
Combining apatinib and temozolomide for brainstem glioblastoma: a case report and review of literature
Introduction
Brainstem glioma comprises a rare and heterogeneous group of brain tumors that primarily occur within the brainstem. Its incidence only accounts for 1–2% of all brain tumors (1). The clinical course of adult brainstem gliomas varies significantly depending on clinicopathologic and radiographic characteristics, but the prognosis is poor with a median overall survival (OS) of 8 months for high-grade gliomas that undergo biopsy (2). A standard treatment for adult brainstem gliomas has not been determined due to the lack of prospective clinical trials. Based on the results of the EORTC-NCIC randomized phase III trial, postoperative radiotherapy with concurrent and adjuvant temozolomide (TMZ) chemotherapy is recommended by NCCN Clinical practice guideline as standard treatment of patients with newly diagnosed glioblastoma and good performance status (PS) (3). However, due to their location in the brainstem, surgical resection of gliomas is not usually considered a safe treatment option, leaving biopsy as the only means for pathological examination. In the subset analysis of 93 patients who had biopsy only in the EORTC-NCIC trial, the addition of concurrent and adjuvant TMZ chemotherapy to radiotherapy achieved a median survival of 9.4 months (4). For this reason, combined management with radiotherapy, chemotherapy, and targeted therapy for patients with brainstem gliomas is of great clinical significance.
MGMT (O6-methylguanine-DNA methyltransferase) is a DNA repair enzyme that exerts its effect by repairing the toxic DNA adducts of O6-methylguanine induced by TMZ, thus abrogating the effects of TMZ chemotherapy (5). A high level of MGMT expression together with MGMT activity is usually observed in populations without methylation of MGMT (6). In several clinical trials, MGMT promoter methylation predicted response to TMZ chemotherapy and was associated with better survival in high-grade gliomas including glioblastoma when compared to unmethylated group (7-10).
Here, we describe the first case of an adult with MGMT methylation-negative refractory brainstem glioblastoma with a TP53 germline mutation who responded to concurrent apatinib and dose-dense temozolomide. We present the following article in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-22/rc).
Case presentation
A previously healthy 28-year-old man was admitted to hospital with progressive dizziness and diplopia of 6 months duration. He had also developed gait instability, tinnitus in the left ear, as well as perioral and tongue paresthesia in 2 weeks. His father had died of a brain tumor and intestinal cancer 20 years prior.
On admission, his cognition was intact. A general physical examination demonstrated no evidence of papilledema or diminution of vision, with the visual fields full to confrontation. Both pupils measured 4 mm and were briskly reactive. Nystagmus was not observed. Signs of increased intracranial pressure were noted. There were decreased light touch and pinprick sensations over the mandibular nerve distribution. The patient had a wide-based, unsteady gait, and his reflexes were noted as 2+. His Karnofsky Performance Scale score was 70.
In June 2020, 12 days after admission, initial contrast-enhanced magnetic resonance imaging (MRI) revealed a brainstem mass lesion as well as a right parietal lobe lesion of abnormal signal intensity. The radiographic differential diagnosis favored either astrocytoma or glioblastoma. Subsequently, a computed tomography (CT)-guided stereotactic biopsy was performed via the transcerebellar approach.
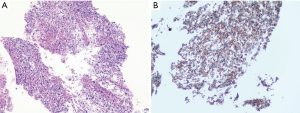
The pathology indicated an astrocytoma morphologically consistent with a high-grade glioma [World Health Organization (WHO) grade IV] (Figure 1). The immunohistochemistry panel showed that tumor cells were H3K27M mutation negative, GFAP positive, p53 positive (80%), and IDH-1 wildtype. The Ki-67 index was estimated to be 40%. However, genetic analysis was not completed for financial reasons.
Diagnosed with brainstem glioblastoma, the patient was treated with volumetric modulated arc therapy (VMAT) with concurrent TMZ (75 mg/m2/day) chemotherapy for 37days from late June to early August 2020. The dose prescription of VMAT was 54 Gy administered in 2 Gy/fraction for the gross tumor volume and 48.6 Gy in 27 fractions for the clinical tumor volume. When the concurrent therapy was completed, the patient’s symptoms and signs greatly improved. Repeated MRI examinations revealed stable disease (SD), and from the end of July 2020 adjuvant TMZ (150 mg/m2) continued to be conventionally administered as a single agent on 5 days per cycle and each cycle lasting for 28 days.
Despite this treatment, the patient was readmitted to hospital 6 weeks later complaining of worsening dizziness and gait instability. MRI study showed that the lesions located in the brainstem and right parietal lobe were unchanged, but a new lesion of the right thalamus was observed and determined to be encephaledema by the radiologists. The treatment regimen was then adjusted to dose-dense TMZ (100 mg/m2 daily; 21 days on, 7 days off) combined with oral apatinib (250 mg/day). Every 28 days was defined as 1 cycle of concomitant therapy.
