are the most common clinical adverse reactions related to VDRA treatment. In this study, paricalcitol did not induce significant hypercalcemia and serum Ca levels remained within normal ranges. There was, however, only a significant increase in serum Ca in Month 12–24 (P=0.0479). occur Hypercalcemia occurred in 18.86% of the participants who received paricalcitol. The risk of hypercalcemia was slightly higher than that reported in the IMPACT study (7.7%, hypercalcemia was defined as serum Ca >2.63 mmol/L) (31). One explanation may be that 8.68% of cases who developed hypercalcemia before treatment and hypercalcemia were subject to a more strictly defined basis serum Ca (Ca >2.5 mmol/L) in this study. We considered 2.63 mmol/L as the cutoff value for hypercalcemia; however, the incidence of hypercalcemia may be lower. Another explanation may be that calcium-based P binders are widely used in Chinese dialysis patients with hyperphosphatemia (27). Overall, P levels showed an insignificant decrease from 1.62±0.37 to 1.60±0.35 mmol/L (P=0.2648); however, P level was decreased in 0.5–3 months of treatment with paricalcitol. These findings are consistent with those seen in previous randomized controlled studies of paricalcitol in HD patients, demonstrating effective iPTH decreases with no significant increases in serum P levels (16,28). Overall, paricalcitol-based treatment was well tolerated in Chinese HD patients.
Subgroup analyses of SHPT patients at higher baseline P levels (>1.78 mmol/L) showed a significant and progressive decrease in iPTH levels (−32.79%, P<0.0001). There is a strong association between PTH and P that was superior to that of PTH and Ca, explaining the effect on P levels (35). This phenomenon was probably due to improvements in bone metabolism or normalization of high bone turnover in SHPT, which decreases P mobilization from bone tissues. This finding confirms previous data on P reduction with paricalcitol treatment that it may be an important and potentially additional protective benefit, contrary to the expectations of significant increases in serum P with receiving a non-selective VDRA. Therefore, it does not appear that hyperphosphatemia (>1.78 mmol/L) is a contraindication to paricalcitol, as some may claim. Conversely, paricalcitol can control blood phosphorus and PTH levels simultaneously in SHPT patients with hyperphosphatemia.
Two RCTs [PRIMO (36) and OPERA (37)], which formed the basis of these KDIGO 2017 (10) guideline suggestions, showed that the use of paricalcitol raises serum calcium levels, increasing the risk of SHPT patients developing hypercalcemia. However, 70% patients in OPERA who were hypercalcemic received concomitant calcium-based phosphate binders. The mechanism of calcification is a complex pathophysiological process involving many factors (38), such as deranged mineral homeostasis, dyslipidemia, compromised scavenging and debris clearing, inflammation, apoptosis, matrix mineralization, and osteogenesis, not simply caused by high calcium or a single factor. Bozic et al. (39) recently demonstrated that SHPT and hyperphosphatasemia are independently associated with cardiovascular incidence in CKD patients, but hypercalcemia does not have a role in the outcomes. In this study, paricalcitol showed the efficacy in the management of iPTH and serum P, which may be one of explanations for calcification prevention. In addition, it was mentioned in China 2019 CKD-MBD guideline that better effectiveness of paricalcitol for mortality in SHPT might be related to its improvement in calcification.
Both KDIGO 2017 guideline (10) and China 2019 CKD-MBD guideline (40) recommended that “In patients with CKD G5D requiring PTH-lowering therapy, we suggest calcimimetics, calcitriol, or vitamin D analogs, or a combination of calcimimetic with calcitriol or vitamin D analogs (2B)”. Some studies (41-43) have demonstrated that the combination of active vitamin D with cinacalcet increases the achieving rate of CKD-MBD biomarkers and may reduce the risk of hypocalcemia that is a common ADR of cinacalcet. However, a few trials evaluated the benefits of combination therapy (paricalcitol plus cinacalcet) compared with paricalcitol alone for SHPT in HD patients. The ACHIEVE study (44) was a RCT study of 173 patients with HD, which showed that the combination therapy (cinacalcet plus paricalcitol/doxercalciferol) had a higher iPTH achieving rate than paricalcitol/doxercalciferol alone. China 2019 CKD-MBD guideline (40) recommended calcitriol/vitamin D analogs combined with calcimimetics for SHPT patients who were under ineffective monotherapy (calcimimetics or calcitriol/vitamin D analogs alone) without contraindication. Further studies are needed to confirm the efficacy and safety of paricalcitol combination therapy for treating SHPT in HD patients.
