
Quality of life among patients with 4 to 10 brain metastases after treatment with whole-brain radiotherapy vs. stereotactic radiotherapy: a phase III, randomized, Dutch multicenter trial
Introduction
Brain metastases (BM) occur in a significant proportion (20–40%) of patients with cancer, and they are an important cause of mortality and morbidity (1). Optimal tumor control and preserved quality of life (QoL) are essential for these patients. However, most previous studies have evaluated only overall survival or progression-free survival as their primary endpoint (2-4).
Traditionally, BM treatment has mainly comprised primarily whole-brain radiotherapy (WBRT). Stereotactic radiotherapy (SRT) has become a widely used and accessible technique to treat patients with BM in recent decades, showing a high rate of local tumor control. Single session radiosurgery (SRS) and (fractionated) stereotactic radiotherapy [(F)SRT] also prevents typical treatment-related fatigue, neurocognitive decline, alopecia, and other side effects associated with whole-brain irradiation. In patients with BM, FSRT can be an option to increase the therapeutic ratio, compared to SRS (2,5,6). To date, few studies have examined QoL changes among patients treated to SRT for BM (7-10). These studies have shown that QoL is likely to improve for such patients, but outcomes are likely to worsen for these patients with a higher number of BM. Studies’ inclusion of QoL metrics could improve patient care (11).
The prognosis for the majority of patients with BM remains poor, but life expectancy for these patients has increased in recent years due to improved systemic-treatment options (9,12). Therefore, BM management no longer solely focuses on survival; rather, maintaining a good QoL for as long as possible is an important objective for this patient group (13-16).
Over the years, clinicians have shifted from WBRT with SRT in order to reduce the risk of declining QoL. Until recently, Dutch guidelines recommended treating patients with one to three BM with SRT and patients with four or more BM with WBRT. A working group was established in 2017 to review these current guidelines. These guidelines now advise SRT for patients with up to 10 BM of low volume (17).
Our NCT02353000 randomized controlled study compared WBRT to SRT to determine whether SRT is a better palliative treatment than WBRT vis-à-vis QoL outcomes for patients with 4 to 10 low-volume BM and a favorable prognosis. The study’s primary endpoints were published previously (18). Its secondary endpoints, which focus on QoL and involve the use of multiple methods, are presented in the current paper. We present the following article in accordance with the CONSORT reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-21-1545/rc).
Methods
Study design and participants
The trial’s materials and methods were reported in a recently published paper that discussed other endpoints than QoL, such as survival and prognostic factors (18). We will briefly outline these materials and methods here. For the entire protocol see (19). In this prospective, randomized, phase III, multicenter trial, patients with 4 to 10 BM from solid tumors, who had been diagnosed using high-resolution, contrast-enhanced MRI scans, were referred for radiotherapy and included as participants. The patients were randomly assigned to one of two treatment groups, WBRT and SRT. All patients who wished to participate provided their written informed consent. The study’s protocol was approved by the medical ethical committee of the Maastricht University Medical Center in the Netherlands (approval number NL53852.068.15/METC153053). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Randomization and masking
After discussions within the multidisciplinary tumor board, patients were selected for participation in the study and allocated to one of the two treatment groups using permutated block randomization and a block size of eight. Neither patients, clinicians, nor study statisticians were masked regarding treatment assignments.
Procedures
Using gadolinium contrast-enhanced MRI (1.0–3 T) with a maximal slice thickness of 1.5 mm, patients’ definitive BM numbers and definitive maximum lesion diameters in any direction for their largest BM were determined. Patients in the WBRT group were treated with five fractions of 4 Gy for a total dose of 20 Gy. Patients’ brains were contoured as the WBRT clinical target volume (CTV) up to the foramen magnum. Hippocampal sparing was not applied in this treatment arm.
For patients in the SRT group, the dose for all BM (15–24 Gy in 1 fraction or 24 Gy in 3 fractions) was determined using the volume of the largest BM or brainstem location. A volume-based dosing strategy was employed, conforming to the National Neuro-Oncology consensus document. If a patient’s V12 Gy of healthy brain tissue surrounding an individual BM exceeded 10 cm3, in addition to 15 Gy in a single fraction, 24 Gy in three fractions was allowed to minimize the risk of radionecrosis (20,21). The planning target volume (PTV) was defined by a 0–2 mm isotropic expansion of the gross tumor volume (GTV) determined by the treating physician’s preference and the center’s set-up uncertainties. If a BM was within or adjacent to a patient’s brainstem, the PTV margin was defined at 0 mm in all centers to minimize the risk of brainstem necrosis.
Outcomes
This paper addresses the study’s QoL-related endpoints: differences in QoL (using the EQ5D EUROQoL questionnaire) at 3, 6, 9, and 12 months post-treatment. Additionally, patients’ Karnofsky scores, World Health Organization (WHO) performance status, toxicity (according to the Common Terminology Criteria for Adverse Events V4.0 criteria), salvage treatment, time to salvage after randomization, and Barthel index scores were evaluated at 3, 6, 9, and 12 months post-treatment. The study’s facultative secondary endpoints were neurocognition using the Hopkins Verbal Learning Test, the QoL European Organisation for Research and Treatment of Cancer (EORTC) BN20 brain module, QoL EORTC QLQ-C30, and the EORTC QLQ-FA13 fatigue scale.
