
Combining ultrasound with bio-indicators reveals progression of carotid stenosis
Introduction
Stroke is the number one cause of disease burden in China, with the highest disability and mortality rates (1), and carotid artery plaque (CAP) is one of the primary causes of stroke. As the degree of carotid artery stenosis (CAS) increases, the risk of ipsilateral ischemic events increases (2). CAS is the leading cause and risk factor for ischemic cerebrovascular disease. The most common location of CAS is the carotid bifurcation, followed by the common carotid artery, internal carotid siphon, middle cerebral artery, and cerebral artery (3). Previous studies have shown that >60% of strokes are caused by carotid stenosis, and these events may lead to disability or even death (4). The Society of Vascular Surgery recommends that carotid endarterectomy should be performed to reduce the risk of stroke for patients with symptomatic CAS if noninvasive imaging determines that stenosis is >70% (Grade I/A) or if angiography determines that stenosis is >50% (Grade I/B) (5). Therefore, accurate assessment of the extent of CAS is essential to prevent stroke (6), and the elucidation of the mechanisms underlying CAS progression is urgently needed.
Digital subtraction angiography (DSA) is generally considered the gold standard for CAS diagnosis. However, the rate of stroke caused by invasive DSA surgery is 0.5–1% (7-9). With the development of noninvasive magnetic resonance and other imaging technology, the diagnostic value of magnetic resonance angiography (MRA), carotid double ultrasound (CDU), and computed tomography angiography (CTA) in CAS has become a highly debated topic. In the diagnosis of CAS, the results of these noninvasive methods have been consistent with those of DSA; therefore, MRA and CDU, as noninvasive screening methods, may be a suitable alternative to DSA. Compared to MRA, CTA, and DSA, CDU is a noninvasive, portable, reliable, low-cost method for examining plaques. Of these noninvasive tests, CDU is a more portable, reliable, and low-cost plaque detection method than MRA or CTA. But accuracy is the biggest limitation of CDU, which is related to the doctor’s experience. CDU can not only detect the degree of carotid stenosis and the plaque area but can also detect the nature of CAP and has become the preferred method for the noninvasive longitudinal evaluation of CAS (10). A preliminary judgment of the nature of the plaque can provide recommendations for subsequent treatment.
High-sensitivity C-reactive protein (hs-CRP) is known as an acute non-specific biomarker of inflammation (11), widely accepted as a predictive factor for risk of cardiovascular diseases (12). Most studies have focused on plaque formation or the severity of plaques and stenosis (13,14) because these factors are associated with cardiovascular and cerebrovascular events. It has been reported that the enhancement degree on is associated with hs-CRP levels. Here, we set out from another perspective to reveal the relationship between hs-CRP level and progression of CAS (15). In the present study, we aimed to retrospectively analyze and determine the predictive ultrasound features and hs-CRP and other clinical factors associated with CAS progression in a cohort study and investigate the relationship between CAS progression and cerebrovascular events. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-2666).
Methods
Patients
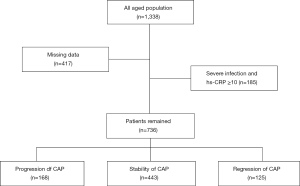
This retrospective study was approved by the institutional review committee of Ruijin Hospital of Shanghai Jiaotong University School of Medicine (Ruijin LL-14-2006). We reviewed the Ruijin Hospital database records from 2009 to 2019 and identified a total of 1,338 patients (a collection of inpatients and outpatients) who had undergone two or more CDUs (Figure 1). Patients with missing data, severe infection, or hs-CRP ≥10 mg/L were excluded from the study, resulting in a final number of 736 patients included in the study. The classification of plaque stenosis was as follows: grade 1: 0–50% (including 50%), grade 2: 50–70%, and grade 3: >70% (including 70%), or patients who had received carotid stent surgery. We divided the population into three categories: the progression group, the stability group, and the regression group (Figure 2), defined as follows: for the progression group, the stenosis grade of the carotid artery assessed by the most recent CDU was greater than that assessed in the previous one; for the stable group, the stenosis grade was maintained at the same level in two or more CDU assessments; and for the regression group, the most recent stenosis grade was lower than the previous stenosis grade assessment of the carotid artery. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). As a retrospective study, it did not involve patients’ privacy, and the written consent of patients was waived by Ruijin Ethical Committee.
