
Comparison of the analgesic efficacy of periarticular infiltration and pericapsular nerve group block for total hip arthroplasty: a randomized, non-inferiority study
Introduction
As osteoarthritis of the hip joint has increased with the increased number of aging individuals in the global population and an increase in the average life expectancy, total hip arthroplasty (THA) surgery trials are increasing (1,2). Postoperative care, including early mobilization and pain control, is important for decreasing the mortality and morbidity of THA (3-6). However, there is no consensus on postoperative pain management due to a variety of innervation of sensory nerves and nociceptive fibers in the hip joints. A number of regional anesthesia administration modalities, such as periarticular infiltration (PAI), have become the mainstay of multimodal approaches used during THA. PAI involves administering analgesics into the tissue surrounding the surgical field and can act locally to reduce peripheral nociception with few systemic adverse effects. The method is simple, safe, and can be performed in the surgical field. Moreover, PAI has been effective for postoperative pain management and reduction of opioid consumption (7-10).
Both the femoral nerve block and the fascia iliaca block are effective in postoperative pain management in THA, but these include motor blocks, which cause the risk of postoperative fall and the limitation of early mobilization (11,12). The pericapsular nerve group (PENG) block has been recently introduced (13) and involves only the sensory nerves of the anterior hip capsule, such as articular branches of the femoral nerve and the accessory obturator nerve (ON), resulting in motor sparing effects. The PENG block has been reported as a postoperative pain control in THA, but prospective and randomized controlled trials are rare (14,15). However, the analgesic effects of PAI and PENG for THA have yet to be compared in clinical research. We hypothesized that the analgesia of PENG was non-inferior to that of PAI. We present the following article in accordance with the CONSORT reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-2785/rc).
Methods
This randomized, single-blind, non-inferiority trial was conducted at the Chungnam National University Hospital, Republic of Korea, from July 2020 to February 2021. The study protocol was approved by the Chungnam National University Hospital Institutional Review Board (IRB CNUH 2020-04-013) and all participants provided written informed consent. The study was registered with Clinical Research information Service (CRIS, https://cris.nih.go.kr, KCT0006049). We enrolled the patients aged 40–80 years with American Society of Anesthesiologists (ASA) physical status I~III scheduled for elective THA. Exclusion criteria included refusal to participate in this study, hypersensitivity or allergies to local anesthetics or morphine, and contraindications to neuraxial block. Study data were collected and managed using the Research Electronic Data Capture (REDCap) software hosted at Chungnam National University Hospital. REDCap is a secure web-based platform designed to support the capture of data for research studies. This study was conducted in accordance with the Consolidated Standards of Reporting Trial statement (16). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Participants were randomly assigned to one of two groups (PAI and PENG) using a computer-generated 2 and 4 block randomization in a 1:1 ratio. To conceal the allocation, the random table was uploaded to the REDCap software in our institution and was accessible only to the researcher who prepared the study drug. The outcome accessor was blinded group allocation.
Spinal anesthesia and perioperative analgesic technique
The patients received intramuscular 2 mg of midazolam as premedication 30 min before surgery. Spinal anesthesia was performed with standardized monitoring. Ten to 12 mg of 0.5% heavy bupivacaine with 100 µg of morphine titrated according to patient height and injected with a 25-gauge spinal needle at the L4-5 or L5-S1 level to achieve sensory block of the T8-10 dermatome.
The PENG block was performed with the patient in the supine position. A linear high-frequency ultrasound probe (HFL50xp: 15–6 MHz, X-Porte) was initially placed in a transverse plane over the anterior inferior iliac spine (AIIS); the probe was turned slightly medial until the hyperechoic continuous shadow of the iliopubic eminence (IPE) (13). The target was the plane between the psoas tendon and pubic ramus. A 22-gauge, 100 mm echogenic needle (SonoPlex cannulas, Pajunk®, Geisingen, Germany) was inserted in an in-plane approach to place the tip in the musculofascial plane between the psoas tendon anteriorly and the pubic ramus posteriorly. Following negative aspiration, 30 mL of the local anesthetic solution (0.5% ropivacaine) was injected in 5 mL increments while observing for adequate fluid spread in this plane.
