
Meta-analysis and systematic review of electronic bronchoscopy in refractory pneumonia
Introduction
Refractory pneumonia, as compared to general pneumonia, refers severe pneumonia with no significant improvement or even worsening after anti-infection treatment, along with a poor prognosis (1). Refractory pneumonia is generally treated with macrolide antibiotics. Patients who are resistant to macrolides or stop taking them before they are cured may experience a relapse, even worse than before treatment. Patients may develop lung abscesses, atelectasis, lung necrosis, and pleural effusion. These diseases lead to a very unsatisfactory lung condition, which is prone to deterioration and should be diagnosed as refractory pneumonia. Refractory pneumonia is a disease that is difficult to treat and can have serious consequences if it occurs, and it is difficult to predict the disease in advance because of its unobvious detection symptoms. Therefore, it is very important to find a way to diagnose refractory pneumonia at an early stage (2). With the increase in the rate of bacterial resistance and changes in the structure of the susceptible population in recent years, clinically refractory pneumonia is becoming more common. Causes of the disease can be classified into 3 main factors: bodily factors, microbial factors, and iatrogenic factors. In clinical treatment, the main contradictions should be based on corresponding treatment measures.
Since its clinical adoption, electronic bronchoscopy (EBS) has become an indispensable tool for the diagnosis and treatment of adult respiratory diseases, as it is soft and bendable (3). It can visually display the morphology and structure of the bronchus, and it can perform bronchoalveolar lavage and clamp foreign bodies through its biopsy hole to aid in further clinical diagnosis and treatment. Therefore, it is widely used in the diagnosis and pathogenic detection of various intractable respiratory diseases and is used to assist in the treatment of clinical respiratory diseases (4). With the continued development of medical technology and accumulation of clinical experience with this procedure, the clinical adoption of EBS has developed rapidly and gradually become favored by clinicians (5). At present, many methods such as bronchoalveolar lavage, bronchoalveolar brushing, bronchial mucosal biopsy, and transbronchial lung biopsy are used in clinical practice. EBS can be placed deep into the lower respiratory tract to collect specimens, while inflicting little damage and providing accurate sampling, which significantly improves the accuracy of lesion detection. It uniquely contributes to the study of the occurrence and development of respiratory diseases and the assessment of curative effects and prognosis (6,7).
However, there is a lack of relevant literature on the efficacy and safety of EBS in the treatment of refractory pneumonia. Therefore, with the aim of providing a theoretical basis for the evaluation of the efficacy of EBS in the treatment of refractory pneumonia, clinical randomized controlled trials (RCTs) of EBS in the treatment of refractory pneumonia were screened to further confirm its efficacy, and a meta-analysis was used to conduct a systematic evaluation. The novelty of this study is the examination of the use of a bronchial electron microscope instead of an ordinary fiberoptic bronchoscope. Refractory pneumonia requires early lavage under bronchial electron microscopy to better observe the cleaning effect. Local lavage unblocks the respiratory tract and clears the secretions of the lower respiratory tract and phlegm thrombus. It is helpful for the diagnosis and treatment of refractory pneumonia, especially in children with sputum blockage combined with atelectasis or persistent atelectasis, which can cause refractory pneumonia. Large flake shadows are quickly discerned by imaging. Therefore, the use of bronchial electron microscopy in the early stage can provide better treatment and cleaning of the respiratory tract. We present the following article in accordance with the PRISMA reporting checklist (available at https://dx.doi.org/10.21037/apm-21-2133).
Methods
Literature inclusion and exclusion criteria
The inclusion criteria were as follows: (I) the participants of the study were patients who were clinically diagnosed with refractory pneumonia; (II) the research type was an RCT published in English-language databases; (III) the treatment method of the experimental group was EBS treatment, the surgical treatment of the control group was conventional treatment, and the baseline data of the experimental group and the control group were comparable; and (IV) the evaluation indicators of the study outcomes included postoperative satisfaction of patients and occurrence of adverse reactions.
