
Correlation between Lactobacillus and expression of E-cadherin, β-catenin, N-cadherin, and Vimentin in postmenopausal cervical lesions
Introduction
Postmenopausal cervical lesions include cervical squamous intraepithelial lesions (SILs) and cervical cancer. The occurrence of cervical cancer is a continuous process, and the development of cervical SILs into invasive cancer takes about 10–15 years. Meanwhile, the progression of carcinoma in situ to invasive carcinoma takes approximately 3–10 years. SIL was previously known as “cervical intraepithelial neoplasia” (CIN). According to the WHO Classification of Female Genital Tumors [2014], low-grade SIL (LSIL) has the same as cytological classification as high-grade SIL (HSIL); LSIL is CIN1, while HSIL includes mostly CIN2 and CIN3 (1). Cervical cancer is considered to be a global health problem, with the fourth highest morbidity and mortality worldwide (2). Among Chinese women, the incidence rate ranks sixth (3), and the mortality rate ranks seventh (3). China’s population is gradually aging, with an increasing number of cervical cancer patients. Multiple factors, including low socioeconomic status, many complications, late diagnosis, difficult treatment, and poor prognosis, the mortality rate accounts for 18% of all malignant tumors (4). The progression from postmenopausal cervical SILs to cervical cancer requires a span of 10–20 years. Human intervention can reduce the occurrence and mortality of postmenopausal cervical cancer, but the diagnosis and treatment of postmenopausal cervical lesions remains challenging. Thus, clarifying the pathogenesis of postmenopausal cervical lesions is of crucial for clinical diagnosis and treatment. From an etiological perspective, persistent high-risk HPV infection is a necessary and inadequate condition for cervical lesions (5). In recent years, abnormal vaginal microecology has been identified as another cause of concern, which can cause cervical lesions (6) and cervical cancer (7). Lactobacillus constitutes the normal female vaginal microenvironment, which can competitively adhere to the vaginal skin, produce lactic acid to change the pH value of the vagina, produce bacteriocin and peroxide and bacteriocin to inhibit the growth of other microorganisms, and maintain the vaginal microecological balance (8). Vaginal microecology is a state of dynamic balance, which is affected and restricted by a variety of external and internal factors. Once the dynamic balance is broken, the risk of reproductive tract infection will be increased (9). The vaginal microecological balance is broken in the following situations: (I) infants and postmenopausal people with low estrogen level. (II) The acidic environment of the vagina is changed, such as frequent vaginal lavage can increase the PH of the vagina, which is not conducive to the growth of Lactobacillus. (III) Long-term application of broad-spectrum antibiotics can inhibit the growth of Lactobacillus (1). This leads to the occurrence of gynecological diseases such as vaginitis, CIN and cervical cancer (10). Studies have shown that CIN and cervical cancer are associated with high relative abundance of Lactobacillus iners and low relative abundance of Lactobacillus jensenii and Lactobacillus crispatus (11). HPV-positive women had a lower abundance of Lactobacillus (12). Studies have shown that the cervical microbial richness of postmenopausal HPV-positive women is lower than that of HPV-negative women, and the difference is significant compared with premenopausal women (13). It is currently believed that a decrease in the number and activity of Lactobacillus leads to an increased risk of HPV infection, and that persistent HPV infection reduces vaginal Lactobacillus abundance, which is an important cause of cervical cancer. Moreover, HPV oncogenes participate in the regulation of lactobacillus to inhibit the survival ability of cervical cancer cells, and block the occurrence and development of cervical cancer by regulating the types and abundance of Lactobacillus (14).
Epithelial-mesenchymal transition (EMT) is involved in the invasion and metastasis of various tumors (15). EMT is characterized by decreased epithelial cell markers, E-cadherin and β-catenin, and increased mesenchymal cell markers, N-cadherin and Vimentin (16). It has been proved that the low expression of e-cadherin and β-catenin is correlated with the pathological degree of SIL (17), which can predict the progression of cervical HSIL and is a risk factor for cervical cancer (18). N-cadherin was highly expressed in cervical squamous cell carcinoma (SCC) tissues, which was significantly higher than that in normal cervical tissues (19).
The expression of Lactobacillus vaginalis in postmenopausal cervical SILs and cervical SCC has not yet been reported. Also, it is not clear whether the mRNA expression of E-cadherin, β-catenin, N-cadherin, and Vimentin is correlated with EMT-related indicators in postmenopausal cervical SCC and cervical SCC. In this study, we aimed to investigate the Lactobacillus changes in the vaginas of postmenopausal normal women with cervical SILs and cervical SCC in the experimental and control groups. We also intend to explore the mRNA expression changes of EMT-related indicators (E-cadherin, β-catenin, N-cadherin, and Vimentin) in both groups. We preliminarily analyzed the correlation between Lactobacillus vaginalis and cervical SILs and cervical SCC EMT, and elucidated the possible mechanism. We present the following article in accordance with the MDAR reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3581/rc).
