
Glutathione combined with mecobalamin in the treatment of chemotherapy-induced peripheral neuropathy in multiple myeloma: a retrospective clinical study
Introduction
The promotion of stem cell transplantation technology and the emergence of new treatment methods, including proteasome inhibitors and immunomodulators, have increased the survival time of MM patients (1-3). Despite increased life spans, PN complications and chemotherapy-induced peripheral neuropathy (CIPN) in MM associated with immunomodulators has attracted attention (4-6). Presently, CIPN caused by bortezomib or thalidomide-based chemotherapy regimens is the most common non-hematological, dose-limiting adverse reaction in MM patients, and significantly reduces their quality of life and survival. Thalidomide-related neurotoxicity occurs in 40% of patients (7), and is frequently characterized by paresthesia and loss of touch and pain. Potential mechanisms include degeneration of dorsal root ganglion, inhibition of angiogenesis and downregulation of tumor necrosis factor-α (TNF-α) which leading to Wallerian degeneration and loss of myelinated fibers (8,9). Bortezomib could damage dorsal root ganglion, mitochondrial and endoplasmic reticulum, inhibit the transcription of nerve growth factor (10). Usually causes painful sensory neuropathy, which commonly presents as a sharp or burning pain in the feet and fingertips, and autonomic dysfunction in 10% of patients (11). Among patients treated with bortezomib, 60–75% showed a complete reversal of symptoms at the 6-month follow-up examination, but only 25% thalidomide-treated patients showed remission (12,13).
Treatment for CIPN is limited. Reducing chemotherapy doses or treatment cycles can partially improve the neurological symptoms associated with MM; however, these measures also inevitably negatively affect MM treatment. Thus, more effective and safer neuroprotective methods urgently need to be identified. Glutathione is known to prevent the accumulation of toxic substances in the peripheral nervous system. The rich sulfhydryl groups of glutathione affect cell metabolism by activating enzymes promoting metabolism. In addition, a combination of sulfhydryl groups and oxygen free radicals increases the excretion of the oxygen free radical, further alleviating the neurotoxic side effects and producing no obvious effects on the efficacy of the chemotherapeutic drug (14). Mecobalamin is an endogenous vitamin B12 that has a high affinity for nervous tissues and can regulate the metabolism of various substances by stimulating lecithin and acetylcholine synthesis. Mecobalamin is generally used in the clinic in the treatment of neurological diseases (15).
Nowadays, there is no report about the efficacy of glutathione combined with mecobalamin on the treatment of MM patients with CIPN. In this study, we retrospectively analyzed the preventive and therapeutic effects of glutathione combined with mecobalamin on MM-related PN to determine whether this combination could be alleviates CIPN.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-3313).
Methods
Research subjects
A total of 158 MM patients diagnosed at the Affiliated Cancer Hospital of Zhengzhou University from September 2008 to September 2018 were selected as the study subjects, who were selected based on baseline data. To be eligible for enrollment in this study, patients had to meet the following inclusion criteria: (I) be aged ≥18 years; (II) have undergone disease diagnosis, classification, and Durie-Salmon (DS) and International Staging System (ISS) staging as per the 2017 Guidelines for the Diagnosis and Treatment of Multiple Myeloma in China; (III) have Eastern Cooperative Oncology Group physical fitness scores of 0, 1, or 2; (IV) have complete information available about their chemotherapy regimen, adverse reactions, examination results, and medical orders; and (V) have completed at least 2 cycles of chemotherapy. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had been diagnosed and treated at other hospitals; (II) had not responded to follow-up telephone calls and or had incomplete medical records; (III) had severe cardiac dysfunction or hepatic insufficiency; (IV) had other malignant solid tumors or hematological tumors; and/or (V) had shown poor compliance with medical advice.
Research methods
In order to reduce CIPN, we take this combination regimen as a preventive measure that administrate it before chemotherapy. Patients were divided into a study group and a control group, and a retrospective analysis was conducted using the method of the control study. Patients in the study group were administered 2.4 g of glutathione intravenously once daily 2–3 days before chemotherapy, combined with 500 µg of mecobalamin administered intravenously once every other day until the end of the chemotherapy cycle. Patients in the control group were not administered glutathione or mecobalamin; however, when hepatic insufficiency occurred, liver-protecting drugs other than glutathione could be selected. The patients were divided into a bortezomib-exposed or bortezomib-unexposed group. The bortezomib-exposed group was further divided into an intravenous and a subcutaneous group based on how the bortezomib was injected. The bortezomib regimens included PD (bortezomib, dexamethasone), PAD (bortezomib, dexamethasone, doxorubicin), and PCD (bortezomib, cyclophosphamide, dexamethasone), while the bortezomib-free regimens included VAD (vindesine, doxorubicin, dexamethasone), DICE (dexamethasone, isocyclophosphamide, etoposide, cisplatin), hyper-CVAD (cyclophosphamide, longSpringdesin, epirubicin, dexamethasone), and DT-PACE (dexamethasone, thalidomide, etoposide, cisplatin, cyclophosphamide, epirubicin). We measure symptoms, signs, ability aspects, and electro-physiologic in MM patients by the Total Neuropathy Score (TNS), which was suggest as a reliable method for assessing both the severity and course of CIPN (16-18). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Affiliated Cancer Hospital of Zhengzhou University (No. 2020021301). Informed consent was taken from all the patients.