Statistical analysis
Patient accrual started on July 1, 2016, but due to poor accrual, the trial closed prematurely in July 2018. Patients’ EQ5D scores were compared using an independent-sample Student’s t-test with a two-sided significance level alpha of 0.05. Secondary-endpoint differences over time were analyzed using Kaplan-Meyer curves, including a log-rank test and analysis of variance (ANOVA) testing. Means were compared using independent-sample Student’s t-tests. Statistical analysis was performed using SPSS®, version 23 (IBM, Armonk, NY, USA).
Results
Baseline characteristics
Between July 2016 and May 2018, 29 patients were enrolled in this study. Twenty patients’ results were suitable for the current analysis; the remaining nine patients did not complete the study’s questionnaires and were, therefore, excluded due to their lack of follow-up data (Figure 1). Ten patients were randomly assigned to WBRT, and 10 patients were randomly assigned to SRT. Our median follow-up time with patients was 26 months. Patients’ most common primary cancer type was non-small-cell lung cancer (NSCLC), at 90% (9 out of 10) of the WBRT group and 80% (8 out of 10) of the SRT group. In both groups, patients were in favorable physical condition, with a median Karnofsky score of 90. Patients’ EQ5D health state and EQ-5D VAS scores at baseline were 0.8 and 77 for the WBRT group, vs. 0.9 and 70 for the SRT group, respectively. No differences were observed in patients’ QoL domains at baseline between the WBRT and SRT groups. The baseline characteristics for both treatment groups are shown in Table 1, and no significant differences were observed between the groups in this regard.
Table 1
| Characteristics | SRT group (n=10) | WBRT group (n=10) | P value |
|---|---|---|---|
| Sex, n [%] | 1.000 | ||
| Female | 4 [40] | 4 [40] | |
| Male | 6 [60] | 6 [60] | |
| Age (years), n [%] | 0.398 | ||
| Median [range] | 56 [51–74] | 64 [51–74] | |
| Mean (± SD) | 59 (±8) | 63 (±8) | |
| <50 | – | – | |
| 51–65 | 7 [70] | 6 [60] | |
| >65 | 3 [30] | 4 [40] | |
| Primary tumor, n [%] | 0.277 | ||
| NSCLC | 8 [80] | 9 [90] | |
| Colorectal | 1 [10] | – | |
| Breast | – | 1 [10] | |
| Others | 1 [10] | – | |
| Number of BM, n [%] | 0.906 | ||
| 4 | 4 [40] | 4 [40] | |
| 5 | 2 [20] | 2 [20] | |
| 6 | 1 [10] | – | |
| 7 | 1 [10] | 2 [20] | |
| 8 | – | 2 [20] | |
| 9 | 2 [20] | – | |
| Cumulative GTV, n [%] | 0.267 | ||
| 0.1–5 cm3 | 4 [40] | 2 [20] | |
| 5–10 cm3 | 2 [20] | 2 [20] | |
| 10–15 cm3 | 2 [20] | 4 [40] | |
| 15–20 cm3 | 2 [20] | 1 [10] | |
| 20–25 cm3 | – | 1 [10] | |
| Symptomatic BM, n [%] | 9 [90] | 10 [100] | 0.331 |
| Dexamethasone use, n [%] | 8 [80] | 6 [60] | 0.171 |
| WHO classification, n [%] | 0.331 | ||
| 0 | 2 [20] | 4 [40] | |
| 1 | 6 [60] | 5 [50] | |
| 2 | 2 [20] | 1 [10] | |
| DS-GPA, n [%] | 0.870 | ||
| 0.5 | 1 [10] | 1 [10] | |
| 1 | 2 [20] | – | |
| 1.5 | 1 [10] | 5 [50] | |
| 2.0 | 4 [40] | 2 [20] | |
| 2.5 | 1 [10] | 2 [20] | |
| 3.0 | 1 [10] | – | |
| RPA, n [%] | 0.696 | ||
| I | 2 [20] | 4 [40] | |
| II | 8 [80] | 5 [50] | |
| III | – | 1 [10] | |
| EQ5D, mean ± SD | |||
| Health state | 0.9±0.1 | 0.8±0.1 | 0.905 |
| VAS score | 70±20 | 77±14 | 0.398 |
SRT, stereotactic radiotherapy; WBRT, whole-brain radiotherapy; SD, standard deviation; NSCLC, non-small-cell lung cancer; BM, brain metastases; GTV, gross tumor volume; DS-GPA, diagnosis-specific graded prognostic assessment; RPA, recursive partitioning analysis; VAS, visual analog scale.