Methods
General information
From January 2016 to January 2020, postmenopausal women who received colposcopy biopsy in the Department of Obstetrics and Gynecology of the First Affiliated Hospital of Inner Mongolia Medical University due to bleeding and abnormal leucorrhea were enrolled. According to pathological results, 30 patients with LSIL (CIN1) were divided into two groups, with an average age of 57.20±6.66 years. Furthermore, there were 18 patients with HSIL (CIN2 and CIN3), with an average age of 59.72±7.81 years. Moreover, 30 patients with SCC of the cervix were reported. The patients were clinical stage I according to the International Federation of Gynecology and Obstetrics (FIGO), with a mean age was 61.33±8.02 years, and the three groups were experimental groups. Thirty postmenopausal women with a pathological diagnosis of normal cervical epithelium or chronic cervicitis were selected as the control group, with an average age of 55.50±6.72 years. Age, age of first sexual intercourse, number of sexual partners, age of menarche, smoking and drinking, and number of pregnancies and childbirths were collected using a structured questionnaire. A pH dipstick was used to detect vaginal pH levels; a cotton swab was used to obtain vaginal secretions to detect the vaginal microbiome; and a HPV special brush was used to obtain vaginal secretions for HPV detection. Next, according to the vinegar white and iodine test under colposcopy, the suspected LSIL, HSIL, and SCC lesions, as well as normal cervical tissue were selected and sent to pathology. Another 20 mg was taken from the same lesion site. The labeled points were placed into numbered 1.5 mL sterile EP tubes without enzyme, quickly placed into the liquid nitrogen tank for storage, and then transferred to the −80 °C refrigerator for storage 24 hours later. After the pathological results were returned, eligible cervical tissues were selected for quantitative real-time polymerase chain reaction (qPCR). qPCR was used to detect the mRNA expression levels of E-cadherin, β-catenin, N-cadherin, and Vimentin.
The inclusion criteria were as follows: (I) postmenopausal women (amenorrhea >1 year), and no sex hormone replacement therapy within 3 months; (II) no vaginal medication or sexual intercourse within 24 hours before the visit; (III) women with no other tumors, and those that had not undergone radiotherapy, chemotherapy, cervical surgery, or physical therapy; and (IV) women with no history of antibiotic use within 2 weeks. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of Inner Mongolia Medical University (No. YKD2015080) and informed consent was taken from all the patients.
Study methods
PH value detection
The vaginal wall was contacted with 3.8–5.4 pH test paper for 1 s, and the pH value was read 30 s later. Next, the precision of the pH test paper was changed to 1.8–9.0 pH to measure again beyond the pH range.
Detection of vaginal secretion
A disposable sterile cotton swab was inserted into the middle and upper third of the lateral vaginal wall under the scope for five turns of wiping. The cotton swab tip was cut into the sterilized frozen storage tube containing DNase inhibitor, and two cotton swab tips were kept for each patient. The samples were frozen at −80 °C, and 16SrRNA sequencing was performed within 1 week. Firstly, DNA was extracted using the CTAB method and identified by agarose gel electrophoresis. Next, the V1–V9 high-variability region sequencing was selected, and barcode specific primers were used. Phusion® high-fidelity PCR Master Mix with GC Buffer (New England Biolabs) was used. PCR and high-fidelity enzyme to ensure the efficiency and accuracy of amplification. Finally, AMpure PB magnetic beads were used to purify and select DNA fragments to construct the SMRT Bell library, which was sequenced by PacBio platform (purchased from Illumina, USA).
HPV testing
Vaginal secretions from the external cervix were collected with a HPV brush, and qualitative typing was performed with a yn-H16 (Shenzhen Aneng Technology Co., Ltd., Shenzhen, China) thermostatic hybridization instrument. This test is a reverse dot hybridization technique that detects high-risk HPV types (16 and 18), and 13 other HPV types (including 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 73, and 82).
qPCR detection E-cadherin, β-catenin, N-cadherin, and Vimentin
According to the results of acetic acid and iodine staining, cervical tissue samples with suspected lesions were taken for sampling and marking under colposcopy, and then sent to pathology. Subsequently, 20 mg tissue samples were taken for marking and numbering, and stored in the refrigerator at −80 °C. qPCR was performed after the pathological results were confirmed. Total RNA was extracted from appropriate tissue samples using Trizol kit (Takara Bio, Japan), and the concentration of extracted RNA was determined by UV analysis. A reverse Transcription Kit (Fermentas, USA) was used for reverse transcription of RNA, and qPCR was then used to analyze the mRNA expression of the target gene. The purified E-cadherin, β-catenin, N-cadherin, and Vimentin genes were quantified in real time by CFX Connect (Bair, USA). Two SYBR Green Mix (Novizan, China) 10 μL, primer Mix (2 mM) 1 μL, template (25 times diluted cDNA) 5 μL, and diethyl coke water [diethylprocarbonate (DEPC) H2O] 4 μL was adjusted to the final reaction of 20 μL. All of the primers were synthesized by biosynthesis. The primer sequences are listed in Table 1. The thermal cycle program consisted of an initial 5 min denaturation at 95 °C, followed by 40 two-step amplification cycles, including denaturation at 95 °C for 10 s and annealing and extension at 60 °C for 30 s. The experiment was repeated three times for each data point. The ACTB gene was selected as the normal gene. Melting curve analysis after each amplification can ensure the specificity of qPCR products and the loss of primer dimers. Data obtained by qPCR were analyzed by 2−ΔΔCt, with ACTB as internal reference [ΔΔCt = (average Ct value of target gene in the test group − average Ct value of reference gene in the test group) − (average Ct value of target gene in the control group − average Ct value of reference gene in the control group); F=2−ΔΔCt].