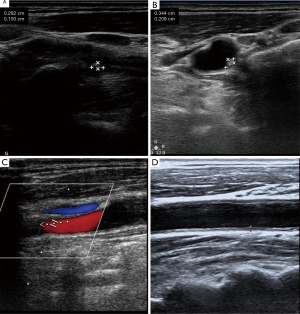
Assessment of CAP
As part of the assessment process, stenosis, intima-media thickness (IMT), ulcer plaque, and echo characteristics were recorded at an average of 18 months of follow-up. The IMT of the carotid arteries was examined bilaterally using a Doppler ultrasound (HD11EX Ultrasound; Philips Medical Systems, Andover, MA, USA). IMT was measured on both the left and right carotid artery starting ~1.5 cm proximal to the carotid artery bulb. We took three recordings and calculated the mean value for each side. The affected side was taken as the standard. The degree of plaque stenosis was graded according to three levels: grade 1: 0–50% (including 50%), grade 2: 50–70%, and grade 3: >70% (including 70%), or patients who had received carotid stent surgery. The echo characteristics of the lesion detected by two-dimensional ultrasound were classified into five types: (I) uniformly echolucent, (II) predominantly echolucent, (III) predominantly echogenic, (IV) uniformly echogenic, and (V) consisted of plaques that could not be classified due to heavy calcification and acoustic shadows. In addition, the degree of CAS was diagnosed according to the widely accepted criteria published in 2003 by the expert panel of the Society of Radiologists in Ultrasound.
Measurement of biochemical parameters
After the subjects were fasted for 6 hours, a venous blood sample was taken in the morning and infused into a vacuum tube containing ethylenediaminetetraacetic acid. The serum concentrations of high sensitivity C-reactive protein (hs-CRP) were determined by particle-enhanced immunonephelometry using N high-sensitivity CRP reagent (Dade Behring Inc., Marburg, Germany). The lower limit of detection was 0.01 mg/L. All participants were further divided into two groups according to the hs-CRP baseline: low risk (<3.0 mg/L) and high risk (≥3.0 mg/L) (16). This article only discusses the effects of high-risk hs-CRP on the disease. High-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol were measured by enzyme-linked immunosorbent assays on a Hitachi 912 analyzer (Roche Diagnostics, Germany).
Measurement of height, weight, and blood pressure
Weight and height were measured at baseline, and body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). Subjects had their blood pressure measured twice with an automatic sphygmomanometer over a period of 10 minutes. The average value of the two measurements was recorded for further analysis.
Statistical analysis
Based on an average 18-month follow-up, the patients were allocated to the following groups: progression, stability, and regression. The demographic factors (sex, age), atherosclerosis-affecting factors [high-risk hs-CRP, coronary artery disease, diabetes, hypertension, statin use and hyperlipidemia (HPL)], plaque characteristics (hypoechoic plaque and ulcerative plaque), and stroke/transient ischemic attacks (TIA) were compared by a chi-square test for categorical variables among the three groups. Comparisons of IMT, HDL-C, LDL-C, and BMI from baseline to follow-up in the three groups of patients were performed by repeated-measures analysis of variance (ANOVA). Following univariate analysis, variables (including sex, high-risk hs-CRP, hypoechoic plaque, hyperlipidemia, and ulcerative plaque) with a P value <0.05 were included in the ordinal regression model to determine the independent predictors of stenosis progression. A P value <0.05 was considered statistically significant. In this study, we used a binary logistic regression model to study the cross-sectional correlation between carotid plaque build-up and stroke/TIA in the group samples. We adjusted for potential confounders in different models: model 1, adjusted for sex; model 2, adjusted for sex, high-risk hs-CRP, hypoechoic plaque, hyperlipidemia, and ulcerative plaque. P<0.05 was considered statistically significant. Statistical analysis was conducted by SPSS Version 22.0 (IBM Corporation, USA).