EQ5D domains
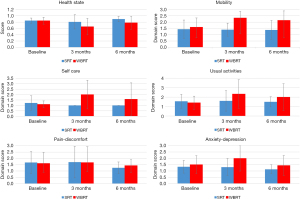
The outcome of patients’ EQ5D domains at 3 months post-treatment is shown in Table 2. A significant difference was observed between the study’s two groups’ scores at 3 months post-treatment for the mobility (P=0.041) and self-care (P=0.028) domains, with poorer results for the WBRT group. In comparing 3 months post-treatment scores to baselines, we found a significant difference in the self-care domain, with poorer results for the patients treated with WBRT (0.9±1.2) compared to an improvement for the SRT group (−0.2±0.7) (P=0.025). No significant differences were observed in other domains compared to baselines. At 6 months post-treatment the compliance was poorer, therefore not suitable for further analysis. Figures 2,3 show the progress of in patients’ scores from the baselines to 6 months post-treatment for the various EQ5D domains among both the WBRT and SRT patient groups.
Table 2
| EQ5D domain | Baseline | 3-month follow up | Δ with respect to baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SRT (n=10) | WBRT (n=10) | P value | SRT (n=9) | WBRT (n=9) | P value | SRT | WBRT | P value | |||
| Mobility | 1.4±0.7 | 1.6±0.8 | 0.674 | 1.4±0.5 | 2.3±1.2 | 0.041* | 0±0.5 | 0.8±1.2 | 0.092 | ||
| Self-care | 1.2±0.7 | 1.1±0.3 | 0.610 | 1.0 | 2.0±1.3 | 0.028* | −0.2±0.7 | 0.9±1.2 | 0.025* | ||
| Usual activities | 1.6±0.7 | 1.4±0.7 | 0.641 | 1.6±1.1 | 2.3±1.5 | 0.234 | 0.1±0.6 | 0.9±1.5 | 0.157 | ||
| Pain and discomfort | 1.7±0.9 | 1.6±0.7 | 0.855 | 1.7±1.3 | 1.7±0.7 | 0.945 | 0.1±1.3 | 0.1±0.8 | 1.0 | ||
| Anxiety and depression | 1.3±0.5 | 1.5±0.7 | 0.565 | 1.3±0.7 | 2.0±1.0 | 0.089 | 0±0.9 | 0.4±1.1 | 0.363 | ||
Data was present as mean ± SD. *, indicates statistical significance. A higher score in the EQ5D domains equals a worse score (e.g., more difficulty with mobility and self-care) among patients treated with WBRT at 3 months post-radiotherapy. SRT, stereotactic radiotherapy; WBRT, whole-brain radiotherapy; Δ, difference; SD, standard deviation.

EORTC questionnaires
To analyze QoL as extensively as possible, we also used QoL EORTC BN20 brain module, the QoL EORTC QLQ-C30, and the fatigue scale EORTC QLQ-FA13, and Table 3 shows these results. At baseline, we noted significant differences in pain on the C30 questionnaire and in seizures on the BN20 questionnaire, with more complaints from the WBRT group (P=0.036 and P=0.031, respectively). Three months post-treatment, multiple categories in all three questionnaires showed significant and clinically relevant differences between the treatment groups. Physical functioning was better preserved among patients who had been treated with SRT vs. patients who had been treated with WBRT (SRT: 89±12; WBRT: 52±35; P=0.016). Similar results occurred for future uncertainty (SRT: 10±12; WBRT: 43±22; P=0.004); patients treated with SRT were less uncertain about their futures and experienced less emotional fatigue (SRT: 4±8; WBRT: 33±31; P=0.026). In comparing 3 months post-treatment scores to baselines, we still found significant differences in physical functioning and social functioning, again favoring SRT.
