
Predicting infected pancreatic necrosis based on influential factors among the most common types of acute pancreatitis: a retrospective cohort study
Introduction
Acute pancreatitis (AP) is one of the common causes of acute abdominal disease and is an indication for hospitalization. Approximately 10% of all cases have persistent organ dysfunction, referred to as severe AP (SAP), which can be a life-threatening condition (1). Unlike western countries (2), hypertriglyceridemic AP (HTGAP) has become the second most common type of AP after biliary AP (BAP) in China (3-5). A recent multicenter study in Beijing collected 2,461 cases over 5 years, and the number of inpatients was shown to increase annually. The most common etiologies by percentage were gallstones (55.75%), hypertriglyceridemia (10.36%), and alcohol (10%) (6). Same trend is reflected in many epidemiological studies (7). In addition to the different causes of BAP and HTGAP, there are significant differences in internal mechanism, population characteristics, severe cases proportion, treatment and prognosis (8).
Two peaks of mortality lie in the course of SAP. Systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) are the major clinical manifestations in the early phase. Treatment focuses on providing intensive care, ensuring a stable internal environment, and protecting organ function. The prognosis of AP patients has dramatically improved due to active and effective etiological management (9). In the late stage, pancreatic and peripancreatic necrosis may occur in combination with infection, sepsis, and deep fungal infection. This second peak of mortality is caused by what is called infected pancreatic necrosis (IPN) (10). The cornerstone of treatment is the control of infection and surgical management of local complications (11). IPN is a serious local complication of SAP with many influencing confounding factors. It needs to be carried out in high-volume center in order to ensure sufficient sample size (12). This study focuses on the in-depth analysis of the two most common etiological types in region, including baseline characteristics, scoring indicators, laboratory examination, imaging manifestation, bacterial spectra distribution and surgical outcomes, so that the evaluation is more objective and complete. The persistent infection or fever is mainly caused by bacteria and fungus. Studying the differences of bacterial spectra is helpful to explore the influencing factors of IPN and provide references for the use of antibiotics. Throughout multivariate regression analysis, we probe into high predictive value factors of IPN to guide subsequent treatment. We present the following article in accordance with the STARD reporting checklist (available at https://dx.doi.org/10.21037/apm-21-2933).
Methods
Patients
In this single-center retrospective study, we recruited 1,746 AP patients (≥18 years of age) admitted to Xuanwu Hospital of Capital Medical University in Beijing between 6 June 2014, and 22 September 2019. Patients with non-biliary and non-hypertriglyceridemic etiologies were excluded, including alcoholic (157 patients), traumatic (18 patients), and idiopathic (174 patients). Recurrent pancreatitis and readmission (248 patients) were also excluded. Based on the guidelines for AP (13), we established the diagnosis when 2 of the following 3 criteria were met: (I) pancreatic-type abdominal pain, (II) elevated serum amylase and/or lipase more than 3 times the upper limit of normal, and (III) imaging findings consistent with AP.
Clinical management protocol
We assigned the participants to either the BAP or HTGAP group and performed critical laboratory tests at admission and during hospitalization. The surgical approach consisted of percutaneous catheter drainage (PCD) followed, if necessary, by video-assisted retroperitoneal debridement (VARD). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Xuanwu Hospital (No. [2017]036). Individual consent for this retrospective analysis was waived.
The diagnosis of BAP needed to be confirmed by documenting gallbladder stones on any cross-sectional imaging, transient fluctuations in liver chemistry values >3× the upper limit of normal, or both. Alanine transaminase (ALT) is probably the single most reliable test, with a positive predictive value of 93% for a biliary etiology when elevated threefold (14,15).
The diagnosis of HTGAP was definitive when serum triglyceride levels were >1,000 mg/dL at clinical onset. The diagnosis remained probable if the increase in serum triglyceride level was between 500 and 1,000 mg/dL, together with emulsion plasma and without any other apparent etiologies (16).
At present, the definition of IPN was taken as the presence of gas within necrotic collections on contrast-enhanced computed tomography (CT). Under the circumstances of deteriorating clinical condition, shown by fever, sepsis, leukocytosis, and persistent illness, IPN may be indicated and require appropriate treatment (17). A positive culture result by PCD or surgery was not a necessary criterion for confirmation of IPN.