Table 1
| Name of the primer | Primer sequence (5'-3') | Products |
|---|---|---|
| E-cadherin F | CACCACGTACAAGGGTCAGGT | 238 bp |
| E-cadherin R | CAGCTGTGAGGATGCCAGTT | |
| β-catenin F | GCGCCATTTTAAGCCTCTCG | 183 bp |
| β-catenin R | AAATACCCTCAGGGGAACAGG | |
| Vimentin F | AAATGGCTCGTCACCTTCGT | 113 bp |
| Vimentin R | AGAAATCCTGCTCTCCTCGC | |
| N-cadherin F | GTGCATGAAGGACAGCCTCT | 263 bp |
| N-cadherin R | GCTGACTCCTTCACTGACTCC | |
| ACTB F | TCCTTCCTGGGCATGGAGT | 208 bp |
| ACTB R | CAGGAGGAGCAATGATCTTGAT |
qPCR, quantitative real-time polymerase chain reaction.
Statistical analysis
Normally distributed measurement data was analyzed using the K-s to normality test, described using , and analysis of variance was used to compare the differences between groups. Median [interquartile range (IQR) was used to describe non-normally distributed data, and the Kruskal-Wallis H test was used to compare the differences. Spearman correlation analysis was used to assess the relationship between the mRNA expressions of E-cadherin, β-catenin, N-cadherin, and Vimentin in groups with postmenopausal cervical lesions. The data were analyzed using SPSS 24.0 (IBM Corp., USA), and P<0.05 was considered statistically significant.
Results
Patient characteristics
Table 2 shows the features of postmenopausal disease. Compared with the control group, the ages of the LSIL, HSIL, and SCC groups increased successively (P<0.001). Vaginal pH was 4.54±0.31 in the control group, 4.73±0.43 in the LSIL group, 5.01±0.26 in the HSIL group, and 5.00±0.30 in the SCC group, with a gradual increase (P<0.001). The positive rates of HPV16 in the control, LSIL, HSIL, and SCC groups were 17.2%, 34.5%, 66.7%, and 61.5%, respectively (P<0.05), and the positive rates of HPV18 were 6.9%, 6.9%, 5.6%, and 23.1%, respectively (P<0.05). Moreover, the smoking rates were 3.3%, 6.7%, 5.6%, and 13.3%, respectively (P<0.05). Compared with the control group, the number of births in LSIL, HSIL, and SCC groups increased successively (P<0.05). There were no significant differences in age of first sexual intercourse, number of sexual partners, and age of menarche between the groups.
Table 2
| Characteristics | NC group | LSIL | HSIL | SCC | F/χ2 | P |
|---|---|---|---|---|---|---|
| Age, | 55.50±6.72 | 57.20±6.66 | 59.72±7.81 | 61.33±8.02 | 3.676 | 0.015 |
| pH, | 4.54±0.31 | 4.73±0.43 | 5.01±0.26 | 5.00±0.30 | 11.673 | <0.001 |
| Age of first-sexual, | 22.83±2.41 | 22.10±2.83 | 22.27±2.67 | 21.13±2.52 | 2.19 | 0.094 |
| Number of sexual partners, | 1.30±0.65 | 1.36±0.61 | 1.22±0.55 | 1.26±0.52 | 0.262 | 0.853 |
| Age of menarche, | 15.30±1.74 | 14.66±1.60 | 15.77±1.89 | 15.76±1.91 | 2.373 | 0.074 |
| Gravidity, | 3.10±1.26 | 3.13±1.65 | 3.72±2.32 | 4.30±2.03 | 2.942 | 0.037 |
| Parity, | 1.83±0.87 | 2.03±1.15 | 2.50±1.29 | 2.90±1.58 | 4.304 | 0.007 |
| HPV, n (%) | 27.371 | <0.001 | ||||
| 16 | 5 (17.2) | 10 (34.5) | 12 (66.7) | 16 (61.5) | ||
| 18 | 2 (6.9) | 2 (6.9) | 1 (5.6) | 6 (23.1) | ||
| Other | 22 (75.9) | 17 (58.6) | 5 (27.8) | 4 (15.4) | ||
| Smoking, n (%) | 2.376 | 0.498 | ||||
| Yes | 1 (3.3) | 2 (6.7) | 1 (5.6) | 4 (13.3) | ||
| No | 29 (96.7) | 28 (93.3) | 17 (94.4) | 26 (86.7) | ||
| Drinking, n (%) | – | – | ||||
| No | 30 (100.0) | 30 (100.0) | 18 (100.0) | 30 (100.0) |
NC, normal control; SIL, squamous intraepithelial lesion; LSIL, low-grade SIL; HSIL, high-grade SIL; SCC, squamous cell carcinoma.