Patients in the PAI group received intraoperative PAI with a mixture of ropivacaine, ketorolac, epinephrine, and normal saline. Ropivacaine [0.75% ropivacaine (20 mL)], ketorolac (60 mg), and epinephrine (1 g) were mixed with normal saline (total volume 100 mL) and divided into two 50-mL syringes. First, approximately 25 mL of the mixture was injected into the subcutaneous tissue and hip abductor muscles before skin incision through a 23-G spinal needle. Second, approximately 25 mL of the mixture was injected into the short external rotator muscles and posterior capsule before capsulotomy. Then, 5 mL of the mixture was injected into the acetabular fossa prior to insertion of the acetabular cup implants. The remaining mixture was injected into the anterior capsule and soft tissues before insertion of the femoral stem.
Surgical technique and postoperative management
Surgery was performed on all patients using a posterolateral approach to the hip in the lateral decubitus position. Acetabular preparation was performed after femoral neck resection, and the R3 Acetabular Cups and Anthology uncemented femoral stem (Smith & Nephew, Memphis, Tennessee, USA) were inserted in order. There were no surgical differences in either group, except for the injection of the mixture in PAI patients.
Postoperative supplemental analgesia was standardized as follows. The patients-controlled analgesia (PCA) devices were applied after sufficient explanation regarding how to use before surgery. PCA devices were set to administer a bolus dose of 20 µg without background infusion with a lockout time of 10 minutes. Total available dose of fentanyl was 1,500 µg and 0.6 mg of ramosetron was mixed in 100 mL of normal saline mixture. PCA was started at the end of the surgery. Furthermore, after surgery, research assistant nurses interview patients in ward and provided education related to the PCA and identified the analgesic effects and side effects of PCA. In part of the multimodal analgesia protocol, all patients were given postoperative oral painkillers (AstraZeneca PLC, VIMOVO® 500/20 mg, London, UK). In cases of high numeric rating scale (NRS) of pain (≥4), intramuscular analgesics (Menarini, Keral® 50 mg, Dublin, Ireland; Sanofi-ventis, Demerol® 25 mg, New York, USA) were used by patients on demand.
Outcomes
The primary outcome was the pain score 12 h after surgery at rest, and the prespecified non-inferiority was 1. Secondary outcomes included pain score during postoperative 24 hours, cumulative opioid consumptions by PCA device, quality of postoperative recovery score, and patient satisfaction. The pain score was assessed using the NRS (0, no pain; 10, worst pain), and nausea and vomiting were assessed by yes or no questions to participants. Further, the use of additional analgesics or the incidence of other adverse effects, such as sweating, dizziness, pruritus, urticaria, and tachycardia, was evaluated in the nursing records. Hypotension was defined as a systolic blood pressure less than 90 mmHg. The used PCA device was returned, and the log records were downloaded in a research computer for evaluation of usage time, bolus frequency, and PCA discontinuation. Patient satisfaction was measured using a Likert scale (1, very dissatisfied; 2, dissatisfied; 3, neutral; 4, satisfied; 5, very satisfied). The quality of postoperative recovery was evaluated using the validated Korean version of the Quality of Recovery-40 (QoR-40) questionnaire (17), which assesses five dimensions of recovery: physical comfort (12 items), emotional state (9 items), physical independence (5 items), psychological support (7 items), and pain (7 items). Each item was rated on a 5-point Likert scale: 1 (none of the time), 2 (some of the time), 3 (usually), 4 (most of the time), or 5 (all of the time). The total score ranged from 40 (poorest quality of recovery) to 200 (best quality of recovery). An assistant researcher administered the QoR-40 three times: the day before surgery and on postoperative day (POD) 1 and POD 2.
Statistical analysis
The sample size was calculated based on the primary outcomes according to non-inferiority hypothesis. In this study, prespecified non-inferiority margin was set to 1 of NRS pain score. Based on a previous study (18), a standard deviation of 1.2, was assumed for the NRS distribution of THA. With alpha =0.025 and power of 90%, the minimum number of patients required in each group was 23. Thus, 60 patients were recruited to allow for dropouts. One-sided non-inferiority testing was performed by comparing the 95% confidence interval (CI) of the mean difference of pain score to the predetermined non-inferiority margin.