Once IPN was confirmed or suspected, we conducted image-guided PCD via the left flank to retroperitoneal peripancreatic necrosis. A step-up VARD surgery was performed if PCD alone did not resolve the IPN. Whenever possible, the intervention was postponed until 4 weeks after the onset of pancreatitis, in line with international guidelines. The tract created from the previously placed drain was used to access the retroperitoneal space, facilitating debridement with traditional laparoscopic instruments under direct visualization (18). Subsequent lavage and fistula control were made more practical by the drains left in the cavity. In general, we obtained specimens either by PCD or by surgery for bacterial sample collection. In clinical practice, the VARD procedure is especially suitable for the patients whose necrotic scope extends down to the left paracolic gutter (19).
Data collection and outcomes
The primary outcomes were the factors influencing IPN and correlations between IPN and AP etiologies. Major comorbidities were recorded in detail (e.g., cardiovascular, pulmonary, and renal complications, and diabetes). The collected data included age; gender; body mass index (BMI); CT severity index (CTSI); SIRS; Acute Physiology and Chronic Health Evaluation II (APACHE II) score; single or multiple organ failure; C-reactive protein (CRP) level; white blood cell count; nutrition; severity; local complications including acute peripancreatic fluid collection (APFC), acute necrotic collection (ANC), pancreatic pseudocyst (PP), walled-off necrosis (WON); bacterial culture results; and complications after VARD (e.g., bleeding, fistulas, ileus, portal venous thrombosis). The predefined secondary outcomes included the etiological distribution ratio, differences in the bacterial spectra, and the incidence of postoperative complications. Outpatient follow-ups took place at 3 and 6 months after discharge. Primary physicians were responsible for collecting data and completing the case-record forms.
Statistical analysis
All data were analyzed using the SAS statistical analysis package version 9.4 (SAS Inc., Cary, NC, USA). Quantitative variables with nonnormal distributions were presented as the medians [interquartile ranges (IQRs)], and those with normal distributions were presented as the mean ± standard deviation. For normally distributed data, the two groups were compared by t-tests, otherwise, the Mann-Whitney test was used. Categorical variables were presented as absolute numbers and percentages and analyzed using a chi-square test or Fisher’s exact test. A multivariate logistic analytic model (stepwise regression) was used to identify independent risk factors of IPN with odds ratios (ORs) and 95% confidence intervals (CIs). Furthermore, receiver operating characteristic (ROC) curves were generated for each of the qualified independent risk factors to assess the predictive ability of each indicator. Significance was set at P<0.05.
Results
Baseline characteristics and clinical features
Between 6 June 2014 and 22 September 2019, 1,746 patients with AP were screened, of whom 1,116 were eligible. The trial profile is shown in Figure 1. The etiological distribution is shown in Figure 2. All participants were divided into two groups according to etiology: BAP (n=919) and HTGAP (n=197). The baseline characteristics of the two groups are displayed in Table 1. The severity categories were mild AP (MAP), moderate-severe AP (MSAP), and SAP based on the modified Atlanta criteria. The HTGAP participants were younger (40 vs. 52 years, P<0.001), had a higher rate of SAP (51.8% vs. 32.0%, P<0.01), and had a higher prevalence of MODS (26.4% vs. 19.0%, P=0.020) than BAP participants. Additionally, HTGAP participants had significantly higher APACHE II scores (8 vs. 7, P<0.001) and CRP levels (111.0 vs. 78.0 mg/L, P<0.001) than BAP participants. The etiological distribution was 919 patients with BAP (52.6%) and 197 with HTGAP (11.3%), those with other etiologies were excluded. Once the AP diagnosis had been confirmed, standard medical treatment was initiated, including fluid resuscitation, enteral and parental nutrition, and vital organ protection. Etiological treatments for BAP included ERCP, cholecystectomy (CCY), and bile duct exploration (462 participants), and lipid-lowering therapies for HTGAP included strict dietary restriction, fibrates, insulin, unfractionated heparin, and apheresis (17 participants).