Adverse reactions
During treatment, blood biochemistry (liver, renal, and coagulation functions and electrolytes), electrocardiograms, and imaging examinations were routinely performed for all patients. If hepatic insufficiency occurred, patients in the study group were given oral medicine to enhance the hepatoprotective effects, and patients in the control group were intravenously administered hepatoprotective drugs other than glutathione. If PN occurred, specific treatments were administered based on the severity of the reaction. If cytopenia occurred, granulocyte colony stimulating factor (G-CSF) or component blood transfusions were administered. If gastrointestinal symptoms occurred, antiemetic, acid suppression, gastrointestinal motility, and other drugs were administered. If creatinine clearance increased, symptomatic kidney protection treatment was administered, and hemofiltration or dialysis was performed as necessary. If a patient experienced a fever, an anti-infection treatment was first administered and then pathogenic examination was performed to treat the symptoms.
Efficacy evaluation and follow-ups
The Guidelines for the Diagnosis and Treatment of Multiple Myeloma in China (revised in 2017) were used as the evaluation criteria. Patients were followed up with by telephone calls or at clinic visits. The final follow-up date was September 1, 2018. The median follow-up period was 27.4 (1.9–100.7) months. The basic clinical data, adverse reactions, PFS, and OS of the groups were analyzed.
Statistical analysis
The data analysis was performed using SPSS 21.0 statistical software. For the general data, non-parametric tests of two independent samples were used to evaluate the quantitative data, and the χ2 test was used to evaluate the qualitative data. The χ2 test and the Fisher exact probability method were used to compare effective rates. The univariate analysis rates were compared using the χ2 test. The multivariate analysis was performed by a logistic regression analysis. PFS and OS curves were plotted using the Kaplan-Meier method, and the differences between the groups were compared using the Log-rank test. The proportional hazard regression model was used for the univariate and multivariate regression analyses, and differences were noted as statistically significant when the P value was <0.05.
Results
Normal information
In total, 158 patients were enrolled in this study. The study group comprised 114 (72.2%) patients [68 male and 46 female with a median age of 63 (range, 29–81) years]. In the study group, there were 64 IgG type patients, 26 IgA type patients, and 24 patients with other types, which concluded IgD type, IgM type, light chain type and non-secretory type. 18 patients (15.8%) showed extramedullary invasion, and 24 patients (21.1%) had diabetes. 97 patients (85.1%) were included in the bortezomib exposure group. The basic clinical data of all the patients included in the study and control groups are shown in Table 1, and there were no significant differences between the two groups (P>0.05). There were 26 (22.8%) stage I patients in the study group, but only 9 (20.5%) in the control group. There were 40 (35.1%) stage II patients in the study group, but only 13 (29.5%) in the control group. There were 48 (42.1%) stage III patients in the study group, but only 22 (50.0%) in the control group. No significant differences were noted in ISS staging between each group (P=0.665). There were 26 stage I + II (22.8%) patients in the study group, but only 5 (11.4%) in the control group. There were 88 stage III (77.2%) patients in the study group, but only 39 (88.6%) in the control group. No significant differences in DS staging were found between the groups (P=0.122l; see Table 1).