Characteristics of vaginal microbiota in postmenopausal cervical lesions
Table 3 shows that the top 10 vaginal microbiota in species level detection in the control, LSIL, HSIL, and SCC groups mainly included: Lactobacillus iners, Lactobacillus gasseri, Lactobacillus crispatus, Streptococcus agalactiae, Lactobacillus delbrueckii, Streptococcus sp HMSC034A12, Mycoplasma hominis, Enterococcus faecalis, Sneathia amnii, and Lactobacillus jensenii. Among these, Lactobacillus iners, Lactobacillus gasseri, Lactobacillus crispatus, Lactobacillus delbrueckii, and Lactobacillus jensenii belong to Lactobacilli. The vaginal flora of postmenopausal women was dominated by Lactobacillus iners. The proportion of Lactobacillus iners in the control, LSIL, HSIL, and SCC groups was 14.84%, 1.17%, 0.27%, and 0.17%, respectively, and the differences were statistically significant (P<0.05).
Table 3
| Name of vaginal microbiome species | NC group, median (IQR) | LSIL, median (IQR) | HSIL, median (IQR) | SCC, median (IQR) | χ2 | P |
|---|---|---|---|---|---|---|
| Lactobacillus iners | 0.1484 (0.0002–0.9886)# | 0.0117 (0.0037–0.5594)# | 0.0027 (0.0002–0.0291) | 0.0017 (0–0.0101) | 13.296 | 0.004 |
| Lactobacillus gasseri | 0 | 0 | 0 (0–0.0001) | 0 (0–0.0007) | 3.347 | 0.341 |
| Lactobacillus crispatus | 0 (0–0.0638) | 0 (0–0.0007) | 0.0002 (0–0.0008) | 0.0001 (0–0.0018) | 1.265 | 0.738 |
| Streptococcus agalactiae | 0 | 0 | 0 | 0 | 0.412 | 0.938 |
| Lactobacillus delbrueckii | 0 | 0 | 0 | 0 | 1.483 | 0.686 |
| Streptococcus sp HMSC034A12 | 0 (0–0.0001) | 0 | 0 | 0 | 0.383 | 0.944 |
| Mycoplasma hominis | 0 (0–0.0039) | 0 (0–0.0097) | 0.0005 (0–0.0447) | 0 (0–0.0026) | 1.641 | 0.650 |
| Enterococcus faecalis | 0 | 0 | 0 | 0 | 1.107 | 0.775 |
| Sneathia amnii | 0 (0–0.0003) | 0 (0–0.0377) | 0.0001 (0–0.0547) | 0.001 (0–0.008) | 6.257 | 0.1 |
| Lactobacillus jensenii | 0 | 0 | 0 | 0 | 1.237 | 0.744 |
#, compared with SCC, P<0.05. NC, normal control; SIL, squamous intraepithelial lesion; LSIL, low-grade SIL; HSIL, high-grade SIL; SCC, squamous cell carcinoma; IQR, interquartile range.
Correlation between total Lactobacillus and mRNA expression of E-cadherin, β-catenin, N-cadherin and Vimentin in postmenopausal cervical lesions
Compared with the control group, E-cadherin and β-catenin mRNA expression in the LSIL group, HSIL group, SCC group decreased with disease progression, while N-cadherin and Vimentin mRNA expression increased with disease progression (Figure 1A). The differences between the LSIL, HSIL, and SCC groups were statistically significant (P<0.05) (Table 4). With the progression of postmenopausal cervical squamous cell disease to cervical SCC, the expression of 16SrRNA in vaginal Lactobacillus iners gradually decreased (Figure 1B). The expression level of total Lactobacillus vaginalis also decreased gradually (Figure 1C) (P<0.05).