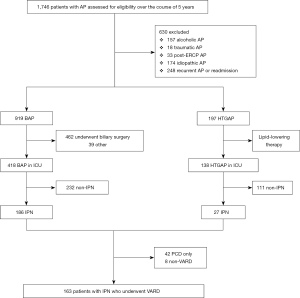
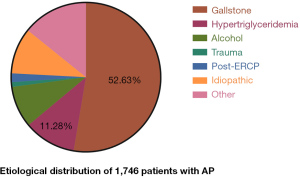
Table 1
| Characteristics | BAP (n=919) | HTGAP (n=197) | P value |
|---|---|---|---|
| Age (year), median [IQR] | 52.0 [39.0, 64.0] | 40.0 [33.0, 52.0] | <0.001 |
| Male gender, n (%) | 518 (56.4) | 154 (78.2) | <0.001 |
| BMI (kg/m2), mean ± SD | 25.3±2.6 | 27.7±2.3 | <0.001 |
| Comorbidities, n (%) | |||
| Cardiovascular disease | 257 (28.0) | 53 (26.9) | 0.763 |
| Pulmonary disease | 74 (8.1) | 16 (8.1) | 0.974 |
| Chronic renal insufficiency | 37 (4.0) | 6 (3.0) | 0.516 |
| Diabetes | 101 (11.0) | 24 (12.2) | 0.630 |
| CT severity index, median [IQR] | 5 [4, 6] | 6 [5, 7] | <0.001 |
| Extent of pancreatic necrosis >50%, n (%) | 239 (26.0) | 57 (28.9) | 0.398 |
| Disease severity, n (%) | |||
| SIRS | 836 (91.0) | 175 (88.8) | 0.351 |
| Admitted to the ICU | 418 (45.5) | 138 (70.1) | <0.001 |
| Single-organ failure | 294 (32.0) | 65 (33.0) | 0.784 |
| Multiple-organ failure | 175 (19.0) | 52 (26.4) | 0.020 |
| Positive blood culture | 248 (27.0) | 67 (34.0) | 0.047 |
| APACHE II score, median [IQR] | 7.00 [4.0, 9.0] | 8.0 [6.0, 10.0] | <0.001 |
| C-reactive protein (mg/L), median [IQR] | 78.0 [59.0, 87.0] | 111.0 [103.0, 130.0] | <0.001 |
| White blood cell count (×109/L), mean ± SD | 12.7±3.3 | 13.1±3.2 | 0.156 |
| Time since onset of symptoms (days), mean ± SD | 11.7±3.9 | 12.2±5.1 | 0.124 |
| Antibiotics treatment, n (%) | 634 (69.0) | 128 (65.0) | 0.272 |
| Nutrition support, n (%) | |||
| Enteral feeding only | 349 (38.0) | 79 (40.1) | 0.578 |
| Parental feeding only | 83 (9.0) | 18 (9.1) | 0.963 |
| Enteral and parental feeding | 202 (22.0) | 47 (23.9) | 0.566 |
| Oral diet | 165 (18.0) | 32 (16.2) | 0.568 |
| Severity (mild/moderate/severe), n (%) | 469 (51.0)/156 (17.0)/294 (32.0) | 55 (27.9)/40 (20.3)/102 (51.8) | <0.001 |
| Local complications, n (%) | |||
| APFC | 239 (26.0) | 61 (30.9) | 0.155 |
| ANC | 332 (36.1) | 83 (42.1) | 0.113 |
| PP | 86 (9.4) | 16 (8.1) | 0.585 |
| WON | 202 (22.0) | 37 (18.8) | 0.321 |
| IPN | 186 (20.2) | 27 (13.7) | 0.034 |
| Confirmed infected necrotic tissue, n (%) | 165 (18.0) | 20 (10.2) | <0.008 |
CT severity index scores range from 0 to 10, with higher scores indicating more extensive pancreatic necrosis and extrapancreatic collections. SIRS was defined according to the consensus-conference criteria of the American College of Chest Physicians and the Society of Critical Care Medicine. APACHE II scores range from 2 to 17, with higher scores indicating more severe disease. Organ failure was defined as a modified Marshall score ≥2 for the renal, respiratory, or cardiovascular system. ANC, acute necrotic collection; AP, acute pancreatitis; APACHE, Acute Physiology and Chronic Health Evaluation; APFC, acute peripancreatic fluid collection; BAP, biliary acute pancreatitis; BMI, body mass index; CT, computed tomography; HTGAP, hypertriglyceridemic acute pancreatitis; ICU, intensive care unit; IPN, infected pancreatic necrosis; IQR, interquartile range; PCD, percutaneous catheter drainage; PP, pancreatic pseudocyst; SD, standard deviation; SIRS, systemic inflammatory response syndrome; VARD, video-assisted retroperitoneal debridement; WON, walled-off necrosis.
The results revealed that IPN had occurred in 186 participants (20.2%) in the BAP group and in 27 participants (13.7%) in the HTGAP group (OR: 1.598, 95% CI: 1.027 to 2.451, P=0.034). We observed no significant differences in other local complications, including APFC (26.0% vs. 30.9%, P=0.155), ANC (36.1% vs. 42.1%, P=0.113), PP (9.4% vs. 8.1%, P=0.585), and WON (22.0% vs. 18.8%, P=0.321).