Table 1
| Clinical features | Study group (n=114) | Control group (n=44) | χ2 | P value |
|---|---|---|---|---|
| Gender | 0.039 | 1.000 | ||
| Male | 68 | 27 | ||
| Female | 46 | 17 | ||
| Age (years) | 0.146 | 0.202 | ||
| ≥60 | 66 | 31 | ||
| <60 | 48 | 13 | ||
| Immunophenotyping | 3.630 | 0.163 | ||
| IgG | 64 | 26 | ||
| IgA | 26 | 14 | ||
| Other types | 24 | 4 | ||
| Extramedullary invasion | 0.500 | 0.618 | ||
| Yes | 18 | 5 | ||
| No | 96 | 39 | ||
| Diabetes | 0.287 | 0.670 | ||
| Yes | 24 | 11 | ||
| No | 90 | 33 | ||
| LDH (U/L) | 258 [123–873] | 236 [106–757] | –1.357 | 0.175 |
| β2 microglobulin (mg/dL) | 5.1 [3.0–8.8] | 5.2 [3.4–8.6] | –0.141 | 0.890 |
| Plasma cell ratio (%) | 33 [12–54] | 33 [15–43] | –0.003 | 0.958 |
| Chemotherapy regimen | 0.709 | 0.473 | ||
| Bortezomib-exposed group | 97 | 35 | ||
| Bortezomib-unexposed group | 17 | 9 | ||
| Bortezomib usage | 0.873 | 0.428 | ||
| Subcutaneous injection | 56 | 17 | ||
| Intravenous injection | 41 | 18 | ||
| ISS staging | ||||
| I | 26 | 9 | ||
| II | 40 | 13 | 0.817 | 0.665 |
| III | 48 | 22 | ||
| DS staging | ||||
| I + II | 26 | 5 | 2.636 | 0.122 |
| III | 88 | 39 |
Efficacy evaluation
The remission rates of complete response (CR) and very good partial response (VGPR) were 18.4% and 15.9% in the study and control groups, respectively (P=0.819). The remission rates of partial response (PR) in the study and control groups were 51.7% and 47.7%, respectively (P=0.724). The overall response rate (ORR) of the study and control groups were 71.2% and 63.6%, respectively (P=0.450).
Adverse reactions
There were no significant differences in the adverse reactions, such as leukopenia, thrombocytopenia, hemoglobin reduction, pulmonary infection/upper respiratory tract infections, digestive tract symptoms, herpes zoster or urinary tract infections, between the study and control groups (P>0.05). The incidence of PN was lower in the study group than the control group (31.6% vs. 41.7%), and the difference between grades 2 and 3 PN was statistically significant (P=0.048 and P=0.040, respectively). The proportions of patients showing hepatic insufficiency in the study and control groups were 19.3% and 36.4%, respectively, and the difference was statistically significant (P=0.037).
In the analysis of the factors affecting neuropathy, PN was characterized as a dependent variable, while gender, age, immune classification, extramedullary invasion, diabetes mellitus, bortezomib exposure, and the bortezomib injection method were characterized as independent variables. The results showed that the proportion of MM patients with diabetes who developed PN during treatment was significantly increased compared with non-diabetes MM patients (P=0.016). PN was then classified into grades 1–5 (see Table 2), and a history of diabetes (P=0.032) and the method of bortezomib injection (P=0.043) was found to affect the PN grade. A further analysis revealed that whether comorbid diabetes (P=0.010) or the route of injection of bortezomib (P=0.008) are associated with the incidence of grade 1 PN.
Table 2
| Clinical characteristics | Grade 1 (n=35) | Grade 2 (n=13) | Grade 3 (n=8) | Grade 4 (n=1) | χ2 | P value |
|---|---|---|---|---|---|---|
| Gender | 1.596 | 0.660 | ||||
| Male | 21 | 6 | 5 | 1 | ||
| Female | 14 | 7 | 3 | 0 | ||
| Age (years) | 0.907 | 0.824 | ||||
| ≥60 | 24 | 8 | 6 | 1 | ||
| <60 | 11 | 5 | 2 | 0 | ||
| Immunophenotyping | 4.044 | 0.671 | ||||
| IgG | 20 | 7 | 4 | 1 | ||
| IgA | 10 | 3 | 4 | 0 | ||
| Other types | 5 | 3 | 0 | 0 | ||
| Extramedullary invasion | 1.718 | 0.633 | ||||
| Yes | 4 | 3 | 2 | 0 | ||
| No | 31 | 10 | 6 | 1 | ||
| Diabetes | 8.824 | 0.032 | ||||
| Yes | 7 | 6 | 5 | 1 | ||
| No | 28 | 7 | 3 | 0 | ||
| Chemotherapy regimen | 0.280 | 0.964 | ||||
| Bortezomib-exposed group | 31 | 11 | 7 | 1 | ||
| Bortezomib-unexposed group | 4 | 2 | 1 | 0 | ||
| Bortezomib usage | 8.157 | 0.043 | ||||
| Subcutaneous injection | 19 | 3 | 1 | 0 | ||
| Intravenous injection | 12 | 8 | 6 | 1 | ||
| DS staging | 1.909 | 0.592 | ||||
| I + II | 8 | 1 | 1 | 0 | ||
| III | 27 | 12 | 7 | 1 | ||
| ISS staging | 3.563 | 0.736 | ||||
| I | 7 | 3 | 0 | 0 | ||
| II | 11 | 5 | 3 | 0 | ||
| III | 17 | 5 | 5 | 1 |
PN, peripheral neuropathy; MM, multiple myeloma.