Table 3
| EORTC questionnaire | Baseline | 3-month follow up | Δ respect to baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SRT (n=10) | WBRT (n=10) | P value | SRT (n=8) | WBRT (n=7) | P value | SRT | WBRT | P value | |||
| EORTC QoL C30 | |||||||||||
| Global health status | 77±11 | 66±20 | 0.135 | 76±15 | 55±23 | 0.051 | −6±19 | −14±32 | 0.566 | ||
| Physical functioning | 83±15 | 83±11 | 1.000 | 89±12 | 52±35 | 0.016* | 0±11 | −33±28 | 0.023* | ||
| Role functioning | 74±31 | 70±27 | 0.743 | 77±29 | 39±39 | 0.058 | −7±46 | −31±29 | 0.305 | ||
| Emotional functioning | 73±19 | 74±20 | 0.938 | 86±13 | 65±19 | 0.026* | 7±20 | −5±21 | 0.305 | ||
| Cognitive functioning | 92±14 | 80±19 | 0.099 | 94±9 | 79±27 | 0.151 | −5±13 | −7±35 | 0.867 | ||
| Social functioning | 80±22 | 83±28 | 0.787 | 96±12 | 67±29 | 0.021* | 19±31 | −19±31 | 0.041* | ||
| Fatigue | 20±15 | 32±19 | 0.122 | 21±26 | 56±35 | 0.056 | 4±28 | 22±33 | 0.300 | ||
| Nausea and vomiting | 2±5 | 0 | 0.353 | 15±25 | 7 ±13 | 0.483 | 17±25 | 7±13 | 0.396 | ||
| Pain | 8±11 | 37±42 | 0.036* | 21±40 | 26±29 | 0.772 | 17±42 | 0±42 | 0.472 | ||
| Dyspnea | 9±22 | 17±36 | 0.561 | 0 | 38±45 | 0.031* | −5±13 | 14±18 | 0.040* | ||
| Insomnia | 27±25 | 37±43 | 0.542 | 17±31 | 38±30 | 0.197 | −10±37 | 0±47 | 0.682 | ||
| Appetite loss | 9±30 | 3±11 | 0.574 | 25±35 | 19±26 | 0.716 | 29±36 | 19±26 | 0.580 | ||
| Constipation | 12±27 | 37±43 | 0.129 | 14±38 | 19±26 | 0.789 | 6±53 | −24±37 | 0.268 | ||
| Diarrhea | 9±30 | 0 | 0.353 | 5±13 | 5±13 | 1 | −11±46 | 5±13 | 0.393 | ||
| Financial difficulties | 9±22 | 10±22 | 0.926 | 0 | 29±30 | 0.018* | 0 | 14±26 | 0.175 | ||
| EORTC QoL BN20 | |||||||||||
| Future uncertainty | 39±19 | 42±25 | 0.817 | 10±12 | 43±22 | 0.004* | −23±25 | 5±33 | 0.107 | ||
| Visual disorder | 12±23 | 16±21 | 0.723 | 4±8 | 33±31 | 0.026* | 5±9 | 17±28 | 0.305 | ||
| Motor dysfunction | 11±14 | 29±26 | 0.061 | 3±5 | 25±17 | 0.003* | −5±17 | 2±24 | 0.572 | ||
| Communication deficit | 12±20 | 16±22 | 0.707 | 6±8 | 10±16 | 0.555 | −8±18 | −10 ±34 | 0.915 | ||
| Headaches | 12±22 | 7±14 | 0.518 | 0 | 33±38 | 0.029 | −10±25 | 24±46 | 0.119 | ||
| Seizures | 0 | 30±43 | 0.031* | 0 | 14±38 | 0.302 | 0 | −29±40 | 0.087 | ||
| Drowsiness | 13±4 | 28±9 | 0.157 | 8±24 | 29±30 | 0.167 | 5±13 | 14±26 | 0.403 | ||
| Itchy skin | 22±6 | 29±9 | 0.076 | 21±35 | 29±23 | 0.630 | 19±26 | 0±27 | 0.207 | ||
| Hair loss | 0 | 0 | – | 21±31 | 43±46 | 0.289 | 24±31 | 43±46 | 0.385 | ||
| Weakness of legs | 14±4 | 22±7 | 0.696 | 0±0 | 38±40 | 0.019* | −6±14 | 29±40 | 0.076 | ||
| EORTC QoL FA13 | |||||||||||
| Physical fatigue | 39±19 | 42±25 | 0.817 | 10±12 | 43±22 | 0.004* | −22±25 | 5±33 | 0.107 | ||
| Emotional fatigue | 12±22 | 16±21 | 0.723 | 4±8 | 33±31 | 0.026* | 5±9 | 17±28 | 0.305 | ||
| Cognitive fatigue | 11±14 | 29±26 | 0.061 | 3±5 | 25±17 | 0.003* | −5±17 | 2±24 | 0.572 | ||
| Interference with daily life | 12±20 | 16±22 | 0.707 | 6±8 | 10±16 | 0.555 | −8±18 | −10±34 | 0.915 | ||
| Social sequelae | 12±22 | 7±14 | 0.518 | 0 | 33±38 | 0.029* | −10±25 | 24±46 | 0.119 | ||
Data was present as mean ± SD. *, indicates statistical significance. The scores for the various EORTC questionnaires at patients’ baseline, 3 months post-treatment, and vs. the baselines. A higher score in the positive elements of the questionnaires (e.g., physical functioning and role functioning) reflects a better score. A higher score in the negative elements (e.g., pain and dyspnea) reflects a worse score—these patients had more complaints and worse QoL. EORTC, European Organisation for Research and Treatment of Cancer; SRT, stereotactic radiotherapy; WBRT, whole-brain radiotherapy; Δ, difference; QoL, quality of life; SD, standard deviation.
Other QoL measurements, toxicity and dexamethasone use
As Table 4 shows, we observed no significant differences between the groups’ Karnofsky scores, EQ6D cognition, Barthel indexes, or Hopkins Verbal Learning Tests post-treatment or compared to baselines.