Influential factors of IPN
We screened many variables, including gender, age, BMI, disease severity, etiologies, APACHE II scores, MODS, CRP levels, and so on. Univariate logistic regression analysis was carried out using the occurrence of IPN as the endpoint, and variables identified as meaningful were entered into multivariate regression. The primary results are shown in Table 2. Independent risk factors identified by the multivariate logistic analytic model (stepwise regression) included etiologies (OR: 20.358, 95% CI: 9.255 to 44.779, P<0.001), CRP levels (OR: 1.009, 95% CI: 1.001 to 1.016, P=0.025), APACHE II scores (OR: 1.837, 95% CI: 1.660 to 2.032, P<0.001), and MODS (1 vs. 0: OR: 31.873, 95% CI: 15.185 to 66.899, P<0.001, and MODS 2 vs. 0: OR: 47.982, 95% CI: 17.749 to 129.707, P<0.001). Adjusted ORs for IPN are shown in Table 3. We calculated the areas under the ROC curves and concluded that APACHE II scores and MODS have the highest value for predicting IPN, with areas under the curve of 0.886 and 0.787, respectively (Figure 3). Among the 4 indicators, APACHE II scores had the highest sensitivity and specificity (Table 4).
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| APACHE II | 1.794 (1.652–1.949) | <0.001 | 1.837 (1.660–2.032) | <0.001 | |
| Etiology (Ref. = HTGAP) | 1.598 (1.032–2.473) | 0.036 | 20.358 (9.255–44.779) | <0.001 | |
| MODS | |||||
| 1 vs. 0 | 21.604 (11.547–40.421) | 0.227 | 31.873 (15.185–66.899) | <0.001 | |
| 2 vs. 0 | 54.467 (26.190–113.274) | 0.168 | 47.982 (17.749–129.707) | <0.001 | |
| CRP | 1.007 (1.002–1.011) | 0.002 | 1.009 (1.001–1.016) | 0.025 | |
1: single-organ failure; 2: multiple-organ failure. APACHE, Acute Physiology and Chronic Health Evaluation; CI, confidence interval; CRP, C-reactive protein; HTGAP, hypertriglyceridemic acute pancreatitis; IPN, infected pancreatic necrosis; MODS, multiple organ dysfunction syndrome; OR, odds ratio.
Table 3
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| APACHE II | 1.837 | 1.660–2.032 | <0.001 |
| MODS 0 vs. 1 | 31.873 | 15.185–66.899 | <0.001 |
| MODS 0 vs. 2 | 47.982 | 17.749–129.707 | <0.001 |
| Etiology 2 vs. 1 | 20.358 | 9.255–44.779 | <0.001 |
| CRP | 1.009 | 1.001–1.016 | 0.025 |
APACHE, Acute Physiology and Chronic Health Evaluation; CI, confidence interval; CRP, C-reactive protein; IPN, infected pancreatic necrosis; MODS, multiple organ dysfunction syndrome; OR, odds ratio.
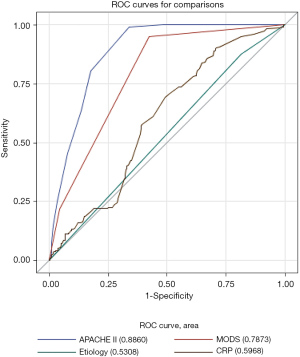
Table 4
| Variables | AUC area | 95% CI | P value | Cutpoint, IPN (“1”) | Youden index | |
|---|---|---|---|---|---|---|
| APACHE II | 0.886 | 0.867 | 0.905 | <0.001 | 8 (0.157) | 0.651 |
| MODS | 0.787 | 0.762 | 0.812 | <0.001 | 1 (0.256) | 0.525 |
| Etiology | 0.531 | 0.505 | 0.557 | 0.036 | 1 (0.182) | 0.197 |
| CRP | 0.597 | 0.559 | 0.635 | <0.001 | 80 (0.182) | 0.197 |
1: IPN occur. APACHE, Acute Physiology and Chronic Health Evaluation; AUC, area under the curve; CRP, C-reactive protein; IPN, infected pancreatic necrosis; MODS, multiple organ dysfunction syndrome; ROC, receiver operating characteristic.