The exclusion criteria were as follows: (I) research types were non-RCT studies, such as retrospective studies, case reports, and cohort studies; (II) the research objects were animals or cells; (III) the literature types were unpublished documents or non-English documents such as degree theses; (IV) the treatment method was EBS; (V) the research participants were patients with refractory pneumonia combined with other diseases; and (VI) the literature had incomplete research data from which a corresponding effect index could not be calculated.
Literature search
Six English-language databases (PubMed, Embase, MEDLINE, Ovid, Springer, and Web of Science) were searched for literature published up until December 31, 2020. Publicly published RCT studies of EBS in the diagnosis and treatment of refractory pneumonia were retrieved. Literature search terms consisted of subject terms and keywords, including “Refractory pneumonia,” “Electronic bronchoscope,” and “Conventional drugs”. “And” or “or” were used for joint searches among search terms, and the literature search was carried out by 2 researchers using independent search methods.
Literature screening
Two researchers independently screened the documents. After the literature search was completed, Note Express 3.2 was used to establish a literature database, and the duplicates of the retrieved literature were then removed. The 2 researchers then manually screened the remaining documents. They were required to read the titles and abstracts of the retrieved documents first and eliminate documents that obviously did not meet the inclusion criteria. The decision whether to include the literature was determined according to the inclusion and exclusion criteria. If the 2 researchers disagreed on the process of document screening, the disagreement would be resolved through discussion. If a consensus was still not reached, a third party would be invited to make a decision after arbitration.
Data extraction
Two hospital researchers were selected to examine patients for disease symptoms, interventions, outcome indicators, and bias assessments based on basic information from the included literature, and data record table was fabricated. Researchers needed to screen literatures from a large number of literatures, extract valid data and information, and screen literatures according to inclusion and exclusion criteria. Researchers can’t exchange methods and review criteria. After the two researchers had completed the examination, they can exchange literature. In the literature screening process, if two researchers disagreed, they should discuss and communicate with each other. If there was no result of communication, the third-party researcher shall be asked to appraise again. The data extracted from the documents that met the inclusion criteria mainly included (I) the title of the document, the title of the document, the first author, the year the document was published, and the author’s main research contributions; (II) the basic information of patients, such as age, nationality, gender, and intervention measures; (III) different methods in the literature, random methods selected in the literature, and whether the literature was blind; (IV) indicators of disease detection and results of the final incidence and total number of patients.
Literature quality assessment
The risk of bias was assessed using the bias analysis provided in 5.0.2 of the Cochrane Intervention Systems Review Manual. Bias risk assessment literature was performed. If the results were good and the risk was low, the literature quality was high. The evaluation indicators were randomization, randomization, blindness, patient awareness, study data integrity, and outcome indicators. If there was no agreement between the two researchers, the two researchers should discuss and communicate with each other. If there was no result, a third researcher was asked to confirm again.
Statistical analysis
The Cochrane Handbook for Systematic Review of Interventions 5.0.2 was used to assess the risk of literature bias. Stata 11.0 (Texas College Station StataCorp, USA) was employed to consolidate statistics in the included literature. Comprehensive statistical data were analyzed using Review Manager 5.3. Forest maps and funnel plots were used to indicate the accuracy, specificity, and sensitivity of the literature. The relative risk (RR) of binary variables in postoperative adverse event count data should be the same. 95% confidence intervals (95% CI) and continuous variables in the measurements, including heart rate (HR), mean arterial pressure (MAP), and visual analog scale (VAS), were calculated. If the units of measurement indicators were the same, the weighted mean difference (WMD) was used as the effect size. Standardized mean difference (SMD) was used as the effect size if the measurement measures were in different units. The I2 test was used to assess heterogeneity in the included literature. The greater the I2 statistic, the greater the heterogeneity. If I2>50% and it failed to explain the source of heterogeneity, a random-effects model (REM) combined with effect size was used for a meta-analysis. If I2<50%, which meant good heterogeneity of the literature, and fixed effects model (FIX) was used for analysis combined with effect size. The combined effect size was tested with a 95% confidence interval. P<0.05 indicated a statistically significant difference. Binary variables were tested with 95% confidence intervals. When 95% CI >1 or <1, the data were statistically significant. When the 95% confidence interval contains 1, the data were not statistically significant. Continuous variables were tested with 95% confidence intervals. When 95% CI >0 or <0, the data were statistically significant.