The normality of continuous data was assessed using the Shapiro-Wilk test. Continuous variables were reported as the mean ± standard deviation and analyzed using independent sample t-tests or as median [interquartile range (IQR)] and analyzed by Mann-Whitney U tests, depending on the results of Shapiro-Wilk tests. Categorical variables were reported as number (%) and analyzed using χ2 or Fisher’s exact test (expected count <5). Statistical significance was set at a two-tailed P value of <0.05. All statistical analyses were performed using R software (version 4.0.3; R Project for Statistical Computing, Vienna, Austria).
Results
In total, 30 patients were allocated to the PAI group and 30 to the PENG group. Three patients in the PAI group and five patients in the PENG group excluded after group allocation due to postoperative delirium and incomplete response to pain outcome evaluation (Figure 1). There were no significant differences in the demographic and clinical characteristics (Table 1).
Table 1
| Variables | PAI (n=27) | PENG (n=25) |
|---|---|---|
| Age (years) | 63.0±11.7 | 60.0±10.9 |
| Sex (F/M) | 11/16 | 10/15 |
| ASA status (2/3) | 20/7 | 23/2 |
| Height (cm) | 160.5±10.1 | 161.8±9.3 |
| Weight (kg) | 62.0 (55.1; 71.5) | 63.0 (58.0; 71.0) |
| Operation side (right/left) | 16/11 | 16/9 |
| Operation time (min) | 98.0 (88.0; 111.5) | 99.0 (87.0; 105.0) |
| Diagnosis | ||
| AVN | 13 (48.1) | 9 (36.0) |
| Dysplastic hip | 0 (0.0) | 3 (12.0) |
| Fracture | 1 (3.7) | 2 (8.0) |
| OA | 13 (48.1) | 11 (44.0) |
Data are expressed as mean ± standard deviation, median (interquartile range), or number (%). PAI, periarticular infiltration; PENG, pericapsular nerve group; ASA, American Society of Anesthesiologists; AVN, avascular necrosis of femoral head; OA, osteoarthritis.
The pain score of postoperative 12 hour was 2.0 (IQR, 1.0; 4.0) in the PAI group and 3.0 (IQR, 1.0; 4.0) in the PENG group. The mean difference and 95% CI for pain score between the PENG and PAI groups was 0.6 (95% CI: −0.8 to 2.0). The upper limit of the 95% CI exceeded the non-inferiority margin of 1 at all time points (Figure 2). Our results do not show that PENG blocks are non-inferior to PAI in terms of postoperative analgesia for THA. The changes in NRS scores over time are shown in Table 2. NRS scores measured during postoperative 24 h showed no significant differences between the two groups. Additionally, the cumulative opioid consumption during the postoperative 48 hours showed no significant differences between the two groups.
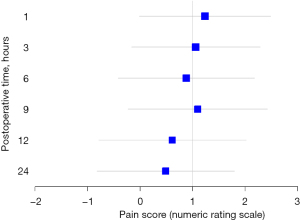
Table 2
| Variables | PAI (n=27) | PENG (n=25) | P |
|---|---|---|---|
| NRS | |||
| 1 h | 1.0 (0.0; 2.5) | 2.0 (1.0; 4.0) | 0.116 |
| 3 h | 1.0 (0.0; 3.0) | 3.0 (1.0; 3.0) | 0.152 |
| 6 h | 1.0 (0.0; 3.0) | 2.0 (1.0; 4.0) | 0.216 |
| 9 h | 2.0 (1.0; 3.0) | 3.0 (1.0; 5.0) | 0.156 |
| 12 h | 2.0 (1.0; 4.0) | 3.0 (1.0; 4.0) | 0.465 |
| 24 h | 2.0 (1.0; 3.5) | 2.0 (1.0; 5.0) | 0.683 |
| Cumulative opioid consumption | |||
| 6 h | 0.0 (0.0; 1.5) | 0.0 (0.0; 1.0) | 0.512 |
| 12 h | 3.0 (0.5; 4.0) | 1.0 (0.0; 4.0) | 0.450 |
| 24 h | 6.0 (3.5; 11.0) | 3.0 (1.0; 8.0) | 0.418 |
| 48 h | 12.0 (6.0; 17.0) | 9.0 (4.0; 22.0) | 0.819 |
Data are expressed as median (interquartile range). NRS, numeric rating scale; PAI, periarticular infiltration; PENG, pericapsular nerve group.