Bacterial spectra
There were 158 pathogenic bacterial cultures among 213 surgeries, of which 138 (87.3%) were in the BAP group and 20 (12.7%) were in the HTGAP group. The most common bacterial strains were Escherichia coli (36 vs. 8, P=0.195), Klebsiella pneumonia (22 vs. 5, P=0.342), Pseudomonas aeruginosa (19 vs. 3, P=1.000), Acinetobacter baumannii (14 vs. 2, P=1.000), Enterococcus (14 vs. 1, P=1.000), and others (33 vs. 1, P=0.078). Drug resistance analysis showed that the gram-negative bacilli were susceptible to carbapenems. The resistance rates for all the antibiotic types in K. pneumonia exceeded 50%. The use of enzyme inhibitors significantly improved bacterial sensitivity to beta-lactams. The gram-positive bacteria were sensitive to vancomycin and tigecycline. No significant difference was shown in the bacterial spectrum between the two etiological groups, and the infections originated mostly from intestinal bacteria (Table 5).
Table 5
| Bacterial spectra | BAP (n=138), n (%) | HGAP (n=20), n (%) | P value |
|---|---|---|---|
| Escherichia coli | 36 (26.1) | 8 (40.0) | 0.195 |
| Klebsiella pneumonia | 22 (15.9) | 5 (25.0) | 0.342 |
| Pseudomonas aeruginosa | 19 (13.8) | 3 (15.0) | 1.000 |
| Acinetobacter baumannii | 14 (10.1) | 2 (10.0) | 1.000 |
| Enterococcus | 14 (10.1) | 1 (5.0) | 1.000 |
| Others | 33 (23.9) | 1 (5.0) | 0.078 |
BAP, biliary acute pancreatitis; HTGAP, hypertriglyceridemic acute pancreatitis.
Complications after surgery
The PCD was performed once IPN was confirmed or suspected. A total of 42 participants (19.7%) underwent PCD alone to alleviate their severe conditions, and VARD was not performed. Meanwhile, “one-step” VARD was performed in 13 participants who did not undergo PCD. In this study, more VARD surgeries (n=163) were carried out than endoscopic or open debridement, and the operation time was usually postponed until 4 weeks after onset (29.0 vs. 29.0 days, P=0.196). The most common systemic complications were bleeding (22.6% vs. 18.5%, P=0.634), enteral fistula (10.2% vs. 11.1%, P=1.000), pancreatic fistula (5.9% vs. 7.4%, P=1.000), ileus (4.3% vs. 3.7%, P=1.000), and portal venous thrombosis (1.1% vs. 3.7%, P=0.336). No difference was found in the median number of debridement procedures, although 6 surgeries were performed in 1 participant. Most non-surviving participants (n=5) had multiple organ failure (Table 6).
Table 6
| Operative outcomes (n=213) | BAP (n=186) | HTGAP (n=27) | P value |
|---|---|---|---|
| Surgery, n (%) | |||
| PCD | 186 (100.0) | 25 (92.6) | 0.016 |
| VARD | 143 (76.9) | 20 (74.1) | 0.748 |
| Debridements | 1 [1.3,1.5] | 1 [1.0,1.7] | 0.448 |
| Complications after surgery, n (%) | |||
| Bleeding | 42 (22.6) | 5 (18.5) | 0.634 |
| Enteral fistula | 19 (10.2) | 3 (11.1) | 1.000 |
| Pancreatic fistula | 11 (5.9) | 2 (7.4) | 1.000 |
| Intestinal obstruction | 8 (4.3) | 1 (3.7) | 1.000 |
| Portal venous thrombosis | 2 (1.1) | 1 (3.7) | 0.336 |
| Operation time since onset (days), median [IQR] | 29.0 [27.9, 29.4] | 29.0 [27.8, 30.5] | 0.196 |
| Mortality, n (%) | 5 (2.7) | 2 (7.4) | 0.487 |
BAP, biliary acute pancreatitis; HTGAP, hypertriglyceridemic acute pancreatitis; IQR, interquartile range; PCD, percutaneous catheter drainage; VARD, video-assisted retroperitoneal debridement.
Discussion
With a mortality rate up to 32%, IPN develops in 33% of patients with SAP (20,21). Therefore, early, accurate prediction of IPN is crucial in determining what interventions should be taken, preventing or delaying severe complications, and reducing mortality. We identified 4 independent risk factors throughout a multivariate logistic analytic model (stepwise regression), including etiologies, CRP levels, APACHE II scores, and MODS, that were relevant to IPN. Among these factors, APACHE II scores and MODS had the highest predictive value (ROC area 0.8860 and 0.7873, respectively).