Sensitivity analysis
Sensitivity analysis was used to evaluate whether the results of meta-analysis were stable and reliable. Specifically, by excluding some controversial studies, low-quality studies, or using different statistical methods/effect models to analyze the same set of data, the changes in the results of meta-analysis were observed. If the sensitivity analysis did not substantially change the results, the results were considered reliable; if the results were substantially changed, this indicated that any interpretation or drawing of conclusions from this data set should be done with caution.
Results
Literature search results
A preliminary search of literatures in six English databases was conducted, and 455 related literatures were found. After the initial search was completed, 198 literatures remained after the copies were deleted according to inclusion and exclusion criteria. Titles and abstracts were carefully screened, and literatures were screened according to inclusion and exclusion criteria. Literatures with high bias should also be deleted. The 2 researchers then read and cross-examined the text of the literature, and screened and excluded the literature accordingly. Finally, a total of 6 articles were included in this study (Figure 1). They were all publicly published RCT studies, and the publication time ranged from 2000 to 2020. The 6 articles contained a total of 796 study participants, and baseline data, such as the age of patients in the experimental group and the control group, were comparable (Table 1). The indicators of the 6 included studies were evaluated according to the main intervention: electronic bronchoscopy. The methodological quality of each study in the intervention results was above average and at the same level of literature quality, and there was no methodological heterogeneity. Therefore, sensitivity analysis was not conducted.
Table 1
| First author | Published year | Group | Sample size | Counter measure |
|---|---|---|---|---|
| Mueller ( |
2007 | Experimental | 52 | Electronic bronchoscopy |
| Control | 52 | Conventional therapy | ||
| Ruiz ( |
2000 | Experimental | 20 | Electronic bronchoscopy |
| Control | 20 | Conventional therapy | ||
| Fagon ( |
2000 | Experimental | 204 | Electronic bronchoscopy |
| Control | 209 | Conventional therapy | ||
| Solé Violán ( |
2000 | Experimental | 43 | Electronic bronchoscopy |
| Control | 45 | Conventional therapy | ||
| Timsit ( |
2001 | Experimental | 47 | Electronic bronchoscopy |
| Control | 47 | Conventional therapy | ||
| Zhang ( |
2014 | Experimental | 22 | Electronic bronchoscopy |
| Control | 35 | Conventional therapy |
Bias risk assessment of included literature
The Cochrane Handbook of Systematic Evaluation of Interventions 5.0.2 was used to assess the risk of bias for the six articles in this study. The deviation risk maps were output using Review Manager 5.3. The risk assessment of bias included seven items. (I) The six literatures included in this study (8-13) were grouped by “different groups of treatment strategies”, indicating low risk. (II) None of the six documents mentioned the existence of “grouping randomization” and did not explain the randomization method, indicating that the risk was not clear. (III) As for whether the subjects were informed, five of the six literatures (8-10,12,13) mentioned that “the patients knew and signed the informed consent form” but did not mention whether the experimenter was blind, so the risk was not clear. (IV) For the blind method of the result assessors, six articles did not mention whether the result assessors were blind, suggesting that the risk was not clear. (V) For the corresponding of methods and results of the literature, the result data of the six articles were complete, indicating a low risk. (VI) For selective reports, there was no selective report in six articles (8-13), indicating a low risk. (VII) For other bias risks, there were three articles (10,11,13) had different numbers in the experimental group and the control group, indicating high risk. In addition, it could not be determined whether there were other deviations in three of the documents (9,12,13), and the risks were considered unclear. The results of the deviation risk assessment were shown in Figures 2 and 3.