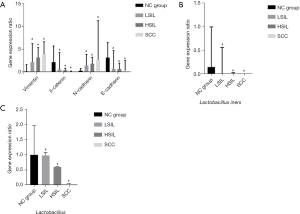
Table 4
| EMT correlation factor | NC group, median (IQR) | LSIL, median (IQR) | HSIL, median (IQR) | SCC, median (IQR) | χ2 | P |
|---|---|---|---|---|---|---|
| Vimentin | 0.35 (0.15–1.56) | 2.79 (2.09–6.00)* | 3.07 (2.06–5.14)* | 3.68 (2.21–6.38)* | 32.964 | <0.001 |
| β-catenin | 2.09 (0.54–5.52) | 0.58 (0.17–4.06)* | 0.36 (0.12–0.78)* | 0.11 (0.06–0.19)* | 35.886 | <0.001 |
| N-cadherin | 0.27 (0.13–0.74) | 1.38 (0.46–3.70)* | 1.70 (1.40–3.12)* | 2.59 (1.01–10.87)* | 33.840 | <0.001 |
| E-cadherin | 3.02 (1.84–6.20) | 0.70 (0.19–4.50)* | 0.63 (0.28–1.88)* | 0.33 (0.07–2.42)* | 22.364 | <0.001 |
| Lactobacillus | 0.9958 (0.9743–1.000) | 0.9769 (0.1041–0.9993)* | 0.5965 (0.0126–0.9965)* | 0.0388 (0.0095–0.9941)* | 15.384 | 0.001 |
*, compared with the control group, P<0.05. EMT, epithelial-mesenchymal transition; NC, normal control; SIL, squamous intraepithelial lesion; LSIL, low-grade SIL; HSIL, high-grade SIL; SCC, squamous cell carcinoma; IQR, interquartile range.
As shown in Table 5, the expression level of total Lactobacillus 16SrRNA was negatively correlated with the progression of postmenopausal cervical lesions (r<0; P<0.05), and positively correlated with the mRNA expressions of Vimentin and N-cadherin (r>0; P<0.05). Also, the mRNA expressions of β-catenin and E-cadherin were negatively correlated (r<0; P<0.05).
Table 5
| EMT correlation factor | Cervical lesion staging after menopause | |
|---|---|---|
| r | P | |
| Vimentin | 0.488 | <0.001 |
| β-catenin | −0.575 | <0.001 |
| N-cadherin | 0.546 | <0.001 |
| E-cadherin | −0.278 | 0.004 |
| Lactobacillus | −0.365 | <0.001 |
EMT, epithelial-mesenchymal transition.
Discussion
Features of postmenopausal cervical SILs and cervical SCC
The etiology of cervical SILs and cervical cancer in postmenopausal women is not unique, but is related to persistent high-risk HPV infection (HPV16, HPV18), economic level, multiple sexual partners, smoking, prematurity, environment, and other factors (1). Seventy percent of cervical lesions in postmenopausal women are caused by HPV16 (20). As age increases, HPV infection will be more persistent and the incidence of cervical cancer will increase (21). Our results showed that the degree of cervical lesions in postmenopausal women was positively correlated with age, pH value, positive ratio of HPV16, number of pregnancies, and smoking. This is consistent with the factors of cervical squamous epithelial lesions and cervical cancer, confirming that multiple etiologies have a synergistic effect on the occurrence and development of cervical cancer from menopausal squamous epithelial lesions. The positive rate of HPV16 was higher than that of HPV18, accounting for 66.7% and 61.5% of cases in the HSIL and SCC groups, respectively. HPV16 was the main type of postmenopausal cervical lesions.
Characteristics of vaginal microbiota in postmenopausal cervical SILs and cervical SCC
The top three species levels in the vaginal flora of healthy postmenopausal women are Lactobacillus heniformis, Lactobacillus inertiorum, and Lactobacillus gianseri (22). The increased abundance of ciliomycetes is associated with cervical SILs, leading to the disorder of vaginal flora (23). With the progress of cervical lesions, the relative abundance of Lactobacillus decreases, and the diversity of flora and anaerobic bacteria increases (24-26). Moreover, the high abundance of Lactobacillus iners is associated with concomitant HPV infection, HSILs, and cervical cancer (23,27). HPV damages the vaginal microecology of postmenopausal women and changes the dominant bacterial community status of Lactobacillus (28).
Our results showed that Lactobacillus iners dominates the vaginal flora of healthy postmenopausal women. With the progression of post-menopausal cervical lesions, the expression of 16SrRNA of Lactobacillus iners and Lactobacillus totalis gradually decreased. The 16SrRNA expression of Lactobacillus crispatus, Mycoplasma hominis, and Sneathia amnii in the vaginal microflora of postmenopausal HSIL patients were 0.0002, 0.0005, and 0.0001, respectively. The 16SrRNA expression of Lactobacillus crispatus and Sneathia amnii in the vaginal flora of SCC patients was 0.0001 and 0.001, respectively. As for Lactobacillus crispatus, resident flora Mycoplasma hominis, and anaerobic bacteria Sneathia amnii, the expression level of 16SrRNA of these three Lactobacillus was higher than that of postmenopausal LSIL patients and normal postmenopausal women, which proved that the expression level of vaginal Lactobacillus decreased with the aggravation of cervical lesions in postmenopausal women. Furthermore, bacterial diversity appeared and anaerobic bacteria significantly increased. Cilium appeared in HSIL and SCC patients, which was consistent with previous studies.