Results
Basic patient characteristics
Among the 1,338 eligible patients, we excluded 417 patients with missing data, and 185 patients with severe infection or hs-CRP ≥10 mg/L. We analyzed the data of the remaining 736 patients by groups. Their average follow-up period is 18 months. Based on the progression of carotid artery stenosis, we divided these 738 patients into three groups: regression, stable and progression (Figure 1). The average age of the 736 included patients was 64.91±9.66 years, and 73.2% of the patients were male. Except for high-risk hs-CRP (P<0.01) and hyperlipidemia (P=0.05), there were no significant differences in other carotid risk factors, such as hypertension, coronary heart disease (CHD), diabetes mellitus (DM), HDL-C, LDL-C, and BMI (Table 1).
Table 1
| Parameters | All patients (n=736) | Regression (n=125) | Stable (n=443) | Progression (n=168) | P |
|---|---|---|---|---|---|
| Age, years | 65.0±7.0 | 65.0±1.0 | 64.6±4.8 | 66.4±6.8 | 0.07 |
| Male | 542 (73%) | 94 (75%) | 317 (72%) | 131 (78%) | 0.04 |
| LDL-C, mmol/L | 2.4±0.9 | 2.4±1.0 | 2.4±0.9 | 2.5±0.9 | 0.95 |
| HDL-C, mmol/L | 1.1±0.3 | 1.1±0.3 | 1.1±0.3 | 1.1±0.3 | 0.56 |
| IMT, mm | 0.8±0.1 | 0.8±0.1 | 0.8±0.1 | 0.8±0.2 | 0.09 |
| BMI, kg/m2 | 25±3.1 | 25±3.3 | 25±2.9 | 25±3.5 | 0.68 |
| High risk hs-CRP | 111 (15.0%) | 12 (9.6%) | 64 (14.4%) | 35 (20.8%) | <0.01 |
| DM | 314 (42.6%) | 55 (44.0%) | 188 (42.4%) | 71 (42.3%) | 0.94 |
| HTN | 632 (85.8%) | 109 (87.2%) | 373 (84.2%) | 150 (89.3%) | 0.50 |
| CHD | 637 (86.5%) | 113 (90.4%) | 376 (84.9%) | 148 (88.1%) | 0.06 |
| HLP | 269 (36.5%) | 45 (36.3%) | 162 (36.6%) | 62 (36.8%) | 0.05 |
| Statin use | 715 (97.1%) | 122 (97.6%) | 430 (97.1%) | 163 (97%) | 0.86 |
| Hypoechoic plaque | 303 (41.1%) | 40 (30.2%) | 182 (41%) | 81 (48.2%) | <0.01 |
| Ulcerative plaque | 115 (15.6%) | 20 (16.0%) | 59 (13.3%) | 36 (21.4%) | 0.02 |
| Stroke/TIA | 200 (27.1%) | 26 (20.8%) | 116 (26.1%) | 58 (34.5%) | <0.01 |
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; IMT, intima-media thickness; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; DM, diabetes mellitus; HTN, hypertension; CHD, coronary heart disease; HLP, hyperlipidemia; TIA, transient ischemic attack.
The use of statin lipid-lowering drugs was almost 100% across the three groups, and thus, there was no difference in statin use. However, the degree of hypoechoic plaque (P<0.01) and ulcerative plaque (P=0.02) was significantly different in the progression group compared with the other two groups (Table 1).
During follow-up, 20 patients displayed increased carotid stenosis and subsequently received carotid stenting.
Independent Factors associated with plaque progression
In the ordinal logistic regression analysis, high-risk hs-CRP (OR, 1.75; 95% CI: 1.17–2.61; P<0.01) and hypoechoic plaque (OR, 1.53; 95% CI: 1.14–2.05; P<0.01) were found to be independent risk factors for CAS progression (Table 2). Other factors, such as ulcerative plaque (OR, 1.43; 95% CI: 0.96–2.12; P=0.08), hyperlipidemia (HLP) (OR, 1.01; 95% CI: 0.74–1.34; P=0.98), and sex (OR, 0.88; 95% CI: 0.64–1.23; P=0.46) were not independent risk factors for CAS progression (Table 2).
Table 2
| Risk factor | B | OR | 95% CI | P |
|---|---|---|---|---|
| Hypoechoic plaque | 0.43 | 1.53 | 1.14–2.05 | <0.01 |
| High risk hs-CRP | 0.56 | 1.75 | 1.17–2.61 | <0.01 |
| Ulcerative plaque | 0.36 | 1.43 | 0.96–2.12 | 0.08 |
| HLP | 0.01 | 1.01 | 0.74–1.34 | 0.98 |
| Sex | −0.12 | 0.88 | 0.64–1.23 | 0.46 |
Ordinal regression analysis is used for the statistics. hs-CRP, high-sensitivity C-reactive protein; HLP, hyperlipidemia; CI, confidence interval.