The logistic regression analysis of the diabetes and bortezomib injection methods revealed that the risk of PN in MM patients with diabetes was 3.484 times higher than that in patients without diabetes mellitus [95% confidence interval (CI): 1.214–10.003]. There was no significant different in the risk of PN in MM patients when bortezomib was injected using different methods (P>0.05; see Table 3).
Table 3
| Factors | B value | Standard error | Wald | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Diabetes | 1.248 | 0.538 | 5.382 | 0.020 | 3.484 | 1.214–10.003 |
| Bortezomib usage | –0.121 | 0.458 | 0.069 | 0.792 | 0.886 | 0.361–2.176 |
PN, peripheral neuropathy; MM, multiple myeloma; OR, odds ratio; CI, confidence interval.
A further analysis revealed that the incidence of PN was lower in patients with diabetes or those who received bortezomib intravenous injections in the study group than in the control group, but the difference was not statistically significant (P>0.05). Glutathione combined with mecobalamin had no significant effect on PN in MM patients with diabetes or those who received bortezomib intravenous injections.
Subsistence analysis
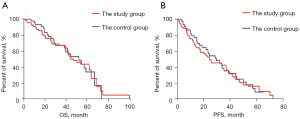
The median follow-up period was 27.4 (1.9–100.7) months. The median OS time was 49.2 months (95% CI: 35.2–59.4 months) in the study group and 51.3 months (95% CI: 35.7–68.9 months) in the control group. There was no significant difference in OS between the study and control groups (P=0.808; see Figure 1A). The median PFS time was 24.6 months (95% CI: 14.1–35.0 months) in the study group and 26.8 months (95% CI: 19.0–40.6 months) in the control group. Similar to OS, there was no significant difference in PFS between the study and control groups (P=0.719; see Figure 1B).
MM prognostic factors
The univariate proportional hazards model revealed that the prognosis of MM was correlated with extramedullary disease (EMD) and elevated LDH (P<0.05). Two factors found to affect prognosis were elevated EMD and LDH (see Tables 4,5). The mortality risk of MM patients with EMD was 2.373 times higher than that of non-MM patients (95% CI: 1.282–4.393), and the median OS of MM patients with EMD and non-MM patients was 23.4 and 52.3 months, respectively (see Figure 2A). The mortality risk of patients with elevated LDH was 1.934 times higher than that of patients with normal LDH levels (95% CI: 1.179–3.173), and the median OS of MM patients with EMD and without EMD was 41.9 and 58.9 months, respectively (see Figure 2B).
Table 4
| Factors | HR | 95% CI | P value |
|---|---|---|---|
| Age | 1.245 | 0.718–2.162 | 0.435 |
| Gender | 0.730 | 0.415–1.285 | 0.275 |
| Immunophenotyping | 0.931 | 0.644–1.345 | 0.702 |
| EMD | 1.966 | 1.088–3.552 | 0.025* |
| Diabetes | 1.224 | 0.873–1.716 | 0.240 |
| Elevated LDH | 1.679 | 1.043–2.702 | 0.033* |
| Plasma cell ratio | 0.965 | 0.555–1.679 | 0.899 |
| Application of bortezomib | 1.091 | 0.608–1.960 | 0.770 |
| DS staging | 1.240 | 0.721–2.131 | 0.437 |
| ISS staging | 0.730 | 0.458–1.165 | 0.187 |
* indicates statistical significance. OS, overall survival; MM, multiple myeloma; HR, hazard ratio; CI, confidence interval; EMD, extramedullary disease; LDH, lactic dehydrogenase.
Table 5
| Factors | Regression coefficient | Standard error | Wald | P value | HR | 95% CI |
|---|---|---|---|---|---|---|
| EMD | 0.864 | 0.314 | 7.569 | 0.006 | 2.373 | 1.282–4.393 |
| LDH | 0.660 | 0.253 | 6.819 | 0.009 | 1.934 | 1.179–3.173 |
MM, multiple myeloma; EMD, extramedullary disease; LDH, lactic dehydrogenase.

Discussion
In this study, the male-to-female ratio was 1.5:1, suggesting that the incidence of MM is higher in males than females. The age of onset ranged from 29 to 81 years, with a median age of 63 years, and a peak age of onset between 55–68 years of age. In our study, 27 (17.1%) MM patients were aged >70 years, and only 6 (3.8%) MM patients were aged <40 years, which differs to the median age of MM patients reported in other studies in China (19,20). In terms of clinical efficacy, the remission rates of VGPR were 18.4% and 15.9% in the study and control groups, respectively. The PR and ORR remission rates were 51.7% and 47.7%, and 71.2% and 63.6% for the study and control groups, respectively. There were no statistically significant differences between the groups. The curative effect of the study group was better than that of the control group, which may be related to there being a slightly lower proportion of clinical stage III patients in the study group than the control group. In addition, the incidence and severity of PN were better in the study group than in the control group, suggesting that reducing the dose of chemotherapy or the treatment cycle would improve the occurrence of PN and thus mitigating toxicities in the treatment of MM (21,22).