Relationship between vaginal microbiome characteristics and HPV in postmenopausal cervical lesions
The amount of Lactobacillus colonized in the vaginas of postmenopausal women is much less than that of pre-menopausal women, and the lactic acid produced by metabolism is reduced, which increases pH value and causes vaginal microbiosis (29). Such changes reduce the competition and inhibition of pathogenic bacteria and increase the probability of reproductive tract infection, which can lead to persistent HPV infection that is difficult to clear and promote postmenopausal CIN and further development into cervical cancer (30). HPV infection causes vaginal microecological imbalance, reduces vaginal Lactobacillus, and destroys local immune function. HPV and vaginal Lactobacillus can be mutually causal and complementary, forming a vicious cycle (31). Our results showed that the HPV16 positive rate and pH value in the LSIL, HSIL and SCC groups increased gradually compared with the control group, and the expression level of total vaginal Lactobacillus decreased, and the difference between the groups was statistically significant. The decrease of vaginal Lactobacillus led to the increase of vaginal pH, which was negatively correlated with HPV16 infection. The synergistic effect of HPV and Lactobacillus vaginalis was demonstrated.
Correlation between Lactobacillus vaginalis and the mRNA expression of EMT-associated protein (E-cadherin β-catenin N-cadherin, and Vimentin) in postmenopausal cervical SILs and cervical cancer
The invasion and metastasis of tumor cells involve multiple molecular biological processes, which are related to EMT. EMT refers to the loss of polarity of epithelial cells, which affects cytoskeletal structure, reduces intercellular adhesion, and converts them into mesenchymal cells that can migrate freely (32-34). E-cadherin, a marker of epithelial cells, is an important factor that inhibits tumor malignant transformation and invasion, and the absence of E-cadherin can make epithelial tumor cells separate from the primary lesion and metastasize to distant areas (35). There are two kinds of epithelial cell marker β-catenin in cells with different functions: one is bound to the cell membrane with E-cadherin and mainly regulates cell-cell adhesion; the other exists in the cytoplasm and nucleus. β-catenin is the core protein molecule of the classical Wnt signaling pathway and participates in this pathway (36). When either E-cadherin or β-catenin is absent, the adhesion between cells is weakened, and cell dissociation ability is enhanced. The cells then become dispersed, separated from the original tissue, leading to the occurrence of invasion, metastasis, and EMT (37). Cadherin switches exist in non-small cell carcinoma and are stimulated by activation of the Wnt/β-catenin pathway, which is characterized by decreased E-cadherin and increased mesenchymal cell marker N-cadherin, thereby enhancing EMT motor capacity (38). The mesenchymal cell marker, Vimentin, is a type III intermediate filament protein. With the decrease of E-cadherin levels in tumor staging of non-small cell lung cancer, the Vimentin level increases and EMT occurs (39). It has been confirmed that EMT occurs in postmenopausal cervical SILs with increasing grade and progression to cervical cancer, and the expression of E-cadherin and β-catenin protein decreases. Meanwhile, the expression of N-cadherin and Vimentin protein increases, and the Wnt/β-catenin signaling pathway is involved (37). The expression of proteins involves multiple steps, such as transcription, post-transcriptional regulation, and translation. In this paper, the mRNA of EMT-related proteins was studied, and our results showed that with the progression of cervical SILs to cervical cancer, the mRNA expression levels of epithelial cell markers, E-cadherin and β-catenin, were negatively correlated with the degree of disease, and the mRNA expression trends of β-catenin and E-cadherin were consistent. We speculated that β-catenin mRNA was bound to E-cadherin and existed on the cell membrane with a consistent expression of β-catenin protein, which needs to be confirmed via combined immunohistochemistry for protein fractionation. The mRNA expression levels of mesenchymal cell markers, N-cadherin and Vimentin, were positively correlated with the degree of disease, and the mRNA expression levels of EMT-related proteins were statistically significant between groups. It was also confirmed that EMT occurred during the progression of postmenopausal cervical SILs to cervical cancer, and the changes of mRNA and protein were consistent. The expression of total Lactobacillus 16SrRNA was positively correlated with mRNA expressions of β-catenin and E-cadherin (r>0; P<0.05), and negatively correlated with mRNA expressions of Vimentin and N-cadherin (r<0; P<0.05). These results suggest that postmenopausal Lactobacillus vaginalis is correlated with EMT and promotes the development of postmenopausal SILs into cervical cancer.
Possible mechanisms through which Lactobacillus vaginalis affects the EMT of postmenopausal cervical SILs and cervical cancer
Although there was no direct evidence to explain how changes in Lactobacillus vaginalis affect the occurrence of EMT, we speculated that the possible mechanisms are as follows. Firstly, the reduction of vaginal Lactobacillus after menopause and the imbalance of vaginal microecology (40). The synergistic effect with HPV destroys the vaginal mucosal barrier and reduces the ability to resist pathogens (41), and also weakens the binding of β-catenin and E-cadherin on the cell membrane, promotes the occurrence of EMT, and leads to the development of postmenopausal pre-cervical cancer lesions into cervical cancer. Secondly, after menopause, vaginal Lactobacillus is reduced, H2O2 production function is reduced, and the immune system is weakened, thereby decreasing IL-2 and increasing IL-10, IL-36γ, SIgA, and IgG (7,42). Furthermore, antibody secretion and the removal of harmful substances are also reduced, and damage of cervical epithelial cells is increased.