Table 4
| Tests | Baseline | 3-month follow-up | Δ with respect to baseline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SRT (n=10) | WBRT (n=10) | P value | SRT (n=9) | WBRT (n=9) | P value | SRT | WBRT | P value | |||
| Karnofsky score | 87±11 | 85±11 | 0.681 | 89±10 | 72±29 | 0.093 | 2±16 | −13±28 | 0.155 | ||
| EQ6D cognition | 1.3±0.5 | 1.5±0.7 | 0.327 | 1.3±0.7 | 1.4±0.7 | 0.659 | −0.1±0.6 | 0±1.2 | 0.810 | ||
| Barthel index | 20±1 | 20±2 | 0.482 | 20±2 | 19±3 | 0.181 | −0.3±2 | −1±2 | 0.309 | ||
| Hopkins Verbal Learning Test | |||||||||||
| Total recall | 22±5 | 18±5 | 0.188 | 24±4 | 22±7 | 0.633 | 4±4 | 3±1 | 0.439 | ||
| Delayed recall | 8±2 | 5±3 | 0.024* | 9±2 | 7±4 | 0.301 | 1±2 | 1±1 | 0.700 | ||
| Retention | 97±13 | 68±20 | 0.001* | 102±33 | 73±29 | 0.149 | −7±24 | 11±19 | 0.260 | ||
| Recognition discrimination index | 11±1 | 10±3 | 0.270 | 11±1 | 10±2 | 0.343 | 1±2 | 0 | 0.503 | ||
Data was present as mean ± SD. *, indicates statistical significance. The outcomes of patients’ Karnofsky scores, EQ6D tests, Barthel indexes, and Hopkins Verbal Learning Test at patients’ baseline, 3 months post-treatment, and vs. the baselines. A higher Karnofsky score reflects better functioning in daily life. The EQ6D test derives from the EQ5D test, which also includes cognition; a lower score is better. A higher score in the Barthel index means more independence. And a higher score in the elements of the Hopkins Verbal Learning Test is better. SRT, stereotactic radiotherapy; WBRT, whole-brain radiotherapy; Δ, difference; SD, standard deviation.
As we have reported previously, adverse events occurred among our study’s patients. Two patients in the SRT group experienced epileptic seizures. Both of these patients had experienced epileptic seizures prior to SRT treatment, and their seizures were controlled with medication. Toxicity among the study participants mainly consisted of hair loss and fatigue among the WBRT group (18). We observed a significant difference in hair loss at 3 months post-treatment between the two study groups, with poorer results for the patients who had been treated with WBRT (P=0.014). Table 5 presents the details of patients’ adverse events and toxicity.
Table 5
| Toxicity | SRT (n=10) | WBRT (n=9) | P value |
|---|---|---|---|
| Hair loss, n [%] | 0.014* | ||
| Grade 1 | 7 [70] | 2 [22] | |
| Grade 2 (> 50% hair loss) | – | 6 [67] | |
| Fatigue, n [%] | 0.281 | ||
| Grade 1 | 3 [30] | 4 [44] | |
| Grade 2 | 2 [20] | 3 [33] | |
| Adverse events#, n [%] | 0.855 | ||
| Epileptic seizure (grade 1–2) | 2 [20] | ||
| Nausea and vomiting (grade 2) | 1 [10] | 1 [11] | |
| Headache (grade 2–3) | 1 [10] | 3 [33] | |
| Blurry vision (grade 1–2) | 1 [10] | 1 [11] | |
| Dizziness (grade 1) | 1 [10] | – | |
| Confusion (grade 1) | – | 1 [11] |
*, indicates statistical significance; #, only adverse events with a (possible) relation to the study’s therapy were included in this table. SRT, stereotactic radiotherapy; WBRT, whole-brain radiotherapy.
At baseline 8 patients in the SRS group use dexamethasone, compared to 6 patients in the WBRT arm. At 3 months post-treatment only 1 patient in the SRS group uses dexamethasone at a dose of 6 mg, compared to
8 patients in the WBRT arm, in which the dose ranges from 1 to 8 mg daily. At 6 months post-treatment 1 patient (8 mg) in the SRS group and 5 patients (range, 1–4 mg) in the WBRT group are still using dexamethasone.
Discussion
Our study showed that the QoL of patients treated with SRT was maintained post-treatment, with significantly better results in the EQ5D domains of mobility and self-care among this patient group. Also, in this group’s EORTC questionnaires, physical functioning was better preserved compared to the WBRT group, and patients treated with SRT were less uncertain about their futures and experienced less emotional fatigue than patients treated with WBRT. These results are clinically relevant—especially in the palliative setting, which makes SRT even more desirable over WBRT.
BM management has undergone dramatic changes over the last decade, mainly through the availability of targeted agents and immunotherapy for several primary tumors and the potential to control BM (22). The biggest concern for these patients is neurocognitive decline, and patients may have neurocognitive impairment due to previous systemic therapies, even before the development of BM. Also, recent technical advances within radiotherapy—such as patient immobilization in an invasive frame and the use of six-degrees-of-freedom robotic couches—have made SRT more efficient and patient-friendly.