The frequency of adverse effects, such as postoperative nausea and vomiting, hypotension, and dizziness, showed no significant differences between the two groups. Additionally, additional analgesics, patient satisfaction scores, and QoR-40 scores for PODs did not show significant differences between the two groups (Tables 3,4).
Table 3
| Variables | PAI (n=27) | PENG (n=25) | P |
|---|---|---|---|
| Additional analgesics | |||
| Ketorolac | 7 (25.9) | 3 (12.0) | 0.296 |
| Meperidine | 1 (3.7) | 2 (8.0) | 0.603 |
| PONV | |||
| 24 h | 7 (25.9) | 3 (12.0) | 0.296 |
| 48 h | 0 (0.0) | 1 (4.0) | 0.481 |
| Hypotension | 1 (3.7) | 4 (16.0) | 0.183 |
| Dizziness | 2 (7.4) | 2 (8.0) | 1.000 |
| Pruritus | 0 (0.0) | 1 (4.0) | 0.481 |
| Urticaria | 0 (0.0) | 1 (4.0) | 0.481 |
| Tachycardia | 0 (0.0) | 0 (0.0) | NA |
| Sweating | 1 (3.7) | 0 (0.0) | 1.000 |
| Patient satisfaction score | 4.0 (4.0; 5.0) | 4.0 (3.0; 5.0) | 0.219 |
Data are expressed as median (interquartile range) or number (%). PAI, periarticular infiltration; PENG, pericapsular nerve group; NA, not applicable.
Table 4
| Variables | PAI (n=27) | PENG (n=25) | P |
|---|---|---|---|
| Preoperative | |||
| Physical comfort | 58.0 (53.0; 59.0) | 54.0 (51.0; 58.0) | 0.342 |
| Emotional state | 39.0 (35.5; 43.0) | 36.0 (33.0; 38.0) | 0.131 |
| Psychological support | 22.0 (19.5; 25.0) | 22.0 (21.0; 25.0) | 0.706 |
| Physical independence | 34.0 (29.5; 35.0) | 32.0 (29.0; 35.0) | 0.363 |
| Pain | 29.0 (27.0; 32.0) | 31.0 (29.0; 32.0) | 0.575 |
| Global QoR-40 | 177.0 (170.5; 191.0) | 174.0 (164.0; 182.0) | 0.355 |
| POD 1 | |||
| Physical comfort | 52.0 (48.0; 57.5) | 54.0 (51.0; 57.0) | 0.526 |
| Emotional state | 39.0 (34.5; 42.5) | 39.0 (35.0; 43.0) | 0.790 |
| Psychological support | 19.0 (16.5; 22.0) | 21.0 (17.0; 24.0) | 0.433 |
| Physical independence | 34.0 (31.0; 35.0) | 33.0 (28.0; 35.0) | 0.177 |
| Pain | 31.0 (26.0; 33.0) | 32.0 (30.0; 32.0) | 0.754 |
| Global QoR-40 | 175.0 (160.5; 183.0) | 174.0 (164.0; 190.0) | 0.728 |
| POD 2 | |||
| Physical comfort | 57.0 (52.5; 59.5) | 55.0 (53.0; 58.0) | 0.306 |
| Emotional state | 41.0 (36.0; 45.0) | 41.0 (38.0; 45.0) | 0.773 |
| Psychological support | 22.0 (19.0; 24.0) | 22.0 (19.0; 25.0) | 0.766 |
| Physical independence | 35.0 (33.5; 35.0) | 34.0 (26.0; 35.0) | 0.018 |
| Pain | 32.0 (30.5; 34.0) | 32.0 (29.0; 34.0) | 0.712 |
| Global QoR-40 | 183.0 (175.0; 191.0) | 181.0 (170.0; 189.0) | 0.436 |
POD, postoperative day; PAI, periarticular infiltration; PENG, pericapsular nerve group; QoR, quality of recovery.