Relationship between carotid progression and stroke/TIA
The number of patients with a prior history of TIA or stroke was significantly different among the groups (P<0.01) (Table 1). We used a logistic regression model to analyze the cross-sectional relationship between the progression of carotid stenosis and stroke/TIA. After adjusting for several potential confounders, the stoke/TIA adjusted odds ratio was 1.80 (95% CI: 1.03–3.13) in the progression group (Table 3). This result proves that the progression of plaque itself is also an independent factor associated with stroke/TIA.
Table 3
| Model | Regression | Stable | Progression | P |
|---|---|---|---|---|
| All | 125 | 443 | 168 | – |
| Stoke/TIA | 26 | 116 | 61 | – |
| Model 1 | Ref | 1.32 (0.81–2.15) | 1.86 (1.07–3.21) | 0.03 |
| Model 2 | Ref | 1.30 (0.79–2.13) | 1.80 (1.03–3.13) | 0.03 |
Model 1: adjusted for sex, age. Model 2: adjusted for sex, age, high risk hs-CRP, hyperlipidemia, ulcerative plaque, and hypoechoic plaque. TIA, transient ischemic attack; CAS, carotid artery stenosis.
Discussion
It is well known that plate vulnerability is a risk factor for future ipsilateral ischemic stroke or TIA. Plaque echolucency is a potential biomarker of plaque vulnerability, and its ultrasound performance is equivalent to a necrotic nucleus (17-19). In this study, hypoechoic plaque proved to be an independent risk factor for progression of CAS, consistent with previous studies.
Hs-CRP is an inflammatory protein synthesized in the liver tissues, and high-risk hs-CRP is an independent predictive factor for cardiovascular disease (20). However, the relationship between hs-CRP and plaque changes has not been previously investigated. In this study, we used ordinal regression to prove that high-risk hs-CRP is closely related to plaque progression. This factor is not only a predictor of plaque production (14) but also a predictor of plaque progression.
Statins are one of the primary drugs for carotid atherosclerosis treatment. In addition to their lipid-lowering effects, these drugs can also improve vascular endothelial function, inhibit inflammatory response, reduce endothelial cell lipid deposition, inhibit platelet aggregation, and stabilize plaques. Statins are an essential component of primary and secondary stroke prevention programs (21). More than 97% of patients in this study had been taking statins for an extended period of time, so the statin use may have reduced the extent of carotid stenosis (22-24). In addition to the statins mentioned, current interventions for CAS include 3-hydroxy-3-methylglutaryl-coenzyme reductase inhibitors, aggressive antihypertensive drugs to control blood pressure, strict blood glucose control in diabetics, smoking cessation, the use of antiplatelet drugs, or surgical treatment including carotid endarterectomy (CEA) and intracranial stenting (25).
In this longitudinal cohort study of CAS patients, we found that the difference in stroke and TIA between the groups was significant, and logistic regression demonstrated that plaque progression had a significant effect on stroke and TIA. Therefore, we concluded that CAS progression is also a predictor of cerebrovascular events, such as stroke and TIA (23,26,27).
Both high-risk hs-CRP and hypoechoic plaque were independent risk factors for the progression of carotid stenosis. Some studies have also shown that the concentration of hs-CRP is positively correlated with the formation of CAP (14); hypoechoic plaque is also an independent risk factor for the progression of carotid stenosis. An increase in the degree of carotid stenosis increases the occurrence of cerebrovascular events. Here, we hypothesized that the characteristics of the plaque itself have a higher impact on the disease than the biochemical indicators. Therefore, our main research direction in the future is to ascertain how to effectively convert hypoechoic plaques into hyperechoic plaques and convert unstable plaques into stable plaques. Studies have been performed from the perspective of platelets and macrophages, changing the chemokines and their receptors that cause interactions (28), thereby changing the composition of plaques or converting macrophage types from M1 to M2. These chemokines can also act on circulating smooth muscle progenitor cells (28,29) and have a beneficial effect on plaque stability. Endothelial progenitor cells (30,31) are important for plaque stabilization, whether they are progenitor cells themselves or exosomes secreted by cells. Studies have also shown that the main features of carotid plaques are highly heritable (32), and the problem of plaques may be solved from a genetic perspective in the future.