We believe that the reduction of estrogen in postmenopausal women leads to changes in vaginal microecology, and lactobacillus vaginalis synergistic with HPV promotes the development of cervical squamous intraepithelial neoplasia into cervical cancer in postmenopausal women. In this process, EMT occurs, mainly manifested by the decreased mRNA expression levels of β-cadherin and E-cadherin, the increase of mRNA expression of Vimintin and n-cadherin, and targeted intervention of any of these processes can slow down the occurrence and development of post-menopausal cervical lesions.
Conclusions
In this study, we found that the level of Lactobacillus iners was dominant in the vaginal microbiota of postmenopausal women, and postmenopausal SILs of the cervix developed into cervical cancer. With the increased grade of cervical lesions, vaginal Lactobacillus decreased, the diversity of vaginal flora increased, and the ability of cervical epithelial cells to clear HPV virus slowed, which aggravated HPV16 infection and promoted the occurrence of EMT. A possible mechanism is the decrease of vaginal Lactobacillus content after menopause and the increase of bacterial diversity, which may damage cervical epithelial tissue, increase the susceptibility of pathogenic bacteria and HPV, induce the decline of the host’s natural immune response, and promote the occurrence of EMT in postmenopausal women. Our results regarding postmenopausal cervical lesions provide a novel understanding. This study also provides a theoretical basis for related biological preparation of Lactobacillus, apply microbial agents is expected to become a new therapeutic target, continuous inhibition of the high-risk type HPV infection rate, in order to inhibit the development of postmenopausal cervical lesions and establish a new diagnostic and treatment strategy.
Acknowledgments
Funding: The study was supported by the National Natural Science (Project No. 81560243, Regional Science Foundation Project).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3581/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-3581/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-3581/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of Inner Mongolia Medical University (No. YKD2015080) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xie X, Kong B, Duan T, et al. Gynecology and Obstetrics. 9th ed. Beijing: People's Medical Publishing House, 2018.
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- International Agency for Research on Cancer (IARC). GLOBOCAN 2020, estimated number of new cases in 2020, China, females, all ages. 2021. Available online: https://acsjournals.onlinelibrary.wiley.com/action/doSearch?AllField=GLOBOCAN+2020&SeriesKey=15424863
- Andersen B, Christensen BS, Christensen J, et al. HPV-prevalence in elderly women in Denmark. Gynecol Oncol 2019;154:118-23. [Crossref] [PubMed]
- Roden RBS, Stern PL. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer 2018;18:240-54. [Crossref] [PubMed]
- Zhang P, Wang D, Zhang Z, et al. Correlation between vaginal microecology and high-risk HPV infection and cervical lesions. Chinese Journal of Nosocomiology 2018;28:3305-7, 3311.
- Zheng JJ, Song JH, Yu CX, et al. Difference in vaginal microecology, local immunity and HPV infection among childbearing-age women with different degrees of cervical lesions in Inner Mongolia. BMC Womens Health 2019;19:109. [Crossref] [PubMed]
- Lu Y, Zhao JW. Research progress on correlation between persistent HR-HPV infection of cervix and vaginal microecology. China Medical Herald 2018;15:26-9.
- Huang YM. Effects of postmenopausal estrogen therapy on vaginal microecology. Chinese Journal of Clinical Rational Drug Use 2019;12:81-2.
- Xu J, Peng JJ, Yang W, et al. Vaginal microbiomes and ovarian cancer: a review. Am J Cancer Res 2020;10:743-56. [PubMed]
- Yang X, Da M, Zhang W, et al. Role of Lactobacillus in cervical cancer. Cancer Manag Res 2018;10:1219-29. [Crossref] [PubMed]
- Niu XX, Li T, Zhang X, et al. Lactobacillus crispatus Modulates Vaginal Epithelial Cell Innate Response to Candida albicans. Chin Med J (Engl) 2017;130:273-9. [Crossref] [PubMed]
- Ritu W, Enqi W, Zheng S, et al. Evaluation of the Associations Between Cervical Microbiota and HPV Infection, Clearance, and Persistence in Cytologically Normal Women. Cancer Prev Res (Phila) 2019;12:43-56. [Crossref] [PubMed]
- Wang ZX, Zhao XX, Gao X, et al. Study progress in Lactobacillus and cervical cancer. Chongqing Medicine 2021;50:1954-7.