The APACHE II scores consist of acute physiology, age index, and chronic health evaluation. This scoring system is the gold standard for risk assessment of critically ill patients. A multivariate logistic regression analysis indicated that APACHE II within 24 h of admission was an independent risk factor for predicting IPN (OR: 4.77, P<0.001), which failed to clarify the predictive value and evaluation accuracy (22). In another study, the area under the curve (AUC) was 0.809 for predicting IPN and the cutoff value was 10.5, sensitivity was 90.9%, and specificity was 48.3% (23). Our study showed that according to the ROC curve analysis, the sensitivity was 99.1%, and the specificity was 66.0%. The Youden index was 0.651. Scoring can be accomplished after admission, unaffected by therapeutic factors, assessing the dynamic changes, and guiding better treatment. It was shown that MODS and IPN are the leading causes of death, and both are closely related. Multivariate analysis showed that early or preoperative MODS was relevant with postoperative IPN (24). Further study revealed that AP patients with persistent organ failure (>48 h) were more susceptible to develop IPN than those with transient organ failure (<48 h) (25,26). Our study found that HTGAP caused more damage than BAP to the circulatory, respiratory, and urinary systems (OR: 1.562, 95% CI: 0.642 to 3.631, P<0.001). The MODS was shown to have a higher predictive value, but the relationship between the types and duration of organ failure and IPN requires further investigation.
For BAP patients, the purported mechanisms underlying the relatively more common IPN occurrence (OR: 1.598, 95% CI: 1.027 to 2.451, P=0.034) were transient or sustained occlusion of the pancreatic duct leading to an increase in intraductal pressure and bile reflux into the pancreatic duct (27). Decreased mucosal integrity increases gut permeability, reduces gut motility, and increases the risk of bacterial translocation. The CRP level is a strong indicator of the degree of inflammation in SIRS patients (28), which may relate to the higher CRP levels in HTGAP than in BAP patients. According to the report, the AUC of CRP for predicting IPN was 0.68, cutoff value ≥430 mg/L, sensitivity was 40%, and specificity was 100% (29). Our study showed that the AUC was 0.5968 with relatively low specificity. The CRP is a non-specific acute phase protein, and other acute inflammatory diseases can also elevate it. Therefore, patients with high APACHE II scores and severe organ failure were the most dangerous. If it is a BAP and CRP continues to increase, the probability of IPN was higher. A combination of APACHE II scores, MODS, etiologies, and CRP can significantly improve predictive accuracy.
As for clinical characteristics of patients, our study showed that HTGAP accounted for 11.3% of all cases of AP, and the proportion of SAP was higher in the HTGAP group than in the BAP group (52% vs. 32%), indicating that HTGAP may lead to relatively more pancreatic microcirculation disorders. According to the demographic analysis, BAP is relatively more common in women (58%), which is related to the lower obesity rate among females compared with males. With regard to the age distribution, HTGAP was more common in the younger population (under 40 years of age) than in the elderly (71% vs. 29%). This result may reflect that hypertriglyceridemia is more common in young adults. Although the precise mechanism underlying HTGAP is not fully understood, an excess of free fatty acids (FFAs) and elevated chylomicron levels are thought to increase the plasma viscosity, which may cause ischemia in the pancreas and trigger inflammation (30).
Most patients with IPN are in hypermetabolic state and extremely weak. The mucosal integrity of the gastrointestinal tract decreases, leading to an increase in gut permeability and subsequent bacterial overgrowth (31). The combined factors increase the possibility of bacterial translocation and infected necrosis. The dominant source of infection was intestinal bacteria, which confirmed this theory. When a culture-proven infection is verified, or the infection is strongly suspected, antibiotics should be used. Under this circumstance, the primary clinical manifestations are the gas collection, bacteremia, sepsis, or worsening of illness. Broad-spectrum antibiotics should be prioritized for penetrating necrosis, including carbapenems, quinolones, and metronidazole (32).
For patients with IPN, PCD undoubtedly provides a direct and effective means for controlling the infection source. The “PANTER” trial showed that 35% of patients did not need further intervention following such treatment (33). In two randomized controlled trials (RCTs) comparing various drainage approaches, it was demonstrated that PCD alone was successful in 35% and 51% of patients. In our study, 19.7% of the participants (9 in the BAP group and 4 in the HTGAP group) underwent the “one-step” method without PCD (34). Another evident advantage of the use of PCD is that other minimally invasive debridement methods can utilize the catheter tract as an entry portal.