Participants were treated with an average dose of 19.69±8.99 µg per week. We also observed a progressive decrease in paricalcitol dosage during the study. Brown et al. (45) also reported a diminished dosage of paricalcitol with stable efficacy as time went on. The iPTH reduced by 414 pg/mL in the lower average weekly dose group (≤15 µg), 564 pg/mL in the middle dose group (15 µg< Dose ≤30 µg), and 752 pg/mL in the higher dose group (>30 µg). Overall, a gradual decrease of iPTH was noted with the average weekly dose increases (P<0.0001).
It is important to consider the limitations of observational analyses in interpreting the results of this study. First, it could have led to selection bias in our recorded data, and missing data were inevitable. The BLFF database relies on the accuracy of documentation by mandatory and regional physicians. The frequency of laboratory testing was based on clinical practices, possibly influencing titration and target achievement. We assessed only biochemical parameters, but not ALP, BALP, or FGF23. None of Ca and P burden, vascular calcification effects of the treatments, or previous/concomitant SHPT therapy were sufficiently considered. Nevertheless, the strengths of this study include its large sample size and the mode of an observational study in real-world clinical practice without restrictions of study protocol. Participants in the study were from 170 dialysis centers in 60 cities in China, and these sites could be geographically representative of China. This study provides new and important information on the use, effectiveness, and safety of paricalcitol in clinical practice in HD patients in China and could be extrapolated to larger populations.
Conclusions
This is the first national real-world observational study since intravenous paricalcitol became available in China in 2014. This study demonstrates that paricalcitol is an effective and well-tolerated treatment for the control of iPTH in real-world clinical practice. The majority of cases of hypercalcemia are transient. As treatment goes on, the serum Ca level tends to be stabilized, and the serum phosphorus level will be improved as iPTH decreases. Our results suggest that intravenous paricalcitol has a significant and sustainable effect on PTH. Physicians and patients can expect significant iPTH decreases within 16–21 weeks after paricalcitol is initiated. Early treatment needs to be emphasized in all SHPT patients.
Acknowledgments
The authors wish to thank all the physicians and medical centers in China that participated in the Better Life for Future (BLFF) project. The abstract and part of Figures/tables had been published in the 57th ERA-EDTA Congress Abstracts and 58th ERA-EDTA Congress Abstracts (57th ERA-EDTA Congress Abstracts: P1425 EFFICACY AND SAFETY OF INTRAVENOUS PARICALCITOL TREATMENT IN CHINESE HEMODIALYSIS PATIENTS WITH SECONDARY HYPERPARATHYROIDISM: A REAL-WORLD DATABASE ANALYSIS; 58th ERA-EDTA Congress Abstracts: MO806 SAFETY OF INTRAVENOUS PARICALCITOL TREATMENT IN CHINESE HEMODIALYSIS PATIENTS: A REAL-WORLD DATABASE ANALYSIS).
Funding: This work was supported by the China Primary Health Care Foundation who launched the BLFF project with the medicine donation by AbbVie. AbbVie participated in the study design, analysis, interpretation of data, review, and approval of the publication.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3966/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3966/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3966/coif). All authors report that this work was supported by the China Primary Health Care Foundation who launched the BLFF project with the medicine donation by AbbVie. AbbVie participated in the study design, analysis, interpretation of data, review, and approval of the publication. YX is an employee of AbbVie Inc. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Xin Hua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (XHEC-D-2020-099). Informed consent was taken from all the patients before inclusion in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815-22. [Crossref] [PubMed]
- Douthat WG, Castellano M, Berenguer L, et al. High prevalence of secondary hyperparathyroidism in chronic kidney disease patients on dialysis in Argentina. Nefrologia 2013;33:657-66. [PubMed]
- Jeloka T, Mali M, Jhamnani A, et al. Are we overconcerned about secondary hyperparathyroidism and underestimating the more common secondary hypoparathyroidism in our dialysis patients? J Assoc Physicians India 2012;60:102-5. [PubMed]
- Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004;15:2208-18. [Crossref] [PubMed]
- Noordzij M, Korevaar JC, Bos WJ, et al. Mineral metabolism and cardiovascular morbidity and mortality risk: peritoneal dialysis patients compared with haemodialysis patients. Nephrol Dial Transplant 2006;21:2513-20. [Crossref] [PubMed]
- Block GA, Port FK. Re-evaluation of risks associated with hyperphosphatemia and hyperparathyroidism in dialysis patients: recommendations for a change in management. Am J Kidney Dis 2000;35:1226-37. [Crossref] [PubMed]
- Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008;52:519-30. [Crossref] [PubMed]
- Danese MD, Belozeroff V, Smirnakis K, et al. Consistent control of mineral and bone disorder in incident hemodialysis patients. Clin J Am Soc Nephrol 2008;3:1423-9. [Crossref] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009;S1-130. [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl (2011) 2017;7:1-59.