These developments call the need for WBRT into question for patients diagnosed with BM. Furthermore, the QUARTZ trial evaluated whether WBRT could be omitted without a significant effect on survival or QoL for patients with intermediate or unfavorable prognoses; the study showed that WBRT did not provide any QoL benefit, survival benefit (median survival, 8.5 weeks), or difference in dexamethasone use vs. the best supportive care for patients with NSCLC and low performance scores (23). This finding led to increasing reluctance among physicians to use WBRT. Especially in a palliative setting, informing patients about available treatment options to individualize the multimodality of their treatment is important. With more treatment options available, shared decision-making is complex and challenging. The goal of this shared decision-making process is to obtain an optimal treatment strategy using shared decision tools based on prognostic models that can be adapted to patients’ characteristics, as well as deliberation between patients and their physicians (22).
SRT is an attractive palliative treatment option for multiple BM because it avoids WBRT and its associated toxicity. Technological improvements have allowed the use of SRT treatment for multiple BM with acceptable treatment times, and SRT is no longer only suitable for patients with a limited number of BM (24). The first matched-pair analysis for potential prognostic factors, comparing SRT vs. WBRT for patients with multiple BM, confirmed these advantages, finding a median overall survival of 16 months (SRT) vs. 8 months (WBRT), while excellent clinical performance and extracranial tumor control were favorable prognostic factors (25).
To our knowledge, our study was is the first phase III, randomized, controlled trial investigating WBRT vs. SRT for patients with 4 to 10 BM that emphasized QoL, using multiple measurement tools—such as the EQ5D and EORTC questionnaires. Crucially, BM treatment must help maintain good QoL and neurological states for patients. Because of effective BM treatments, such as SRT, the cause of death for BM patients is mainly extracranial disease progression (26,27). Over the years, the literature has reflected an increasing interest in QoL as an indicator of patient outcomes. Since WBRT carries a risk of inducing fatigue, neurocognitive deterioration, and alopecia, we consider our finding that QoL maintenance was not inferior for patients treated with SRT vs. WBRT in a multiple-BM setting to be very important. Therefore, our chosen endpoints in this trial considered QoL post-radiotherapy, and we hypothesized that QoL was better preserved after SRT treatment for even longer than 3 months post-radiotherapy. This study showed that QoL was maintained after SRT and that significant and clinically relevant differences occurred post-treatment in multiple EQ5D domains and EORTC questionnaires between the two treatment groups, with poorer results for patients treated with WBRT. When we examined the significant differences at 3 months post-treatment, we identified several differences that were undesirable in our palliative setting. Patients treated with WBRT showed higher scores in the EQ5D domains self-care and mobility, which means these patients experienced more difficulty in these domains of daily life. Also, patients treated with WBRT scored significantly worse in multiple questions of the EORTC questionnaires, which means these patients faced a higher burden in daily life (e.g., motor dysfunction, headaches, and cognitive, physical, and emotional fatigue), as well as visual disorders. Additionally, patients treated with SRT scored significantly higher in physical, emotional, and social functioning on the EORTC questionnaire—a desirable outcome for this patient population.
Multiple studies have shown that QoL is preserved or even, likely, improved among patients treated with SRT. Additionally, upfront WBRT independently predicts QoL deterioration, and increased BM numbers were associated with worsening in the overall EQ5D (7,28). More mature randomized-trial results are needed to confirm our hypothesis.
Contrary to our expectations, we observed relatively high patient compliance with the QoL questionnaires in our study. To evaluate the rate at which—and the reasons why—cancer patients do not participate in QoL questionnaires, the Italian Group for Evaluation of Outcomes in Oncology (IGEO) conducted a study at 79 medical oncology and radiotherapy centers. Almost 88% of patients filled out questionnaires, and the most important reasons for non-compliance were refusal (29%), poor eyesight (17%), and illiteracy (18%). Older patients with low performance status or locally advanced disease completed questionnaires less frequently (29). Leung et al. reviewed the use of the EORTC questionnaires (QLQ-BN20 and QLQ-C30) for patients with BM. In total, 13 studies were identified, and the QLQ-BN20 had mainly been used in conjunction with the QLQ-C30 questionnaire. Compliance issues were commonly mentioned, and QoL changes varied during the study periods. Leung et al. concluded that QoL assessments should be conducted using disease-specific questionnaires and that patients’ burden should be minimized in order to maximize accrual and data collection (30).
Our study’s relatively high compliance was probably due to the involvement of a research nurse who contacted each patient by telephone, as well as the clarity of the questionnaires. Although our patients generally had poor prognoses, this outcome supports the use of these questionnaires with this patient population.