After only 1 week of combined chemotherapy and antiangiogenic therapy, the patient reported objective clinical improvement of his symptoms. MRI obtained 4 months following the concurrent treatment revealed that the size of the brainstem lesion had slightly decreased but the lesion of the right parietal lobe remained unchanged. Furthermore, the lesion of the right thalamus had disappeared, suggesting that the patient achieved a partial response to the treatment. He subsequently underwent MRI follow-up approximately every 3 months. Thus far, he has completed more than 11 cycles of concomitant treatment and has experienced nausea, rash, and elevation of alanine transaminase hepatic enzymes. All these adverse events (AEs) well tolerated and manageable. They were evaluated as grade I/II using the Common Terminology Criteria for Adverse Events (CTCAE version 5.0).
His last MRI follow-up in August 2021, showed stability of the tumor (Figure 2). At that time, we performed whole-exome sequencing and a 6-gene panel (Genetron Health; Beijing, China) using paraffin-embedded samples of biopsy tissue. A negative result was found for MGMT and isocitrate dehydrogenase 1 (IDH 1). No loss of heterozygosity was present in either 1p or 19q. The TP53 germline mutation was identified, but no approved agents yet exist that target it. These results indicated a refractory malignancy with extremely poor prognosis. He was recently followed-up on January 5, 2022 in good condition but refused to perform MRI examinations of the head himself. The treatment line, results of molecular genetics screening, and AEs are shown in Figure 3, Table 1, and Table 2, respectively.
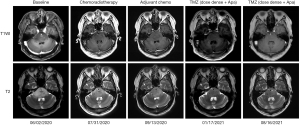
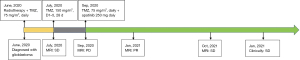
Table 1
| Item | Result |
|---|---|
| MGMT promoter methylation | Negative |
| Loss of heterozygosity of chromosome 1p | Negative |
| Loss of heterozygosity of chromosome 19q | Negative |
| IDH1 R132 mutation | Negative |
| IDH2 R172 mutation | Negative |
| TERT C228T mutation | Negative |
| TERT C250T mutation | Negative |
| BRAF V600E mutation | Negative |
| H3F3A K27M mutation | Negative |
| HISTIH3B K27M mutation | Negative |
| TP53 mutation | Positive |
MGMT, O6-methylguanine-DNA methyltransferase; IDH, isocitrate dehydrogenase; TERT, telomerase reverse transcriptase; BRAF, V-Raf murine sarcoma viral oncogene homolog B.
Table 2
| Adverse event | Grade |
|---|---|
| Nausea | Grade 2 |
| Fatigue | Grade 1 |
| Rash | Grade 2 |
| Elevation of alanine transaminase | Grade 1 |
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Adult brainstem gliomas are uncommon and have various prognoses depending on the clinical, radiological, and histological characteristics. Guillamo et al. found WHO high-grade pathology (grade III/IV) to be associated with an extremely poor prognosis for brainstem glioma patients despite aggressive treatment with radiotherapy and chemotherapy (11). Nevertheless, the patient in our study failed standard Stupp’s regimen but achieved progression-free survival (PFS) of nearly 16.0 months following a combination of dose-dense TMZ chemotherapy and apatinib treatment after resistance to adjuvant temozolomide chemotherapy. The patient is still alive and without disease progression.
It’s well established that angiogenesis is involved in tumor progression and metastasis. Glioblastomas are usually distributed with numerous microscopic microvessels, and endotheliosis has been defined as a pathological feature. In the growth, invasion, and metastasis of glioblastoma, angiogenesis plays a critical role. Thus, antiangiogenesis is a potential target for patients with glioblastoma. The processes of angiogenesis are primarily driven by the interaction of vascular endothelial growth factor (VEGF) and its tyrosine kinase receptor (VEGFR family), especially VEGFR-2 (12). As a result, new blood vessels are formed that contribute to tumor growth and metastasis. Additionally, these vessels are structurally and functionally abnormal, leading to the formation of a tumor microenvironment characterized by low oxygen tension and high interstitial fluid pressure, which is typical of histological subtypes with more aggressive clinical behavior (13). Apatinib is an oral small-molecule inhibitor that potently targets VEGFR-2 to inhibit angiogenesis and can cross the blood–brain barrier (14). As an antiangiogenic agent, apatinib can normalize tumor vessels and prevent tumor metastasis. It has been approved for the treatment of metastatic adenocarcinoma of the stomach or gastroesophageal junction in China based on the results of randomized phase III trial (15). However, apatinib treatment has not been well studied in gliomas, especially adult brainstem glioblastoma.