- Liu ZH, Li G, Zhang L, et al. Executive Summary: Clinical Practice Guideline of Chronic Kidney Disease - Mineral and Bone Disorder (CKD-MBD) in China. Kidney Dis (Basel) 2019;5:197-203. [Crossref] [PubMed]
- National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003;42:S1-201. [Crossref] [PubMed]
- Schömig M, Ritz E. Management of disturbed calcium metabolism in uraemic patients: 1. Use of vitamin D metabolites. Nephrol Dial Transplant 2000;15:18-24. [Crossref] [PubMed]
- Brancaccio D, Bommer J, Coyne D. Vitamin D receptor activator selectivity in the treatment of secondary hyperparathyroidism: understanding the differences among therapies. Drugs 2007;67:1981-98. [Crossref] [PubMed]
- Coyne D, Acharya M, Qiu P, et al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis 2006;47:263-76. [Crossref] [PubMed]
- Martin KJ, González EA, Gellens M, et al. 19-Nor-1-alpha-25-dihydroxyvitamin D2 (Paricalcitol) safely and effectively reduces the levels of intact parathyroid hormone in patients on hemodialysis. J Am Soc Nephrol 1998;9:1427-32. [Crossref] [PubMed]
- Coyne DW, Grieff M, Ahya SN, et al. Differential effects of acute administration of 19-Nor-1,25-dihydroxy-vitamin D2 and 1,25-dihydroxy-vitamin D3 on serum calcium and phosphorus in hemodialysis patients. Am J Kidney Dis 2002;40:1283-8. [Crossref] [PubMed]
- Sprague SM, Llach F, Amdahl M, et al. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int 2003;63:1483-90. [Crossref] [PubMed]
- Llach F, Yudd M. Paricalcitol in dialysis patients with calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis 2001;38:S45-50. [Crossref] [PubMed]
- Sprague SM, Lerma E, McCormmick D, et al. Suppression of parathyroid hormone secretion in hemodialysis patients: comparison of paricalcitol with calcitriol. Am J Kidney Dis 2001;38:S51-6. [Crossref] [PubMed]
- Cardús A, Panizo S, Parisi E, et al. Differential effects of vitamin D analogs on vascular calcification. J Bone Miner Res 2007;22:860-6. [Crossref] [PubMed]
- Brancaccio D, Cozzolino M, Cannella G, et al. Secondary hyperparathyroidism in chronic dialysis patients: results of the Italian FARO survey on treatment and mortality. Blood Purif 2011;32:124-32. [Crossref] [PubMed]
- Messa P, Cozzolino M, Brancaccio D, et al. Effect of VDRA on survival in incident hemodialysis patients: results of the FARO-2 observational study. BMC Nephrol 2015;16:11. [Crossref] [PubMed]
- Xie Y, Su P, Sun Y, et al. Comparative efficacy and safety of paricalcitol versus vitamin D receptor activators for dialysis patients with secondary hyperparathyroidism: a meta-analysis of randomized controlled trials. BMC Nephrol 2017;18:272. [Crossref] [PubMed]
- Liu Y, Liu LY, Jia Y, et al. Efficacy and safety of paricalcitol in patients undergoing hemodialysis: a meta-analysis. Drug Des Devel Ther 2019;13:999-1009. [Crossref] [PubMed]
- Geng X, Shi E, Wang S, et al. A comparative analysis of the efficacy and safety of paricalcitol versus other vitamin D receptor activators in patients undergoing hemodialysis: A systematic review and meta-analysis of 15 randomized controlled trials. PLoS One 2020;15:e0233705. [Crossref] [PubMed]
- Liu ZH, Yu XQ, Yang JW, et al. Prevalence and risk factors for vascular calcification in Chinese patients receiving dialysis: baseline results from a prospective cohort study. Curr Med Res Opin 2018;34:1491-500. [Crossref] [PubMed]
- Ross EA, Tian J, Abboud H, et al. Oral paricalcitol for the treatment of secondary hyperparathyroidism in patients on hemodialysis or peritoneal dialysis. Am J Nephrol 2008;28:97-106. [Crossref] [PubMed]