Many experimental studies have examined the mechanisms behind CIPN. Poruchynsky et al. suggested that bortezomib increases microtubule aggregation and hinders signaling by affecting the proteins that regulate the stability of microtubules (23). In vitro experiments performed by Csizmadia et al. showed that when proteasome inhibitors were present, proteins with erroneous folding accumulated, impairing cell function and leading to neurotoxicity (24). A study investigating the mechanism behind bortezomib-induced PN in rats found that rats treated with a combination of reduced glutathione showed neuropathological favorable changes compared to rats treated with saline. This confirmed that reactive oxygen species (ROS) release leads to CIPN production. Glutathione alleviated systemic toxicity in rats and reduced ROS release in neuronal cells, but did not reverse CIPN. A clinical study investigating the effect of glutathione on neurotoxicity, oxaliplatin pharmacokinetics, and platinum-deoxyribonucleic acid (Pt-DNA) adduct formation in 27 colorectal cancer patients treated with FOLFOX4 adjuvant therapy found that patients treated with glutathione showed significantly (P=0.0037) lower than the placebo group between above contents; however, there were no significant differences in the pharmacokinetic parameters. Thus, this work shows that glutathione is an effective strategy for reducing oxaliplatin-induced PN without affecting the pharmacokinetics of oxaliplatin and the formation of Pt-DNA adducts (25). However, a double-blind, placebo-controlled trial of 185 patients treated with paclitaxel and carboplatin failed to show that glutathione was beneficial. As carboplatin is the least neurotoxic platinum drug, glutathione did not effectively prevent paclitaxel-induced PN (26). Further, the results of the North Central Cancer Treatment Group/Combined Trial n08ca did not support the use of glutathione in preventing paclitaxel/carboplatin-induced CIPN (14). In addition to glutathione, there is evidence that vitamins B3, B6, and B12 can protect patients from the occurrence, development or severity of CIPN (27). In a prospective, randomized, placebo-controlled trial, 71 patients underwent chemotherapy with paclitaxel, oxaliplatin or vincristine. The main results of this study showed that vitamin B did not significantly reduce the incidence of CIPN (P=0.22), but did significantly improve sensory neuropathy perception (P=0.03 at 12 weeks; P=0.005 at 24 weeks; P=0.021 at 32 weeks). Additionally, the risk estimates for sensory neuropathy perception were also statistically different at the time chemotherapy was stopped and after the 12-week follow-up period [odds ratio (OR): 5.78, 95% CI: 1.63–20.5, and OR: 8.1 at 32 weeks, 95% CI: 1.23–53.2] (28). In this study, we confirmed that glutathione combined with mecobalamin reduced the incidence of CIPN (31.6% vs. 47.7%), especially level 2 and level 3 CIPN (P=0.048 and 0.040, respectively). Thus, reduced glutathione combined with mecobalamin not only reduced the incidence of MM-related PN, but also reduced the severity of its occurrence.
Next, we analyzed PN as a dependent variable and found that patients with diabetes had a significantly higher incidence of PN (P=0.016) and a more severe form of PN (P=0.032). In study group, there was a higher incidence of grade 2–4 PN than grade 1 PN, and the difference in severity was statistically significant (P=0.010). The logistic regression analysis revealed that MM patients with diabetes were 3.484 times more likely to develop PN than patients without diabetes. It is likely that diabetes increases the incidence of MM-related PN and aggravates neurological damage in patients (29).
In this study, the incidence of subcutaneously injecting bortezomib was 46%, which was significantly lower than that observed for an intravenous injection (54%). This difference was not statistically significant; however, the analysis confirmed that changing the route of administration affected the severity of PN (P=0.043). A further analysis revealed that the incidence of grade 2 and higher PN in the subcutaneous injection group was significantly lower than grade 1 PN (P=0.008). Moreau et al. confirmed that plasma exposure to bortezomib was similar for subcutaneous and intravenous injections as shown by two randomized studies of MMY-3021 and CAN-1004 in which the pharmacodynamics were comparable. In addition, bortezomib has been shown to reduce the overall incidence of grade 3 adverse events, especially the incidence of PN (30). The initial analysis of MMY-3021 was performed after the last patient completed 4 cycles of bortezomib treatment, with a median follow-up period of <1 year. Arnulf et al. reported the final survival analysis of the last patient after 1 year randomization (31). A significant decrease in the incidence of PN comparing subcutaneous and intravenous injections (all grades: 38% vs. 53%; P=0.044; Level 2 and above: 24% vs. 41%; P=0.012; Level 3 and above: 6% vs. 16%; P=0.026) was also observed. Minarik et al. conducted a retrospective analysis of a randomized trial (32). The cohort included primary patients, patients treated with bortezomib regimens, and patients treated with autologous stem cell transplantation (ASCT). When comparing the two groups of patients receiving different types of bortezomib injection methods, no difference was found in the incidence of PN between the two groups. Specifically, the intravenous injection group had a 48% incidence of PN and the subcutaneous injection group had a 41% incidence of PN. The incidence of grade 2 PN in the intravenous injection group compared to the subcutaneous injection group was 20% versus 18%, and the incidence of grade 3 PN in these groups was 6% vs. 4%. Conversely, the present analysis showed that the incidence of PN was dose-dependent and could be reduced by lowering the treatment intensity (to bortezomib once a week) rather than by the route of administration. Thus, additional research needs to be conducted to confirm the significance of subcutaneously injecting bortezomib in reducing the occurrence of PN.