In fact, there’ve been several clinical studies focusing on combined apatinib and TMZ treatment for high-grade gliomas. In single-arm phase II trial which included 20 patients with recurrent glioblastoma who were experiencing relapse from standard chemoradiotherapy (TMZ and radiotherapy), treatment with apatinib (500 mg/daily) and dose-dense TMZ (100 mg/m2) was instead administered and demonstrated an objective response rate of 45%, a disease control rate of 90%, a median PFS of 6 months, and a median OS of 9 months (16). A prospective single-arm exploratory study including 15 recurrent glioblastoma patients thereafter yielded similar results, with a median PFS of 3.1 months and median OS of 8.7 months (17). These results were further confirmed in a retrospective analysis by Ge et al. that showed a median PFS of 4.9 months and a median OS of 8.2 months (18). In addition, in a case report similar to ours, an adult with a brainstem anaplastic astrocytoma benefited from an initial treatment of combined radiotherapy, TMZ, and apatinib (500 mg/day) and had PFS of over 8 months (19). However, the patient in our report was pathologically diagnosed with glioblastoma and treated with low dose apatinib after resistance to temozolomide chemotherapy. Previous studies have shown that combined antiangiogenic therapy plus TMZ can significantly improve the survival rate when compared with TMZ monotherapy (20,21). In addition, the efficiency and safety of a low dose have been confirmed in other solid tumors, including non-small cell lung cancer, ovarian cancer, and gastric carcinoma (22-24). That is to say, to the best of our knowledge, we present the first case of low-dose apatinib combined with dose-dense TMZ for the treatment of adult brainstem glioblastoma.
Gliomas are typically subdivided into four grades (I–IV) according to the WHO. Among them, grade IV gliomas are the most malignant. Surgical resection is considered the optimal treatment, and postoperative radiotherapy together with adjuvant therapy (i.e., “Stupp’s regimen”: adjuvant TMZ chemotherapy for at least 6 cycles post-concurrent TMZ and radiotherapy) are strongly recommended in the guidelines (4). Despite this, the PFS and OS for high-grade gliomas are unsatisfactory. It has been reported that the 5-year survival rate in patients with glioblastoma is only 9.8% after receiving standard Stupp’s regimen (9). Furthermore, only a subset of malignant gliomas respond to TMZ. The sensitivity to TMZ chemotherapy involves complex molecular mechanisms in which MGMT plays an important role. MGMT methylation is associated with better survival outcomes in patients with high-grade gliomas who have received TMZ chemotherapy. Our patient had a MGMT methylation-negative WHO grade IV brainstem glioblastoma, so he could not undergo complete surgical resection due to the tumor’s location, and therefore the disease progressed after 1 cycle of adjuvant TMZ chemotherapy. Additionally, radiological findings revealed a new lesion of encephaledema, which may explain the insensitivity to TMZ monotherapy following chemoradiotherapy observed in this case. As solid tumors, malignant gliomas such as glioblastoma are also highly angiogenic with many VEGFR-2 molecules distributed on the surface of the gliomal microvascular endothelial cells (25,26), which can therefore be targeted by apatinib. Furthermore, increasing evidence suggests a better response to antiangiogenic agents in malignant tumors with TP53 mutation than TP53 wide-type. A study by Wheler et al demonstrated that the TP53 mutation upregulated VEGF-A and VEGFR-2 in multiple cancers and was significantly associated with favorable outcomes in patients who have received VEGF/VEGFR inhibitors (27). The TP53 mutation has been reported in 32% of all glioblastomas, but whether it predict favorable prognosis to Apatinib in brainstem gliomas patients remains explored. In addition to inhibiting angiogenesis, apatinib has been shown to enhance the antitumor activity of TMZ in glioma cells by improving the anaerobic tumor microenvironment in vitro (28). This suggests synergistic effects of concurrent TMZ and apatinib treatment and highlights the benefits for patients with malignant gliomas.
Conclusions
This report is the first case study to demonstrate the effectiveness of using low-dose apatinib and dose-dense TMZ for brainstem glioblastoma and the mild level of toxicity that results from the treatment. Although drawing definitive conclusions is difficult on the basis of such limited experience, apatinib combined with TMZ may be an alternative treatment for patients with clinically inoperable and MGMT methylation-negative malignant gliomas. Further studies are required to elucidate the efficiency and safety of combined apatinib and TMZ for brainstem malignant gliomas.