Clinical efficacy
In this study, a total of 3 documents (9,11,13) analyzed the clinical efficacy of EBS in patients. A total of 185 patients with refractory pneumonia were included, including 85 in the experimental group and 100 in the control group. The heterogeneity test results showed that I2=65% and P=0.06, which suggested that there was heterogeneity in the articles. Therefore, the REM was used for analysis (results in Figure 4). The results showed that the combined effect of meta-analysis was as follows: OR =3.8, 95% CI: 1.11–12.99, Z=2.13, and P=0.03. The diamond in the forest plot was on the right side of the vertical line, which indicated that the clinical efficacy of EBS in patients with refractory pneumonia was higher than that in the control group.

Mortality rate
A total of 3 articles (8-10) in this study analyzed the mortality rate of patients, and 557 patients with refractory pneumonia were included, including 276 in the experimental group and 281 in the control group. The heterogeneity test results showed that I2=0% and P=0.48, which suggested that there was heterogeneity in the articles. Therefore, FEM was used for analysis (results in Figure 5). The results showed that the combined effect of meta-analysis was the following: OR =0.7, 95% CI: 0.3–1.63, Z=0.82, and P=0.41. The diamond in the forest plot was on the left of the vertical line, which indicated that the mortality rate of patients with refractory pneumonia treated by EBS was lower than that of the control group.
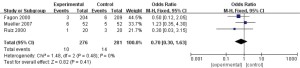
Infection
In this study, a total of 3 documents (8,11,12) analyzed patients’ infection status. A total of 286 patients with refractory pneumonia were enrolled, including 142 in the experimental group and 144 in the control group. The heterogeneity test results were I2=0% and P=0.86, which suggested that there was heterogeneity in the articles. Therefore, the FEM was used for analysis (results in Figure 6). The combined effect of meta-analysis was as follows: OR =0.84, 95% CI: 0.5–1.39; Z=0.69, and P=0.49. The diamond in the forest plot was on the left side of the vertical line, which indicated that the infection rate of patients with refractory pneumonia treated by EBS was lower than that of the control group.
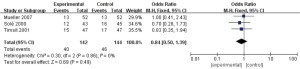
ICU hospitalization days
A total of 4 articles (8-10,13) in this study analyzed the length of stay of patients in the ICU. A total of 574 patients with refractory pneumonia were enrolled, including 278 in the experimental group and 296 in the control group. The heterogeneity test results were I2=17% and P=0.3, which suggested that there was heterogeneity in the articles. Therefore, the FEM was used for analysis (results in Figure 7). The combined effect of meta-analysis was as follows: OR =0.59; 95% CI: 0.36–0.98, Z=2.04, and P=0.04. The diamond in the forest plot was on the left side of the vertical line, which indicated that the number of days of ICU hospitalization for patients with refractory pneumonia treated by EBS was lower than that of the control group.
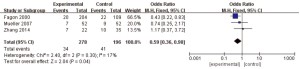
Days of antibiotic use
In this study, a total of 3 documents (8,10,11) analyzed the number of days patients took antibiotics. A total of 605 patients with refractory pneumonia were enrolled, including 299 in the experimental group and 306 in the control group. The heterogeneity test results were I2=62% and P=0.07, suggesting that there was a certain degree of heterogeneity among the studies. Therefore, the REM was used for analysis, (results in Figure 8). The combined effect of meta-analysis was as follows: OR =0.39, 95% CI: 0.18–0.84, Z=2.41, and P=0.02. The diamond in the forest plot was on the left side of the vertical line, which indicated that the number of days of antibiotic use in patients with refractory pneumonia treated by EBS was lower than that in the control group.

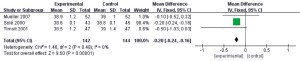
Body temperature
In this study, a total of 3 documents (8,11,12) analyzed the body temperature of patients during treatment, and 286 patients with refractory pneumonia were enrolled, including 142 in the experimental group and 144 in the control group. The heterogeneity test results showed that I2=0% and P=0.48, which suggested that there was heterogeneity in the articles. Therefore, the FEM was used for analysis (results in Figure 9). The combined effect of meta-analysis was as follows: MD =−0.20, 95% CI: −0.24 to −0.16, Z=9.5, and P<0.00001. The diamond in the forest plot was on the left side of the vertical line, which indicated that the body temperature of patients with refractory pneumonia treated with EBS was lower than that of the control group.