We further explored whether reduced glutathione combined with mecobalamin reduced the incidence of CIPN in patients with diabetes or those administered bortezomib intravenously. The incidence of PN in both groups was decreased (the former was 45.8% vs. 72.7%, the latter was 39.0% vs. 61.1%); however, the differences were not statistically significant. Glutathione combined with mecobalamin may improve chemotherapy-related PN, but may not significantly improve PN caused by patients with diabetes or patients experiencing different modes of administration.
The prognostic factors of MM were also analyzed in this study. EMD mostly occurs when MM progresses or recurs, and is a sign of poor prognosis. MM patients with EMD have a significantly shorter survival time than who without EMD (33-35). The univariate analysis showed that the risk of death in EMD-positive patients was 2.373 times higher than that in EMD-negative (95% CI: 1.282–4.393), and the median OS was 23.4 vs. 52.3 months (P=0.006), respectively. Presently, there is no clear plan for the treatment of MM patients with extramedullary infiltration, and there are few relevant reports on the evaluation of its treatment effects. We also found that the risk of death for patients with elevated LDH was 1.934 times higher than that of patients with normal LDH (95% CI: 1.179–3.173), and the median OS for both was 41.9 and 58.9 months, respectively (P=0.009). Many studies have reported that an increase in LDH is related to the serum concentrations of β2-microglobulin, creatinine, and thymidine kinase, which predicts the proliferation of myeloma cells, the occurrence of extramedullary lesions, and a shorter survival time (36,37). Some have pointed out that a 17p13 deletion is associated with LDH levels and a 17p13 deletion is associated with a high risk of a poor prognosis in MM patients. New drugs, such as lenalidomide and bortezomib, cannot overcome the adverse effects of TP53 gene deletions caused by 17p deletions on MM prognosis (38,39).
Multiple myeloma-associated antigen-1 (MMSA-1) is a recently discovered MM-associated antigen. A multivariate Proportional hazards model analysis predicted that MMSA-1 and LDH levels are independently associated with poor PFS and OS in patients with myeloma (40). Chim et al. observed 77 Chinese myeloma patients eligible for transplantation who received continuous thalidomide maintenance therapy with ASCT. After 3 induction regimens (19 underwent a PAD regimen, 33 a VTD regimen, and 25 a staged treatment), the 5-year OS and PFS rates were 66.4% and 36.2%, respectively. The univariate analysis showed that elevated LDH, ISS stage III, high β2 microglobulin, and failure to obtain CR/ near complete response (nCR) after ASCT were all risk factors affecting OS and EFS. The multivariate analysis revealed that elevated LDH and age were risk factors for a poor prognosis of OS, and elevated LDH is the only negative factor for EFS. Chim found that the risk of death in patients with elevated LDH was 4.289 times higher than that in patients with normal LDH levels (95% CI: 1.500–12.260) (41). Our study suggested that the mortality risk for elevated LDH was 1.934. The question of whether glutathione combined with mecobalamin alleviates CIPN and improves prognosis still needs to be investigated. The sample size used in this study is small and related reports are limited. Further validation needs to be performed with a larger sample size.
In this study, we can conclude glutathione combined with mecobalamin can treat CIPN, heighten patients’ live quality and not affect chemotherapy effects. But our study have some limits, such as selection bias, heterogeneity in patient management and poor data quality. Our results provide direction and focus for prospective studies, which can verify the effect of glutathione plus mecobalamin treatment of CIPN.
Conclusions
In this study, reduced glutathione combined with mecobalamin was used to treat MM-related PN, and was found to not only reduce the incidence of PN but also to reduce the severity of disease. It had no significant effect on disease remission, or the PFS or OS of patients. This drug combination also had a good safety profile. Diabetes and intravenously administered bortezomib increases the incidence and severity of CIPN. Additionally, if reduced glutathione is combined with mecobalamin, blood glucose levels need to be controlled and bortezomib needs to be subcutaneously injected to reduce the incidence and severity of PN. In addition, this study showed that elevated EMD and LDH are independent prognostic factors of MM, and glutathione combined with mecobalamin improves patient prognosis.