- Coyne DW, Goldberg S, Faber M, et al. A randomized multicenter trial of paricalcitol versus calcitriol for secondary hyperparathyroidism in stages 3-4 CKD. Clin J Am Soc Nephrol 2014;9:1620-6. [Crossref] [PubMed]
- Zawierucha J, Malyszko J, Malyszko JS, et al. Three Therapeutic Strategies: Cinacalcet, Paricalcitol or Both in Secondary Hyperparathyroidism Treatment in Hemodialysed Patients During 1-Year Observational Study-A Comparison. Front Endocrinol (Lausanne) 2019;10:40. [Crossref] [PubMed]
- Ketteler M, Martin KJ, Wolf M, et al. Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: results of the IMPACT SHPT study. Nephrol Dial Transplant 2012;27:3270-8. [Crossref] [PubMed]
- Ketteler M, Ambühl P. Where are we now? Emerging opportunities and challenges in the management of secondary hyperparathyroidism in patients with non-dialysis chronic kidney disease. J Nephrol 2021;34:1405-18. [Crossref] [PubMed]
- Tabibzadeh N, Karaboyas A, Robinson BM, et al. The risk of medically uncontrolled secondary hyperparathyroidism depends on parathyroid hormone levels at haemodialysis initiation. Nephrol Dial Transplant 2021;36:160-9. [Crossref] [PubMed]
- Robinson J, Malone S, Hurtado TB, et al. Lack of Testing, Urgency to Treat, Consensus, and Concrete Guidelines All Contribute to Subpar Management of Secondary Hyperparathyroidism and Vitamin D Insufficiency for Non-Dialysis Patients with CKD. ASN Kidney Week 2021:PUB074.
- Rodriguez M, Salmeron MD, Martin-Malo A, et al. A New Data Analysis System to Quantify Associations between Biochemical Parameters of Chronic Kidney Disease-Mineral Bone Disease. PLoS One 2016;11:e0146801. [Crossref] [PubMed]
- Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 2012;307:674-84. [Crossref] [PubMed]
- Wang AY, Fang F, Chan J, et al. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol 2014;25:175-86. [Crossref] [PubMed]
- Jahnen-Dechent W, Heiss A, Schäfer C, et al. Fetuin-A regulation of calcified matrix metabolism. Circ Res 2011;108:1494-509. [Crossref] [PubMed]
- Bozic M, Diaz-Tocados JM, Bermudez-Lopez M, et al. Independent effects of secondary hyperparathyroidism and hyperphosphatemia on chronic kidney disease progression and cardiovascular events: an analysis from the NEFRONA cohort. Nephrol Dial Transplant 2021; Epub ahead of print. [Crossref] [PubMed]
- National Shenzang Disease Clinical Medical Research Center. Guidelines for diagnosis and treatment of mineral and bone abnormalities of chronic kidney disease in China. Chinese Journal of Nephrology Dialysis & Transplantation 2019;28.
- Ureña P, Fouque D, Brunet P, et al. Cinacalcet treatment for secondary hyperparathyroidism in dialysis patients in real-world clinical practice - the ECHO observational study: French experience. Nephrol Ther 2012;8:527-33. [PubMed]
- Fukagawa M, Fukuma S, Onishi Y, et al. Prescription patterns and mineral metabolism abnormalities in the cinacalcet era: results from the MBD-5D study. Clin J Am Soc Nephrol 2012;7:1473-80. [Crossref] [PubMed]
- Ureña-Torres P, Bridges I, Christiano C, et al. Efficacy of cinacalcet with low-dose vitamin D in incident haemodialysis subjects with secondary hyperparathyroidism. Nephrol Dial Transplant 2013;28:1241-54. [Crossref] [PubMed]
- Fishbane S, Shapiro WB, Corry DB, et al. Cinacalcet HCl and concurrent low-dose vitamin D improves treatment of secondary hyperparathyroidism in dialysis patients compared with vitamin D alone: the ACHIEVE study results. Clin J Am Soc Nephrol 2008;3:1718-25. [Crossref] [PubMed]
- Brown AJ, Finch J, Takahashi F, et al. Calcemic activity of 19-Nor-1,25(OH)(2)D(2) decreases with duration of treatment. J Am Soc Nephrol 2000;11:2088-94. [Crossref] [PubMed]
(English Language Editor: J. Jones)