Acknowledgments
We thank the patient and his family for their consent to publish this case.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-22/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-22/coif). LZ is from Genetron Health Inc., which supports the whole-exome sequencing and a 6-gene panel examination in this study. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol 2015;17:iv1-iv62. [Crossref] [PubMed]
- Doyle J, Khalafallah AM, Yang W, et al. Association between extent of resection on survival in adult brainstem high-grade glioma patients. J Neurooncol 2019;145:479-86. [Crossref] [PubMed]
- Nabors LB, Portnow J, Ahluwalia M, et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:1537-70. [Crossref] [PubMed]
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96. [Crossref] [PubMed]
- Kaina B, Christmann M, Naumann S, et al. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6:1079-99. [Crossref] [PubMed]
- Christmann M, Nagel G, Horn S, et al. MGMT activity, promoter methylation and immunohistochemistry of pretreatment and recurrent malignant gliomas: a comparative study on astrocytoma and glioblastoma. Int J Cancer 2010;127:2106-18. [Crossref] [PubMed]
- Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res 2004;10:1871-4. [Crossref] [PubMed]
- Hegi ME, Diserens A, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997-1003. [Crossref] [PubMed]
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66. [Crossref] [PubMed]
- Bell EH, Zhang P, Fisher BJ, et al. Association of MGMT Promoter Methylation Status With Survival Outcomes in Patients With High-Risk Glioma Treated With Radiotherapy and Temozolomide: An Analysis From the NRG Oncology/RTOG 0424 Trial. JAMA Oncol 2018;4:1405-9. [Crossref] [PubMed]
- Guillamo JS, Monjour A, Taillandier L, et al. Brainstem gliomas in adults: prognostic factors and classification. Brain 2001;124:2528-39. [Crossref] [PubMed]
- Melincovici CS, Bosca AB, Susman S, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol 2018;59:455-67. [PubMed]
- Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci 2007;8:610-22. [Crossref] [PubMed]
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374-80. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Wang Y, Meng X, Zhou S, et al. Apatinib Plus Temozolomide for Recurrent Glioblastoma: An Uncontrolled, Open-Label Study. Onco Targets Ther 2019;12:10579-85. [Crossref] [PubMed]
- Yao H, Liu J, Zhang C, et al. Clinical study of apatinib plus temozolomide for the treatment of recurrent high-grade gliomas. J Clin Neurosci 2021;90:82-8. [Crossref] [PubMed]
- Ge J, Li C, Xue F, et al. Apatinib Plus Temozolomide: An Effective Salvage Treatment for Recurrent Glioblastoma. Front Oncol 2021;10:601175. [Crossref] [PubMed]
- Yu D, Han G, Liu H, et al. Treatment of adult brainstem glioma with combined antiangiogenic therapy: a case report and literature review. Onco Targets Ther 2019;12:1333-9. [Crossref] [PubMed]
- Lorgis V, Maura G, Coppa G, et al. Relation between bevacizumab dose intensity and high-grade glioma survival: a retrospective study in two large cohorts. J Neurooncol 2012;107:351-8. [Crossref] [PubMed]
- Levin VA, Chan J, Datta M, et al. Effect of angiotensin system inhibitors on survival in newly diagnosed glioma patients and recurrent glioblastoma patients receiving chemotherapy and/or bevacizumab. J Neurooncol 2017;134:325-30. [Crossref] [PubMed]
- Chen W, Li Z, Zheng Z, et al. Efficacy and safety of low-dose apatinib in ovarian cancer patients with platinum-resistance or platinum-refractoriness: A single-center retrospective study. Cancer Med 2020;9:5899-907. [Crossref] [PubMed]
- Du Y, Cao Q, Jiang C, et al. Effectiveness and safety of low-dose apatinib in advanced gastric cancer: A real-world study. Cancer Med 2020;9:5008-14. [Crossref] [PubMed]
- Geng Q, Shen H, Zhu W, et al. Safety and Efficacy of Low-Dosage Apatinib Monotherapy in Advanced Lung Squamous-Cell Carcinoma: A Prospective Cohort Study. Onco Targets Ther 2020;13:11529-35. [Crossref] [PubMed]
- El Hallani S, Boisselier B, Peglion F, et al. A new alternative mechanism in glioblastoma vascularization: tubular vasculogenic mimicry. Brain 2010;133:973-82. [Crossref] [PubMed]
- Zhou D, Zhan S, Zhou D, et al. A study of the distribution and density of the VEGFR-2 receptor on glioma microvascular endothelial cell membranes. Cell Mol Neurobiol 2011;31:687-94. [Crossref] [PubMed]
- Wheler JJ, Janku F, Naing A, et al. TP53 Alterations Correlate with Response to VEGF/VEGFR Inhibitors: Implications for Targeted Therapeutics. Mol Cancer Ther 2016;15:2475-85. [Crossref] [PubMed]
- Wang C, Jiang M, Hou H, et al. Apatinib suppresses cell growth and metastasis and promotes antitumor activity of temozolomide in glioma. Oncol Lett 2018;16:5607-14. [Crossref] [PubMed]
(English Language Editor: K. Brown)