Acknowledgments
The authors would like to thank all the patients for their cooperation. We would also like to thank Medical Record Department, Henan Cancer Hospital for providing us with facilities and technical support.
Funding: This study was supported by grants from the National Natural Science Foundation of China (No. 81470287) and the Henan Outstanding Person Foundation (No. 184200510007).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-3313
Data Sharing Statement: Available a https://dx.doi.org/10.21037/apm-21-3313
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-3313). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics board of Affiliated Cancer Hospital of Zhengzhou University (No. 2020021301). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oriol A, Abril L, Ibarra G, et al. Limited treatment options in refractory multiple myeloma: promising therapeutic developments. Expert Rev Anticancer Ther 2020;20:31-44. [Crossref] [PubMed]
- Xu P, Zhou R, Xu J, et al. Higher single dose of bortezomib plus thalidomide and dexamethasone is a promising therapy for newly diagnosed multiple myeloma. Transl Cancer Res 2019;8:2099-106. [Crossref]
- Zhang HM, Liu XY, Liu YZ, et al. Analysis of the efficacy and safety of bortezomib for treating newly diagnosed multiple myeloma through different administration methods. Cancer Manag Res 2019;11:8295-302. [Crossref] [PubMed]
- Tacchetti P, Terragna C, Galli M, et al. Bortezomib- and thalidomide-induced peripheral neuropathy in multiple myeloma: clinical and molecular analyses of a phase 3 study. Am J Hematol 2014;89:1085-91. [Crossref] [PubMed]
- Staff NP, Grisold A, Grisold W, et al. Chemotherapy-induced peripheral neuropathy: A current review. Ann Neurol 2017;81:772-81. [Crossref] [PubMed]
- Haryani H, Fetzer SJ, Wu CL, et al. Chemotherapy-Induced Peripheral Neuropathy Assessment Tools: A Systematic Review Oncol Nurs Forum 2017;44:E111-23. [Crossref] [PubMed]
- Johnson DC, Corthals SL, Walker BA, et al. Genetic factors underlying the risk of thalidomide-related neuropathy in patients with multiple myeloma. J Clin Oncol 2011;29:797-804. [Crossref] [PubMed]
- De Logu F, Trevisan G, Marone IM, et al. Oxidative stress mediates thalidomide-induced pain by targeting peripheral TRPA1 and central TRPV4. BMC Biol 2020;18:197. [Crossref] [PubMed]
- Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia 2012;26:595-608. [Crossref] [PubMed]
- Azoulay D, Lavie D, Horowitz N, et al. Bortezomib-induced peripheral neuropathy is related to altered levels of brain-derived neurotrophic factor in the peripheral blood of patients with multiple myeloma. Br J Haematol 2014;164:454-6. [Crossref] [PubMed]
- Zhi WI, Ingram E, Li SQ, et al. Acupuncture for Bortezomib-Induced Peripheral Neuropathy: Not Just for Pain. Integr Cancer Ther 2018;17:1079-86. [Crossref] [PubMed]
- Zajączkowska R, Kocot-Kępska M, Leppert W, et al. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int J Mol Sci 2019;20:1451. [Crossref] [PubMed]
- Kerckhove N, Collin A, Condé S, et al. Chemotherapy-induced peripheral neuropathy: Symptomatology and epidemiology. Bull Cancer 2018;105:1020-32. [Crossref] [PubMed]
- Leal AD, Qin R, Atherton PJ, et al. North Central Cancer Treatment Group/Alliance trial N08CA-the use of glutathione for prevention of paclitaxel/carboplatin-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled study. Cancer 2014;120:1890-7. [Crossref] [PubMed]
- Solomon LR. Functional vitamin B12 deficiency in advanced malignancy: implications for the management of neuropathy and neuropathic pain. Support Care Cancer 2016;24:3489-94. [Crossref] [PubMed]
- Cavaletti G, Bogliun G, Marzorati L, et al. Grading of chemotherapy-induced peripheral neurotoxicity using the Total Neuropathy Scale. Neurology 2003;61:1297-300. [Crossref] [PubMed]
- Cavaletti G, Frigeni B, Lanzani F, et al. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst 2007;12:210-5. [Crossref] [PubMed]
- Argyriou AA, Iconomou G, Kalofonos HP. Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood 2008;112:1593-9. Erratum in: Blood 2009;113:4478. [Crossref] [PubMed]
- Sun CY, Li JY, Chu ZB, et al. Efficacy and safety of bortezomib maintenance in patients with newly diagnosed multiple myeloma: a meta-analysis. Biosci Rep 2017;37:BSR20170304. [Crossref] [PubMed]