White blood cell count
In this study, a total of 3 documents (8,11,12) analyzed the patients’ white blood cell counts, and 286 patients with refractory pneumonia were enrolled, including 142 in the experimental group and 144 in the control group. The heterogeneity test results showed that I2=0% and P=0.93, which suggested that there was heterogeneity in the articles. Therefore, the FEM was used for analysis (results in Figure 10). The combined effect of meta-analysis was as follows: MD =0.55, 95% CI: −0.57 to 1.67, Z=0.96, and P=0.34. The diamond in the forest plot was on the right side of the vertical line, which indicated that the white blood cell count of patients with refractory pneumonia treated with EBS was higher than that of the control group.
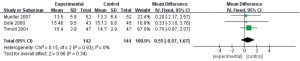
PaO2:FIO2 ratio
In this study, a total of 3 documents (8,11,12) analyzed the PaO2:FIO2 ratio of patients, and 286 patients with refractory pneumonia were enrolled, including 142 in the experimental group and 144 in the control group. The heterogeneity test results showed that I2=0% and P=0.49, which suggested that there was heterogeneity in the articles. Therefore, the FEM was used for analysis (results in Figure 11). The combined effect of meta-analysis was as follows: MD =−9.96, 95% CI: −13.31 to −6.61, Z=5.83, and P<0.00001. The diamond in the forest plot was on the left side of the vertical line, which indicated that the PaO2:FIO2 ratio of patients with refractory pneumonia treated by EBS was lower than that of the control group.
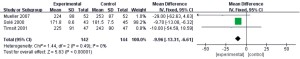
Publication bias analysis
The results of postoperative adverse reaction indexes were analyzed in terms of publication bias using Review Manager 5.3 (results in Figure 12). The clinical efficacy, mortality, infection, ICU hospitalization days, antibiotic use days, body temperature, white blood cell count, and PaO2:FIO2 ratio were basically distributed within the credible interval, and the literature bias was low.
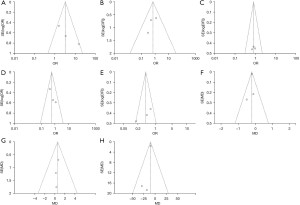
Discussion
Refractory pneumonia is not a single disease, and the reasons for intractable pneumonia vary from person to person. Basic diseases or immune damage often make the clinical manifestations of pneumonia imperceptible and atypical, which can delay diagnosis and treatment or lead to more complications and increased complexity of treatment (14-16). In clinical treatment, the pathogens must first be identified to avoid bacterial infection in the upper respiratory tract. Bacterial sampling must be carried out in the lower respiratory tract, so precision instruments are required (17).
The emergence of bronchoscopy has provided convenience in clinical diagnosis and treatment, but early bronchoscopy has disadvantages including large diameter and poor lighting. With the rise in improvement of glass optical fiber technology, the progress of related technologies, and the demand of clinical needs, the resolution of bronchoscopes has continued to increase, the field of view has widened, and the diameter of the insertion point has grown smaller. At present, the smallest diameter of the bronchoscope can be 1.2 mm (18-20). As clinical experience in China has grown and medical technology has advanced, EBS has been able to provide increasing benefit in the diagnosis and treatment of clinical symptoms, with greater higher safety (21,22). Patients with refractory mycoplasma pneumoniae pneumonia often have respiratory mucus obstruction, atelectasis, and other conditions. Early bronchoscopy and bronchoalveolar lavage treatment to clear respiratory secretions and phlegm thrombus has an important role in reducing symptoms such as high fever, promoting lung recruitment, and reducing the occurrence of sequelae. After bronchoscopy lavage treatment is applied in patients with refractory pneumonia, the symptoms of fever and cough are significantly reduced, and the absorption time of lung shadows and the length of hospitalization are significantly shortened.