Our study faced several important limitations. First, our trial closed prematurely due to poor accrual. Therefore, only 29 out of a planned 230 patients were randomized into groups, of whom 20 patients were included in this QoL analysis. Due to this small study population, no major significant differences could be determined between the two treatment groups’ survival and a possible outlier, which we did not found, could possible influence our data (18). In radiation oncology, clinical-trial failure is common. To date, the factors that contribute to this failure are not well understood. Nguyen et al. reviewed 134 studies of randomized, controlled trials involving radiotherapy, and they compared complete and incomplete trials to identify predictors of trial failure. They observed an increase in failure over time, with rates up to 40% in 2012. A third of the reviewed trials failed, and more than 50% failed due to poor accrual (31). The main reason why our study closed prematurely appeared to be patients’ and referrers’ preference for SRT, and the most important consideration was that WBRT side effects were undesirable—especially for patients with very favorable performance status (Karnofsky performance status of 90–100). Furthermore, in our study design we did not use hippocampal sparing or memantine when patients are treated with WBRT. Hippocampal sparing could provide the preservation of neurocognitive functions and memantine has shown to be neuro-protective which prolonged the time to cognitive decline. Second, in hindsight, we note that our inclusion criteria may have been too strict, in particular the maximum diameter of the BM. A considerable number of patients could not be included in our trial because they did not meet these inclusion criteria (having fewer than ten BM or exceeding our BM volume criteria). Additionally, in the Netherlands, SRT has become the preferred treatment choice over WBRT for patients with up to 10 BM (17). Third, the majority of patients (85%) who enrolled in this trial had NSCLC as their primary tumor. However, no evidence has suggested that cognitive effects and QoL vary across primary tumors, and NSCLC patients are the majority in almost all BM trials (32-34). Finally, participants and clinicians could not be blinded to treatment allocation in our trial, which is a typical approach for such phase III trials evaluating radiotherapy.
We conclude that patients with 4 to 10 BM who were treated with SRT alone, compared to WBRT, maintained higher QoL post-treatment, with significant and clinically relevant differences in multiple EQ5D domains and in the EORTC questionnaires favoring SRT over WBRT.
Acknowledgments
This paper’s authors thank our data safety commission (Dr. An Hoeben, Dr. Monique Anten, and Dr. Peter Koehler), and particularly Rody Zuidema and Ruud Hoeben, for their commitment and contribution to this trial.
Funding: MAASTRO has a research agreement with Varian Medical Systems, Palo Alto, which provided funding for this trial. The Data Center was responsible for collecting and maintaining the study’s data. Authors acknowledge financial support from ERC advanced grant (ERC-ADG-2015 No. 694812 - Hypoximmuno), ERC-2020-PoC: 957565-AUTO.DISTINCT. Authors also acknowledge financial support from SME Phase 2 (RAIL No. 673780), the European Union’s Horizon 2020 research and innovation programme under grant agreement: ImmunoSABR No. 733008.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-21-1545/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-21-1545/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-21-1545/coif). Dr. JZ reports that MAASTRO Clinic has a research agreement with Varian Medical Systems, which include financial support of the data management of this trial. Varian was not involved in the analysis of the results, nor the writing of this manuscript. Dr. PL reports, within and outside the submitted work, grants/sponsored research agreements from Radiomics, ptTheragnostic/DNAmito, Health Innovation Ventures. He received an advisor/presenter fee and/or reimbursement of travel costs/external grant writing fee and/or in kind manpower contribution from Oncoradiomics, BHV, Varian, Elekta, ptTheragnostic and Convert pharmaceuticals. Dr. PL has shares in the company Radiomics SA, Convert pharmaceuticals SA and Comunicare SA and is co-inventor of two issued patents with royalties on radiomics (PCT/NL2014/050248, PCT/NL2014/050728) licensed to Radiomics SA and one issue patent on mtDNA (PCT/EP2014/059089) licensed to ptTheragnostic/DNAmito, three non-patented invention (softwares) licensed to ptTheragnostic/DNAmito, Radiomics SA and Health Innovation Ventures and three non-issues, non licensed patents on Deep Learning-Radiomics and LSRT (N2024482, N2024889, N2024889). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the medical ethical committee of the Maastricht University Medical Center in the Netherlands (approval number NL53852.068.15/METC153053) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. [Crossref] [PubMed]
- Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014;15:387-95. [Crossref] [PubMed]
- Trifiletti DM, Lee CC, Kano H, et al. Stereotactic Radiosurgery for Brainstem Metastases: An International Cooperative Study to Define Response and Toxicity. Int J Radiat Oncol Biol Phys 2016;96:280-8. [Crossref] [PubMed]