Discussion
In this randomized trial, we compared the PAI and PENG blocks in patients undergoing THA. Our results do not show that PENG blocks are non-inferior to PAI in terms of postoperative analgesia for THA. Although our results suggest that both techniques were effective in postoperative analgesia for THA, coupled with the time consumption and cost effectiveness of the PENG block, contribute to recommending PAI as the preferred anesthetic technique for use during THA.
PAI is known for postoperative pain management and reduction in opioid consumption (7-10). PAI can be performed easily and quickly during surgery, with a theoretically low risk of injury to the nerves or blood vessels (18). Additionally, PAI may lead to early ambulation and reduced inflammation, resulting in a reduced risk of deep venous thrombosis. However, direct infiltration into the joint has the disadvantage of possible infection. Additional injections are not possible after surgery. The PENG block is a plane block technique that involves the injection of an anesthetic solution into the space between the iliopsoas muscle and IPE (13). It is a new regional anesthesia method based on blocking the articular branches of the femoral nerve and accessory ON in the region between the AIIS and IPE. Although the PENG block is known to be an effective hip surgery, little research has been conducted on the effective drug dosage, injection site, and volume of local anesthetics. Thus, there is no consensus on the most effective administration method using the PENG block.
The ON plays an important role in pain management during hip surgery (19). The ON is located close to the inferomedial acetabulum, which is far medial to the injection point of the PENG block. In the first introduction of PENG, it was not possible to comment on whether the local anesthetic would spread medially enough to reach the subpectineal plane between the pectineus and obturator externus muscles where the articular branches of the ON can be found. Recently, high-volume injectate (20 mL) may spread to the main trunk of the ON as the nerve courses along the lateral wall within the true pelvis through the intermuscular septum between the pectineus and psoas (20). Also, the dye includes the terminal articular branch of the inferomedial side of acetabulum (21). Moreover, several clinical reports have demonstrated the potential of the ON blocks in the case of a high-volume PENG block (22-24). We believe that 30 mL of our PENG block protocol sufficiently blocks the ON, both cephalad spreading to the proximal main trunk or distal articular branch in the inferomedial acetabulum.
The PENG block has been deemed as an effective regional technique for postoperative pain after hip surgery, targeting only the anterior capsule of the hip joint. Thus, it does not include pain originating from the posterior capsule of the hip joint. One of the differences between the two groups in our study was whether the innervation of the posterior capsule was blocked. In the posterior capsule, articular branches from the sciatic nerve and nerves to the quadratus femoris innervate the posteromedial section of the hip joint capsule. Additionally, articular branches of the superior gluteal nerve innervate the posterolateral section of the hip joint capsule (25). Conversely, mechanoreceptors of the posterior capsule are one- tenth of the anterior capsule, and there are no sensory fibers (26). The neural end organs of the hip include mechanoreceptors and free nerve endings, which play critical roles in joint proprioceptive and sensory functions. The anterior capsule is the most richly innervated section of the joint, suggesting that these nerves are the main targets for hip analgesia.
We hypothesized that PENG blocks would be non-inferior to PAI blocks based on the high volume of 30 mL injection and lower innervation of nociception in the posterior capsule. However, there was no statistically significant difference, and pain scores were slightly higher in the PENG blocks than in the PAI blocks. An explanation for why we did not obtain as many positive results as PAI blocks possibly lies in the regions of the incision covered by the two methods. The area of the posterolateral skin incision is innervated by the iliohypogastric, subcostal, and superior cluneal nerves (27,28). PAI includes the region of skin incision by subcutaneous injection, but PENG does not. It is important to manage postoperative pain originating from surgical trauma to the skin and soft tissues (29). In cases of hip fracture, femoral nerve block is an effective analgesic technique preoperatively, but it is not sufficient for postoperative pain management. Thus, postoperative pain may originate primarily from incisional trauma to cutaneous and subcutaneous tissues.