In our study, males were overrepresented (73% vs. 27%), which could be due to several reasons. First, in China, male smoking rates are much higher than those of females, which may explain the higher rate of extracranial stenosis in men (33). Second, the incidence of male stroke is significantly higher in all age groups in China than in females (34). Finally, Ruijin Hospital is a comprehensive tertiary medical center that accepts patients nationwide. Our analysis of patients’ demographic profiles (both inpatient and outpatient service) from 2009 to 2019 revealed that more males than females were treated in our neurosurgery department, which may be due to referral patterns and local demographics.
There are some limitations to our study. First, this is essentially a retrospective cohort study and future prospective studies are needed to confirm our results. Second, during follow-up, we did not provide more detailed information on vascular risk factors (like hypertension, diabetes, or hyperlipidemia).
Acknowledgments
Funding: This work was supported by funding from the SJTU National Infrastructures for Translational Medicine (Shanghai) Research Fund (No. TMSK-2020-112), National Natural Science Foundation of China (NSFC) [82101532 (BC)] and the Science and Technology Commission of Shanghai Municipality # 201409003100 (BC).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-2666
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-2666
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-2666). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the institutional review committee of Ruijin Hospital of Shanghai Jiaotong University School of Medicine (Ruijin LL-14-2006). As a retrospective study, it did not involve patients’ privacy, and the written consent of patients was waived by Ruijin Ethical Committee.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- GBD 2016 Lifetime Risk of Stroke Collaborators; Feigin VL, Nguyen G, et al. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N Engl J Med 2018;379:2429-37.
- Paraskevas KI. Prevention and treatment of strokes associated with carotid artery stenosis: a research priority. Ann Transl Med 2020;8:1260. [Crossref] [PubMed]
- Dix JE, Evans AJ, Kallmes DF, et al. Accuracy and precision of CT angiography in a model of carotid artery bifurcation stenosis. AJNR Am J Neuroradiol 1997;18:409-15. [PubMed]
- Flaherty ML, Kissela B, Khoury JC, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology 2013;40:36-41. [Crossref] [PubMed]
- Ricotta JJ, Aburahma A, Ascher E, et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg 2011;54:e1-31. [Crossref] [PubMed]
- Debrey SM, Yu H, Lynch JK, et al. Diagnostic accuracy of magnetic resonance angiography for internal carotid artery disease: a systematic review and meta-analysis. Stroke 2008;39:2237-48. [Crossref] [PubMed]
- Elias RM, Wald JT, Kallmes DF. Diagnosis of carotid artery stenosis with oculopneumoplethysmography alone and in combination with MRA. Vasc Health Risk Manag 2012;8:631-9. [Crossref] [PubMed]
- Zhao H, Wang J, Liu X, et al. Assessment of carotid artery atherosclerotic disease by using three-dimensional fast black-blood MR imaging: comparison with DSA. Radiology 2015;274:508-16. [Crossref] [PubMed]
- Guédon A, Clarençon F, Di Maria F, et al. Very late ischemic complications in flow-diverter stents: a retrospective analysis of a single-center series. J Neurosurg 2016;125:929-35. [Crossref] [PubMed]
- Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: grayscale and Doppler ultrasound diagnosis--Society of Radiologists in Ultrasound consensus conference. Ultrasound Q 2003;19:190-8. [Crossref] [PubMed]
- Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol 2004;29:439-93. [PubMed]
- Fonseca FA, Izar MC. High-Sensitivity C-Reactive Protein and Cardiovascular Disease Across Countries and Ethnicities. Clinics (Sao Paulo) 2016;71:235-42. [Crossref] [PubMed]