- Emery A, Trifiletti DM, Romano KD, et al. More than Just the Number of Brain Metastases: Evaluating the Impact of Brain Metastasis Location and Relative Volume on Overall Survival After Stereotactic Radiosurgery. World Neurosurg 2017;99:111-7. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Pinkham MB, Sanghera P, Wall GK, et al. Neurocognitive Effects Following Cranial Irradiation for Brain Metastases. Clin Oncol (R Coll Radiol) 2015;27:630-9. [Crossref] [PubMed]
- Sheehan JP, Grills I, Chiang VL, et al. Quality of life outcomes for brain metastasis patients treated with stereotactic radiosurgery: pre-procedural predictive factors from a prospective national registry. J Neurosurg 2018;131:1848-54. [Crossref] [PubMed]
- Skeie BS, Eide GE, Flatebø M, et al. Quality of life is maintained using Gamma Knife radiosurgery: a prospective study of a brain metastases patient cohort. J Neurosurg 2017;126:708-25. [Crossref] [PubMed]
- Verhaak E, Gehring K, Hanssens PEJ, et al. Health-related quality of life in adult patients with brain metastases after stereotactic radiosurgery: a systematic, narrative review. Support Care Cancer 2020;28:473-84. [Crossref] [PubMed]
- Habets EJ, Dirven L, Wiggenraad RG, et al. Neurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: a prospective study. Neuro Oncol 2016;18:435-44. [Crossref] [PubMed]
- Ariello K, Tan H, Soliman H. Narrative review of neurocognitive and quality of life tools used in brain metastases trials. Ann Palliat Med 2021;10:923-35. [Crossref] [PubMed]
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep 2012;14:48-54. [Crossref] [PubMed]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013;31:65-72. [Crossref] [PubMed]
- Pham A, Lo SS, Sahgal A, et al. Neurocognition and quality-of-life in brain metastasis patients who have been irradiated focally or comprehensively. Expert Rev Qual Life Cancer Care 2016;1:45-60. [Crossref]
- Wong J, Hird A, Kirou-Mauro A, et al. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol 2008;15:25-45. [Crossref] [PubMed]
- Chiu L, Chiu N, Zeng L, et al. Quality of life in patients with primary and metastatic brain cancer as reported in the literature using the EORTC QLQ-BN20 and QLQ-C30. Expert Rev Pharmacoecon Outcomes Res 2012;12:831-7. [Crossref] [PubMed]
- Hilkens NA, Enting RH, Hendriks LEL, et al. Revised guideline 'Brain metastases': More treatment options. Ned Tijdschr Geneeskd 2020; [PubMed]
- Hartgerink D, Bruynzeel A, Eekers D, et al. A Dutch phase III randomized multicenter trial: whole brain radiotherapy versus stereotactic radiotherapy for 4-10 brain metastases. Neurooncol Adv 2021;3:vdab021.
- Zindler JD, Bruynzeel AME, Eekers DBP, et al. Whole brain radiotherapy versus stereotactic radiosurgery for 4-10 brain metastases: a phase III randomised multicentre trial. BMC Cancer 2017;17:500. [Crossref] [PubMed]
- Zindler JD, Thomas CR Jr, Hahn SM, et al. Increasing the Therapeutic Ratio of Stereotactic Ablative Radiotherapy by Individualized Isotoxic Dose Prescription. J Natl Cancer Inst 2015;108:djv305. [Crossref] [PubMed]
- Zindler JD, Schiffelers J, Lambin P, et al. Improved effectiveness of stereotactic radiosurgery in large brain metastases by individualized isotoxic dose prescription: an in silico study. Strahlenther Onkol 2018;194:560-9. [Crossref] [PubMed]
- Hartgerink D, van der Heijden B, De Ruysscher D, et al. Stereotactic Radiosurgery in the Management of Patients With Brain Metastases of Non-Small Cell Lung Cancer: Indications, Decision Tools and Future Directions. Front Oncol 2018;8:154. [Crossref] [PubMed]
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004-14. [Crossref] [PubMed]
- Sahgal A, Ma L, Chang E, et al. Advances in technology for intracranial stereotactic radiosurgery. Technol Cancer Res Treat 2009;8:271-80. [Crossref] [PubMed]
- El Shafie RA, Celik A, Weber D, et al. A matched-pair analysis comparing stereotactic radiosurgery with whole-brain radiotherapy for patients with multiple brain metastases. J Neurooncol 2020;147:607-18. [Crossref] [PubMed]
- Yamamoto M, Kawabe T, Sato Y, et al. A case-matched study of stereotactic radiosurgery for patients with multiple brain metastases: comparing treatment results for 1-4 vs ≥ 5 tumors: clinical article. J Neurosurg 2013;118:1258-68. [Crossref] [PubMed]
- Yamamoto M, Kawabe T, Sato Y, et al. Stereotactic radiosurgery for patients with multiple brain metastases: a case-matched study comparing treatment results for patients with 2-9 versus 10 or more tumors. J Neurosurg 2014;121:16-25. [Crossref] [PubMed]
- Bunevicius A, Lavezzo K, Shabo L, et al. Quality-of-life trajectories after stereotactic radiosurgery for brain metastases. J Neurosurg 2020;134:1791-9. [Crossref] [PubMed]
- Patient compliance with quality of life questionnaires. Italian Group for Evaluation of Outcomes in Oncology (I.G.E.O.). Tumori 1999;85:92-5. [PubMed]
- Leung A, Lien K, Zeng L, et al. The EORTC QLQ-BN20 for assessment of quality of life in patients receiving treatment or prophylaxis for brain metastases: a literature review. Expert Rev Pharmacoecon Outcomes Res 2011;11:693-700. [Crossref] [PubMed]
- Nguyen TK, Nguyen EK, Warner A, et al. Failed Randomized Clinical Trials in Radiation Oncology: What Can We Learn? Int J Radiat Oncol Biol Phys 2018;101:1018-24. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016;316:401-9. [Crossref] [PubMed]
- Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429-37. [Crossref] [PubMed]