- Das V, Bhattacharya S, Chikkaputtaiah C, et al. The basics of epithelial-mesenchymal transition (EMT): A study from a structure, dynamics, and functional perspective. J Cell Physiol 2019; Epub ahead of print. [Crossref] [PubMed]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003;15:740-6. [Crossref] [PubMed]
- Zacapala-Gómez AE, Navarro-Tito N, Alarcón-Romero LDC, et al. Ezrin and E-cadherin expression profile in cervical cytology: a prognostic marker for tumor progression in cervical cancer. BMC Cancer 2018;18:349. [Crossref] [PubMed]
- Zhang JJ, Chen YP, Zhang TF, et al. Expression and the Significance of E-cadherin and β-catenin in Cervical Intraepithelial Neoplasia. Progress in Modern Biomedicine 2011;11:3491-3.
- Fan Q, Bao W, Yang TT, et al. Expression and clinical significance of Twist, E-cadherin and N-cadherin in cervical squamous cell carcinoma. Journal of Shanghai Jiaotong University 2012;32:783-7. (Medical Science).
- Hao M, Lin L. Clinical Characteristics of Postmenopausal Patients with Cervical Intraepithelial Neoplasia Grade II or Above. Chinese Journal of Minimally Invasive Surgery 2017;17:155-8.
- Ma QF, Guo YL, Gao H, et al. Prevalence and Determinants of High-risk HPV Infection among 11549 Women from an Opportunistic Screening in Hubei Province. Curr Med Sci 2019;39:622-30. [Crossref] [PubMed]
- Ma J. Vaginal microbiota in postmenopausal women and its correlation with transvaginal probiotics intervention. Beijing: Peking Union Medical College, 2019.
- Audirac-Chalifour A, Torres-Poveda K, Bahena-Román M, et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS One 2016;11:e0153274. [Crossref] [PubMed]
- Chen Y, Qiu X, Wang W, et al. Human papillomavirus infection and cervical intraepithelial neoplasia progression are associated with increased vaginal microbiome diversity in a Chinese cohort. BMC Infect Dis 2020;20:629. [Crossref] [PubMed]
- Zhang C, Liu Y, Gao W, et al. The direct and indirect association of cervical microbiota with the risk of cervical intraepithelial neoplasia. Cancer Med 2018;7:2172-9. [Crossref] [PubMed]
- Godoy-Vitorino F, Romaguera J, Zhao C, et al. Cervicovaginal Fungi and Bacteria Associated With Cervical Intraepithelial Neoplasia and High-Risk Human Papillomavirus Infections in a Hispanic Population. Front Microbiol 2018;9:2533. [Crossref] [PubMed]
- Seo SS, Oh HY, Lee JK, et al. Combined effect of diet and cervical microbiome on the risk of cervical intraepithelial neoplasia. Clin Nutr 2016;35:1434-41. [Crossref] [PubMed]
- Du H. Effects of HPV infection on vaginal microecology, cervical lesions and immune function in postmenopausal women. Modern Practical Medicine 2020;32:641-3, 647.
- Lu Y. Correlation between vaginal microecologic changes and human papillomavirus infection in postmenopausal women. Chinese Journal of Microecology 2021;33:224-6, 235.
- Bai L, Song J. Relationship between cervical lesions and vaginal microecology and Cervical HPV infection in postmenopausal women. World Latest Medicine Information 2018;18:111-2.
- Chen W, Wu L, Yan Y. Relationship between human papillomavirus infection and vaginal microecology. Chinese Journal of Microecology 2017;29:832-4.
- Smith BN, Bhowmick NA. Role of EMT in Metastasis and Therapy Resistance. J Clin Med 2016;5:17. [Crossref] [PubMed]
- Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer 2016;15:18. [Crossref] [PubMed]
- Nakaya Y, Sheng G. EMT in developmental morphogenesis. Cancer Lett 2013;341:9-15. [Crossref] [PubMed]
- Qiao D, Song J. The Activated Wnt/β-catenin Pathway and Cervical Lesions. Journal of International Reproductive Health/Family Planning 2016;35:510-4.
- Chen Y, Zhang C, Chen S, et al. Molecular cell biology. 3rd ed. Beijing: Higher Education Press, 2019.
- Xu J. Correlation between postmenopausal cervical lesions and Wnt/β-catenin signaling pathway. Hohhot: Inner Mongolia Medical University, 2018.
- Pan S, An L, Meng X, et al. MgCl2 and ZnCl2 promote human umbilical vein endothelial cell migration and invasion and stimulate epithelial-mesenchymal transition via the Wnt/β-catenin pathway. Exp Ther Med 2017;14:4663-70. [Crossref] [PubMed]
- Luo T, Wang L, Wu P, et al. Downregulated vimentin and upregulated E-cadherin in T1 stage non-small-cell lung cancer: does it suggest a mesenchymal-epithelial transition? Neoplasma 2017;64:693-9. [Crossref] [PubMed]
- Liu J, Ning Y. Survival status of lactobacilli in vaginal microenvironment: Research progress. Chinese Journal of Microecology 2016;28:733-6.
- Mitra A, MacIntyre DA, Marchesi JR, et al. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome 2016;4:58. [Crossref] [PubMed]
- Łaniewski P, Barnes D, Goulder A, et al. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci Rep 2018;8:7593. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