- Qian X, Chen H, Xia J, et al. Real-World Clinical Outcomes in Elderly Chinese Patients with Multiple Myeloma: A Single-Center Experience. Med Sci Monit 2018;24:5887-93. [Crossref] [PubMed]
- Wadia RJ, Stolar M, Grens C, et al. The prevention of chemotherapy induced peripheral neuropathy by concurrent treatment with drugs used for bipolar disease: a retrospective chart analysis in human cancer patients. Oncotarget 2018;9:7322-31. [Crossref] [PubMed]
- Piccolo J, Kolesar JM. Prevention and treatment of chemotherapy-induced peripheral neuropathy. Am J Health Syst Pharm 2014;71:19-25. [Crossref] [PubMed]
- Poruchynsky MS, Sackett DL, Robey RW, et al. Proteasome inhibitors increase tubulin polymerization and stabilization in tissue culture cells: a possible mechanism contributing to peripheral neuropathy and cellular toxicity following proteasome inhibition. Cell Cycle 2008;7:940-9. [Crossref] [PubMed]
- Csizmadia V, Raczynski A, Csizmadia E, et al. Effect of an experimental proteasome inhibitor on the cytoskeleton, cytosolic protein turnover, and induction in the neuronal cells in vitro. Neurotoxicology 2008;29:232-43. [Crossref] [PubMed]
- Milla P, Airoldi M, Weber G, et al. Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity. Anticancer Drugs 2009;20:396-402. [Crossref] [PubMed]
- Bakogeorgos M, Georgoulias V. Risk-reduction and treatment of chemotherapy-induced peripheral neuropathy. Expert Rev Anticancer Ther 2017;17:1045-60. [Crossref] [PubMed]
- Schloss J, Colosimo M. B Vitamin Complex and Chemotherapy-Induced Peripheral Neuropathy. Curr Oncol Rep 2017;19:76. [Crossref] [PubMed]
- Schloss JM, Colosimo M, Airey C, et al. A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN). Support Care Cancer 2017;25:195-204. [Crossref] [PubMed]
- Delforge M, Bladé J, Dimopoulos MA, et al. Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. Lancet Oncol 2010;11:1086-95. [Crossref] [PubMed]
- Moreau P, Karamanesht II, Domnikova N, et al. Pharmacokinetic, pharmacodynamic and covariate analysis of subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma. Clin Pharmacokinet 2012;51:823-9. [Crossref] [PubMed]
- Arnulf B, Pylypenko H, Grosicki S, et al. Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica 2012;97:1925-8. [Crossref] [PubMed]
- Minarik J, Pavlicek P, Pour L, et al. Subcutaneous bortezomib in multiple myeloma patients induces similar therapeutic response rates as intravenous application but it does not reduce the incidence of peripheral neuropathy. PLoS One 2015;10:e0123866. [Crossref] [PubMed]
- Beksac M, Seval GC, Kanellias N, et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: analysis of parameters that improve outcome. Haematologica 2020;105:201-8. [Crossref] [PubMed]
- Billecke L, Murga Penas EM, May AM, et al. Cytogenetics of extramedullary manifestations in multiple myeloma. Br J Haematol 2013;161:87-94. [Crossref] [PubMed]
- Chisholm KM, Bangs CD, Bacchi CE, et al. Expression profiles of MYC protein and MYC gene rearrangement in lymphomas. Am J Surg Pathol 2015;39:294-303. [Crossref] [PubMed]
- Kiba T, Ito T, Nakashima T, et al. Bortezomib and dexamethasone for multiple myeloma: higher AST and LDH levels associated with a worse prognosis on overall survival. BMC Cancer 2014;14:462. [Crossref] [PubMed]
- Huang B, Lu J, Wang X, et al. Prognostic value of lactate dehydrogenase in Chinese patients with newly diagnosed transplant eligible multiple myeloma. Leuk Lymphoma 2017;58:1740-2. [Crossref] [PubMed]
- Bergsagel PL, Mateos MV, Gutierrez NC, et al. Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high-risk multiple myeloma. Blood 2013;121:884-92. [Crossref] [PubMed]
- Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J Clin Oncol 2010;28:4630-4. [Crossref] [PubMed]
- Meng S, Lu C, Zhang W, et al. MMSA-1 expression pattern in multiple myeloma and its clinical significance. Clin Exp Med 2016;16:599-609. [Crossref] [PubMed]
- Chim CS, Sim J, Tam S, et al. LDH is an adverse prognostic factor independent of ISS in transplant-eligible myeloma patients receiving bortezomib-based induction regimens. Eur J Haematol 2015;94:330-5. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