To systematically evaluate the application value of EBS in the diagnosis and treatment of refractory pneumonia, a total of 6 reports were included in this study. A meta-analysis was performed to systematically evaluate the efficacy of EBS in the treatment of refractory pneumonia. The results showed that the clinical efficacy of treatment in the experimental group was significantly higher than that of the control group, the adverse outcomes, such as infection and death, were lower than those of the control group. Studies have revealed that EBS directly inspect the bronchial lumen of grade III to IV pneumonia (23). Direct suction and removal of sputum and secretions at the target location can be realized by EBS, and the lavage of sputum stasis with normal saline can dilute the sputum to stimulate cough and facilitate the expectoration and aspiration of sputum (24), which greatly reduces the survival rate and reproduction of bacteria. EBS thus facilitates the rapid recovery of patients (25,26). It was also found that the body temperatures of patients in the experimental group were lower during treatment, and the results of biochemical examinations were better than those of the control group, indicating that EBS had a good therapeutic effect and resulted in less damage to the body and stress responses, which was conducive to the improvement of the disease. Bronchial electron microscopy can also be used in patients with COVID-19. Patients with COVID-19 are weak in coughing and sputum production, which leads to a further decline in lung oxygenation. In critically ill patients, phlegm and mucus plugs cause treatment difficulties and aggravate their condition. Sputum suction and lavage with the assistance of bronchoscopy are important treatments for symptomatic relief and recovery of critically ill patients. Most critically ill patients with COVID-19 require tracheal intubation or tracheotomy for ventilator-assisted ventilation, and bronchoscopy can be used for guidance and monitoring.
Conclusions
In this study, a total of 6 articles on EBS diagnosis and treatment of refractory pneumonia were included in the meta-analysis, involving 796 patients. The results showed that EBS can significantly reduce the incidence of adverse postoperative outcomes and treatment time for patients compared with conventional treatment, while improving the clinical efficacy; this demonstrates the significant advantages of EBS treatment. However, this study also has certain limitations, which mainly includes the considerable publication bias of some of the included literature. In addition, due to differences in the research directions of the authors of the literature, some analysis indicators contained a small number of samples, and the meta-analysis results are thus not sufficiently accurate. Therefore, in follow-up studies, more large samples and high-quality articles will be included to verify the clinical effect of EBS in the treatment of refractory pneumonia.
Acknowledgments
Funding: This study was supported by funding from the Hunan Provincial Health Commission Project (No. 20200855).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-2133
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-2133). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhai YY, Wu SZ, Yang Y, et al. An analysis of 20 clinical cases of refractory mycoplasma pneumonia in children. Ann Palliat Med 2020;9:2592-9. [Crossref] [PubMed]
- Musher DM, Abers MS, Bartlett JG. Evolving Understanding of the Causes of Pneumonia in Adults, With Special Attention to the Role of Pneumococcus. Clin Infect Dis 2017;65:1736-44. [Crossref] [PubMed]
- Saldias Peñafiel F, Gassmann Poniachik J, Canelo López A, et al. Features of community-acquired pneumonia in immunocompetent hospitalized adults according to the causal agent. Rev Med Chil 2018;146:1371-83. [PubMed]
- Petroianni A, Ceccarelli D, Conti V, et al. Aspiration pneumonia. Pathophysiological aspects, prevention and management. A review. Panminerva Med 2006;48:231-9. [PubMed]