This study has several limitations. First, the sample size was small. Therefore, we did not determine the non-inferiority of the PENG block, but also cannot conclusively state that the PAI is superior. A well-designed study is needed to secure an appropriate number of samples so that the difference felt clinically can be statistically proven. Second, to evaluate whether the postoperative pain control method is effective, functional outcomes such as accelerated rehabilitation and length of hospital stay should be included as well as pain assessment, our study did not evaluate anything other than that related to postoperative pain and quality of recovery. Future study needed including functional outcomes. Third, although the outcome assessors were blinded to patient allocation, the patients were not. Although the placebo PENG block or PAI would have allowed double-blinding, it was not ethical to use a placebo injection. Considering a single nerve block is categorized into a high Serious Harm and Morbidity (SHAM) scale, the plane block or periarticular injection are considered to be similar scale (30). Fourth, the content of the local anesthesia mixture used for PENG differed from that of the PAI mixture. The PAI mixture is a standardized formulation containing ketorolac, epinephrine, and ropivacaine, whereas the local anesthetic for the PENG block in our study contained only ropivacaine.
Conclusions
Both PAI and PENG block provided analgesia following THA, with similar effectiveness as assessed using 10-point pain scores and opioid consumption. It is not conclusive that PENG is inferior or non-inferior to PAI based on our study.
Acknowledgments
Funding: This research was supported by the National Research Foundation of Korea (NRF-2019R1G1A1099660) and Project of Education Department of the Jilin province of China (JJKH20210583KJ).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-2785/rc
Trial Protocol: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-2785/tp
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-2785/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-2785/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Chungnam National University Hospital Institutional Review Board (IRB CNUH 2020-04-013) and all participants provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Singh JA. Epidemiology of knee and hip arthroplasty: a systematic review. Open Orthop J 2011;5:80-5. [Crossref] [PubMed]
- Sloan M, Premkumar A, Sheth NP. Projected Volume of Primary Total Joint Arthroplasty in the U.S., 2014 to 2030. J Bone Joint Surg Am 2018;100:1455-60. [Crossref] [PubMed]
- Guerra ML, Singh PJ, Taylor NF. Early mobilization of patients who have had a hip or knee joint replacement reduces length of stay in hospital: a systematic review. Clin Rehabil 2015;29:844-54. [Crossref] [PubMed]
- Okamoto T, Ridley RJ, Edmondston SJ, et al. Day-of-Surgery Mobilization Reduces the Length of Stay After Elective Hip Arthroplasty. J Arthroplasty 2016;31:2227-30. [Crossref] [PubMed]
- Baer M, Neuhaus V, Pape HC, et al. Influence of mobilization and weight bearing on in-hospital outcome in geriatric patients with hip fractures. SICOT J 2019;5:4. [Crossref] [PubMed]
- Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty 2007;22:12-5. [Crossref] [PubMed]
- Ma HH, Chou TA, Tsai SW, et al. The efficacy of intraoperative periarticular injection in Total hip arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord 2019;20:269. [Crossref] [PubMed]
- Jiang J, Teng Y, Fan Z, et al. The efficacy of periarticular multimodal drug injection for postoperative pain management in total knee or hip arthroplasty. J Arthroplasty 2013;28:1882-7. [Crossref] [PubMed]
- Johnson RL, Amundson AW, Abdel MP, et al. Continuous Posterior Lumbar Plexus Nerve Block Versus Periarticular Injection with Ropivacaine or Liposomal Bupivacaine for Total Hip Arthroplasty: A Three-Arm Randomized Clinical Trial. J Bone Joint Surg Am 2017;99:1836-45. [Crossref] [PubMed]