- Gao S, Zhao D, Qi Y, et al. Circulating Oxidized Low-Density Lipoprotein Levels Independently Predict 10-Year Progression of Subclinical Carotid Atherosclerosis: A Community-Based Cohort Study. J Atheroscler Thromb 2018;25:1032-43. [Crossref] [PubMed]
- Xu R, Zhang Y, Gao X, et al. High-Sensitivity CRP (C-Reactive Protein) Is Associated With Incident Carotid Artery Plaque in Chinese Aged Adults. Stroke 2019;50:1655-60. [Crossref] [PubMed]
- Xiong L, Sun WJ, Cai HY, et al. Correlation of enhancement degree on contrast-enhanced ultrasound with histopathology of carotid plaques and serum high sensitive C-reactive protein levels in patients undergoing carotid endarterectomy. J Huazhong Univ Sci Technolog Med Sci 2017;37:425-8. [Crossref] [PubMed]
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499-511. [Crossref] [PubMed]
- Kakkos SK, Griffin MB, Nicolaides AN, et al. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg 2013;57:609-618.e1; discussion 617-8. [Crossref] [PubMed]
- Russell DA, Wijeyaratne SM, Gough MJ. Relationship of carotid plaque echomorphology to presenting symptom. Eur J Vasc Endovasc Surg 2010;39:134-8. [Crossref] [PubMed]
- Giannakopoulos TG, Moulakakis K, Sfyroeras GS, et al. Association between plaque echogenicity and embolic material captured in filter during protected carotid angioplasty and stenting. Eur J Vasc Endovasc Surg 2012;43:627-31. [Crossref] [PubMed]
- Wang A, Tian X, Wu S, et al. Metabolic Factors Mediate the Association Between Serum Uric Acid to Serum Creatinine Ratio and Cardiovascular Disease. J Am Heart Assoc 2021;e023054: [Crossref] [PubMed]
- Artom N, Montecucco F, Dallegri F, et al. Carotid atherosclerotic plaque stenosis: the stabilizing role of statins. Eur J Clin Invest 2014;44:1122-34. [Crossref] [PubMed]
- Amarenco P, Bogousslavsky J, Callahan A 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549-59. [Crossref] [PubMed]
- Du R, Zhao XQ, Cai J, et al. Changes in carotid plaque tissue composition in subjects who continued and discontinued statin therapy. J Clin Lipidol 2016;10:587-93. [Crossref] [PubMed]
- Shanmugam N, Román-Rego A, Ong P, et al. Atherosclerotic plaque regression: fact or fiction? Cardiovasc Drugs Ther 2010;24:311-7. [Crossref] [PubMed]
- Gokaldas R, Singh M, Lal S, et al. Carotid stenosis: from diagnosis to management, where do we stand? Curr Atheroscler Rep 2015;17:480. [Crossref] [PubMed]
- Sabeti S, Schlager O, Exner M, et al. Progression of carotid stenosis detected by duplex ultrasonography predicts adverse outcomes in cardiovascular high-risk patients. Stroke 2007;38:2887-94. [Crossref] [PubMed]
- Conrad MF, Boulom V, Mukhopadhyay S, et al. Progression of asymptomatic carotid stenosis despite optimal medical therapy. J Vasc Surg 2013;58:128-35.e1. [Crossref] [PubMed]
- Akhtar S, Gremse F, Kiessling F, et al. CXCL12 promotes the stabilization of atherosclerotic lesions mediated by smooth muscle progenitor cells in Apoe-deficient mice. Arterioscler Thromb Vasc Biol 2013;33:679-86. [Crossref] [PubMed]
- Döring Y, Noels H, van der Vorst EPC, et al. Vascular CXCR4 Limits Atherosclerosis by Maintaining Arterial Integrity: Evidence From Mouse and Human Studies. Circulation 2017;136:388-403. [Crossref] [PubMed]
- Li X, Chen C, Wei L, et al. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy 2016;18:253-62. [Crossref] [PubMed]
- Maki T, Morancho A, Martinez-San Segundo P, et al. Endothelial Progenitor Cell Secretome and Oligovascular Repair in a Mouse Model of Prolonged Cerebral Hypoperfusion. Stroke 2018;49:1003-10. [Crossref] [PubMed]
- Tarnoki AD, Baracchini C, Tarnoki DL, et al. Evidence for a strong genetic influence on carotid plaque characteristics: an international twin study. Stroke 2012;43:3168-72. [Crossref] [PubMed]
- Jiang B, Wang WZ, Chen H, et al. Incidence and trends of stroke and its subtypes in China: results from three large cities. Stroke 2006;37:63-8. [Crossref] [PubMed]
- Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke 2009;40:1082-90. [Crossref] [PubMed]