- Herold CJ, Sailer JG. Community-acquired and nosocomial pneumonia. Eur Radiol 2004;14:E2-20. [Crossref] [PubMed]
- Cunha CB. The first atypical pneumonia: the history of the discovery of Mycoplasma pneumoniae. Infect Dis Clin North Am 2010;24:1-5. [Crossref] [PubMed]
- Hsu-Kim C, Hoag JB, Cheng GS, et al. The microbiology of postobstructive pneumonia in lung cancer patients. J Bronchology Interv Pulmonol 2013;20:266-70. [Crossref] [PubMed]
- Mueller EW, Croce MA, Boucher BA, et al. Repeat bronchoalveolar lavage to guide antibiotic duration for ventilator-associated pneumonia. J Trauma 2007;63:1329-37; discussion 1337. [Crossref] [PubMed]
- Ruiz M, Torres A, Ewig S, et al. Noninvasive versus invasive microbial investigation in ventilator-associated pneumonia: evaluation of outcome. Am J Respir Crit Care Med 2000;162:119-25. [Crossref] [PubMed]
- Fagon JY, Chastre J, Wolff M, et al. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med 2000;132:621-30. [Crossref] [PubMed]
- Solé Violán J, Fernández JA, Benítez AB, et al. Impact of quantitative invasive diagnostic techniques in the management and outcome of mechanically ventilated patients with suspected pneumonia. Crit Care Med 2000;28:2737-41. [Crossref] [PubMed]
- Timsit JF, Cheval C, Gachot B, et al. Usefulness of a strategy based on bronchoscopy with direct examination of bronchoalveolar lavage fluid in the initial antibiotic therapy of suspected ventilator-associated pneumonia. Intensive Care Med 2001;27:640-7. [Crossref] [PubMed]
- Zhang Y, Chen Y, Chen Z, et al. Effects of bronchoalveolar lavage on refractory Mycoplasma pneumoniae pneumonia. Respir Care 2014;59:1433-9. [Crossref] [PubMed]
- Don M, Canciani M, Korppi M. Community-acquired pneumonia in children: what's old? What's new? Acta Paediatr 2010;99:1602-8. [Crossref] [PubMed]
- Lee KY, Youn YS, Lee JW, et al. Mycoplasma pneumoniae pneumonia, bacterial pneumonia and viral pneumonia. J Pediatr (Rio J) 2010;86:448-50. [Crossref] [PubMed]
- Wang Y, Ma L, Li Y, et al. Epidemiology and clinical characteristics of pathogens positive in hospitalized children with segmental/lobar pattern pneumonia. BMC Infect Dis 2020;20:205. [Crossref] [PubMed]
- Crnich CJ, Safdar N, Maki DG. The role of the intensive care unit environment in the pathogenesis and prevention of ventilator-associated pneumonia. Respir Care 2005;50:813-36; discussion 836-8. [PubMed]
- Malek E, Lebecque P. Etology and treatment of community acquired pneumonia in children. J Pharm Belg 2007;62:21-4. [PubMed]
- Ishida T, Hashimoto T, Arita M, et al. Evaluation of community-acquired pneumonia guidelines of Japanese Respiratory Society: differentiation of atypical pneumonia and bacterial pneumonia. Nihon Kokyuki Gakkai Zasshi 2002;40:929-35. [PubMed]
- Gauchan E, Adhikari S. C-reactive Protein Versus Neutrophil/lymphocyte Ratio in Differentiating Bacterial and Non-bacterial Pneumonia in Children. J Nepal Health Res Counc 2016;14:154-8. [PubMed]
- Padilla Ygreda J, Lindo Pérez F, Rojas Galarza R, et al. Etiology of community acquired pneumonia in children 2-59 months old in two ecologically different communities from Peru. Arch Argent Pediatr 2010;108:516-23. [PubMed]
- Ito I, Ishida T, Hashimoto T, et al. Clinical comparison of Chlamydia pneumoniae pneumonia, ornithosis, and Mycoplasma pneumoniae pneumonia. Nihon Kokyuki Gakkai Zasshi 2001;39:172-7. [PubMed]
- Almirall J, Bolíbar I, Toran P, et al. Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest 2004;125:1335-42. [Crossref] [PubMed]
- Yankov IV, Shmilev TI. Ventilator-associated pneumonias in children (I)--diagnostic criteria, etiology and pathogenesis. Folia Med (Plovdiv) 2012;54:5-11. [Crossref] [PubMed]
- Vilibić Cavlek T, Mlinarić Galinović G, Turković B, et al. Etiology of atypical pneumonias in 2002. Results of the Croatian Institute of Public Health. Acta Med Croatica 2004;58:187-92. [PubMed]
- Hussain H, Waters H, Omer SB, et al. The cost of treatment for child pneumonias and meningitis in the Northern Areas of Pakistan. Int J Health Plann Manage 2006;21:229-38. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)