- Pandazi A, Kanellopoulos I, Kalimeris K, et al. Periarticular infiltration for pain relief after total hip arthroplasty: a comparison with epidural and PCA analgesia. Arch Orthop Trauma Surg 2013;133:1607-12. [Crossref] [PubMed]
- Gasanova I, Alexander JC, Estrera K, et al. Ultrasound-guided suprainguinal fascia iliaca compartment block versus periarticular infiltration for pain management after total hip arthroplasty: a randomized controlled trial. Reg Anesth Pain Med 2019;44:206-11. [Crossref] [PubMed]
- Hunt KJ, Bourne MH, Mariani EM. Single-injection femoral and sciatic nerve blocks for pain control after total knee arthroplasty. J Arthroplasty 2009;24:533-8. [Crossref] [PubMed]
- Girón-Arango L, Peng PWH, Chin KJ, et al. Pericapsular Nerve Group (PENG) Block for Hip Fracture. Reg Anesth Pain Med 2018;43:859-63. [Crossref] [PubMed]
- Remily EA, Hochstein SR, Wilkie WA, et al. The pericapsular nerve group block: a step towards outpatient total hip arthroplasty? Hip Int 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Kukreja P, Avila A, Northern T, et al. A Retrospective Case Series of Pericapsular Nerve Group (PENG) Block for Primary Versus Revision Total Hip Arthroplasty Analgesia. Cureus 2020;12:e8200. [Crossref] [PubMed]
- Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [Crossref] [PubMed]
- Lee JH, Kim D, Seo D, et al. Validity and reliability of the Korean version of the Quality of Recovery-40 questionnaire. Korean J Anesthesiol 2018;71:467-75. [Crossref] [PubMed]
- Choi JS, Kim JH, Gwak HC, et al. Pain Control after Total Hip Replacement Arthroplasty Using Periarticular Multimodal Drug Injection. J Korean Hip Soc 2010;22:273-82. [Crossref]
- Lee S, Hwang JM, Lee S, et al. Implementation of the Obturator Nerve Block into a Supra-Inguinal Fascia Iliaca Compartment Block Based Analgesia Protocol for Hip Arthroscopy: Retrospective Pre-Post Study. Medicina (Kaunas) 2020;56:150. [Crossref] [PubMed]
- Tran J, Agur A, Peng P. Is pericapsular nerve group (PENG) block a true pericapsular block? Reg Anesth Pain Med 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Yamak Altinpulluk E, Galluccio F, Salazar C, et al. Peng block in prosthetic hip replacement: A cadaveric radiological evaluation. J Clin Anesth 2020;65:109888. [Crossref] [PubMed]
- Ahiskalioglu A, Aydin ME, Ahiskalioglu EO, et al. Pericapsular nerve group (PENG) block for surgical anesthesia of medial thigh. J Clin Anesth 2020;59:42-3. [Crossref] [PubMed]
- Ahiskalioglu A, Aydin ME, Celik M, et al. Can high volume pericapsular nerve group (PENG) block act as a lumbar plexus block? J Clin Anesth 2020;61:109650. [Crossref] [PubMed]
- Aydin ME, Borulu F, Ates I, et al. A Novel Indication of Pericapsular Nerve Group (PENG) Block: Surgical Anesthesia for Vein Ligation and Stripping. J Cardiothorac Vasc Anesth 2020;34:843-5. [Crossref] [PubMed]
- Birnbaum K, Prescher A, Hessler S, et al. The sensory innervation of the hip joint--an anatomical study. Surg Radiol Anat 1997;19:371-5. [Crossref] [PubMed]
- Gerhardt M, Johnson K, Atkinson R, et al. Characterisation and classification of the neural anatomy in the human hip joint. Hip Int 2012;22:75-81. [Crossref] [PubMed]
- Nielsen TD, Moriggl B, Barckman J, et al. Cutaneous anaesthesia of hip surgery incisions with iliohypogastric and subcostal nerve blockade: A randomised trial. Acta Anaesthesiol Scand 2019;63:101-10. [Crossref] [PubMed]
- Nielsen TD, Moriggl B, Barckman J, et al. Randomized trial of ultrasound-guided superior cluneal nerve block. Reg Anesth Pain Med 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Lee SM, Yun DJ, Lee SH, et al. Continuous wound infiltration of ropivacaine for reducing of postoperative pain after anterior lumbar fusion surgery: a clinical retrospective comparative study. Korean J Pain 2021;34:193-200. [Crossref] [PubMed]
- Jarman J, Marks N, Fahy CJ, et al. Anaesthetists' risk assessment of placebo nerve block studies using the SHAM (Serious Harm and Morbidity) scale. Anaesthesia 2012;67:361-6. [Crossref] [PubMed]