Extensive studies have confirmed that early debridement (first 2 weeks) is associated with higher morbidity and mortality. Delayed procedure (after 4 weeks) should be the best strategy when the peripancreatic collection is well walled-off and has an evident indication (35). Minimally invasive surgical techniques, including VARD, laparoscopic transgastric debridement, and open transgastric debridement, are feasible and practical. The features of the disease and multidisciplinary discussion determine the choice of approach. Laparoscopic-guided VARD is most commonly performed in our center due to its evident advantages (e.g., widened field of vision, easy bleeding control, available equipment). The “TENSION” trial proved that the endoscopic step-up approach was not superior to the surgical step-up method in decreasing major complications and death. In the endoscopic group, the rate of pancreatic fistulas and length of hospitalization were relatively lower (36). Either endoscopic or surgical intervention should be selected according to individual clinical characteristics. Minimally invasive interventions were not shown to aggravate the severity of trauma in patients who were already in a fragile condition. Trauma control can maximize the improvement in prognosis.
Several limitations existed in this study. As a single-center retrospective study, these results represent regional clinical characteristics. Prediction for IPN is a challenging task involving clinical, laboratory, imaging indicators, and a scoring system. After admission, patients with SAP should be diagnosed under multidisciplinary team (MDT) discussion as soon as possible. In the early stage, doctors should focus on controlling the systemic inflammatory response and organ function protection. Once IPN occurs, PCD and minimally invasive surgery should be arranged after 4 weeks. Laparoscopy or endoscopy can be the first choice for debridement, but it should be carefully selected according to the location of necrosis and surgical conditions. The best effect can be achieved only by individualized treatment on the basis of principles.
Conclusions
In our study, BAP developed into IPN more frequently than did HTGAP. Etiologies, APACHE II scores, MODS, and CRP levels contributed to predicting IPN. The APACHE II scores had the highest sensitivity and specificity. Our study presents a new method of predicting IPN in the late stage. Laparoscopic-guided VARD had advantages in specific cases. The implementation of reasonable management leads to improved prognosis.
Acknowledgments
Funding: This research was supported by grants from Shanxi Scholarship Council of China, Beijing Municipal Science & Technology Commission (Z171100001017077), the Cutting-Edge Clinical Medicine Construction Project of Capital Medical University (1192070312), and the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (XMLX201404). The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-2933
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-2933
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-2933). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of the Xuanwu Hospital (No. [2017]036). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van Dijk SM, Hallensleben NDL, van Santvoort HC, et al. Acute pancreatitis: recent advances through randomised trials. Gut 2017;66:2024-32. [Crossref] [PubMed]
- Forsmark CE, Vege SS, Wilcox CM. Acute Pancreatitis. N Engl J Med 2016;375:1972-81. [Crossref] [PubMed]
- Guo YY, Li HX, Zhang Y, et al. Hypertriglyceridemia-induced acute pancreatitis: progress on disease mechanisms and treatment modalities. Discov Med 2019;27:101-9. [PubMed]
- Liu M, Liu L. Meta-analysis of changes in etiological composition ratio of alcohol acute pancreatitis in recent 20 years. Chinese Journal of Pancreatology 2020;20:221-8.
- Yin G, Cang X, Yu G, et al. Different Clinical Presentations of Hyperlipidemic Acute Pancreatitis: A Retrospective Study. Pancreas 2015;44:1105-10. [Crossref] [PubMed]
- Zheng Y, Zhou Z, Li H, et al. A multicenter study on etiology of acute pancreatitis in Beijing during 5 years. Pancreas 2015;44:409-14. [Crossref] [PubMed]
- Fan J, Ding L, Lu Y, et al. Epidemiology and Etiology of Acute Pancreatitis in Urban and Suburban Areas in Shanghai: A Retrospective Study. Gastroenterol Res Pract 2018;2018:1420590 [Crossref] [PubMed]
- Li X, Ke L, Dong J, et al. Significantly different clinical features between hypertriglyceridemia and biliary acute pancreatitis: a retrospective study of 730 patients from a tertiary center. BMC Gastroenterol 2018;18:89. [Crossref] [PubMed]
- Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology 2013;144:1180-93. [Crossref] [PubMed]
- van Grinsven J, van Brunschot S, Bakker OJ, et al. Diagnostic strategy and timing of intervention in infected necrotizing pancreatitis: an international expert survey and case vignette study. HPB (Oxford) 2016;18:49-56. [Crossref] [PubMed]
- Fong ZV, Fagenholz PJ. Minimally Invasive Debridement for Infected Pancreatic Necrosis. J Gastrointest Surg 2019;23:185-91. [Crossref] [PubMed]
- Bai X, Jin M, Zhang H, et al. Evaluation of Chinese updated guideline for acute pancreatitis on management of moderately severe and severe acute pancreatitis. Pancreatology 2020;20:1582-6. [Crossref] [PubMed]
- Working Group IAP/APA Acute Pancreatitis Guidelines. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013;13:e1-15. [Crossref] [PubMed]
- Lévy P, Boruchowicz A, Hastier P, et al. Diagnostic criteria in predicting a biliary origin of acute pancreatitis in the era of endoscopic ultrasound: multicentre prospective evaluation of 213 patients. Pancreatology 2005;5:450-6. [Crossref] [PubMed]
- Liu CL, Fan ST, Lo CM, et al. Clinico-biochemical prediction of biliary cause of acute pancreatitis in the era of endoscopic ultrasonography. Aliment Pharmacol Ther 2005;22:423-31. [Crossref] [PubMed]
- Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology 2020;20:795-800. [Crossref] [PubMed]
- Ricci C, Pagano N, Ingaldi C, et al. Treatment for Infected Pancreatic Necrosis Should be Delayed, Possibly Avoiding an Open Surgical Approach: A Systematic Review and Network Meta-analysis. Ann Surg 2021;273:251-7. [Crossref] [PubMed]
- Thomson JE, Van Dijk SM, Brand M, et al. Managing Infected Pancreatic Necrosis. Chirurgia (Bucur) 2018;113:291-9. [Crossref] [PubMed]
- Baron TH, DiMaio CJ, Wang AY, et al. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020;158:67-75.e1. [Crossref] [PubMed]
- Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016;59:128-40. [Crossref] [PubMed]
- Petrov MS, Shanbhag S, Chakraborty M, et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010;139:813-20. [Crossref] [PubMed]
- Thandassery RB, Yadav TD, Dutta U, et al. Hypotension in the first week of acute pancreatitis and APACHE II score predict development of infected pancreatic necrosis. Dig Dis Sci 2015;60:537-42. [Crossref] [PubMed]
- Lin ZQ, Guo J, Xia Q, et al. Human leukocyte antigen-DR expression on peripheral monocytes may be an early marker for secondary infection in severe acute pancreatitis. Hepatogastroenterology 2013;60:1896-902. [PubMed]
- Rau BM, Bothe A, Kron M, et al. Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis. Clin Gastroenterol Hepatol 2006;4:1053-61. [Crossref] [PubMed]
- Thandassery RB, Yadav TD, Dutta U, et al. Dynamic nature of organ failure in severe acute pancreatitis: the impact of persistent and deteriorating organ failure. HPB (Oxford) 2013;15:523-8. [Crossref] [PubMed]
- Lytras D, Manes K, Triantopoulou C, et al. Persistent early organ failure: defining the high-risk group of patients with severe acute pancreatitis? Pancreas 2008;36:249-54. [Crossref] [PubMed]
- Papapanagiotou A, Sgourakis G, Peristeraki S, et al. Potential Prediction of Acute Biliary Pancreatitis Outcome on Admission. Pancreas 2018;47:454-8. [Crossref] [PubMed]
- Riché FC, Cholley BP, Laisné MJ, et al. Inflammatory cytokines, C reactive protein, and procalcitonin as early predictors of necrosis infection in acute necrotizing pancreatitis. Surgery 2003;133:257-62. [Crossref] [PubMed]
- Rau BM, Kemppainen EA, Gumbs AA, et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg 2007;245:745-54. [Crossref] [PubMed]
- Garg R, Rustagi T. Management of Hypertriglyceridemia Induced Acute Pancreatitis. Biomed Res Int 2018;2018:4721357 [Crossref] [PubMed]
- Peng YB, Huang J, Qin S, et al. Investigation of distribution of bacteria and fungi in severe acute pancreatitis. Zhonghua Wai Ke Za Zhi 2010;48:496-501. [PubMed]
- Wu Y, Li F, Cao F, et al. Compositional and drug-resistance profiling of pathogens in patients with infected pancreatic necrosis. Chinese Journal of Hepatobiliary Surgery 2018;24:253-7.
- van Santvoort HC, Besselink MG, Bakker OJ, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med 2010;362:1491-502. [Crossref] [PubMed]
- Cao F, Duan N, Gao C, et al. One-Step verse Step-Up Laparoscopic-Assisted Necrosectomy for Infected Pancreatic Necrosis. Dig Surg 2020;37:211-9. [Crossref] [PubMed]
- Besselink MG. The 'step-up approach' to infected necrotizing pancreatitis: delay, drain, debride. Dig Liver Dis 2011;43:421-2. [Crossref] [PubMed]
- van Brunschot S, van Grinsven J, van Santvoort HC, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet 2018;391:51-8. [Crossref] [PubMed]
(English Language Editor: J. Jones)


