
Serum 25-hydroxy vitamin D level is associated with cognitive impairment in people aged 65 years and older
Introduction
Recent studies have shown that vitamin D not only effects calcium and bone metabolism, but also protects cognitive function by multiple mechanisms (1-4). The evidence of the neuroprotective properties of vitamin D was primarily derived from previous studies in vivo and in vitro.
Vitamin D is a steroid hormone that is acquired through dietary intake and Vitamin D3 can be produced from 7-dehydrocholesterol in the dermis and epidermis upon exposure to ultraviolet B radiation. Ergocalciferol (D2) and cholecalciferol (D3) are 2 major forms of vitamin D. Vitamin D requires activation through a 2-step enzymatic pathway: in step 1, vitamin D is activated by enzyme 25 hydroxylase, producing 25-hydroxyvitamin D (25-OH-D), and in step 2, vitamin D activation produces an active form of 1,25(OH)D in the kidney. The level of 25-OH-D in the serum is widely used as a measure of vitamin D in the body (5).
Vitamin D deficiency is now recognized as a worldwide concern. Older adults are especially susceptible to becoming vitamin D deficient due to age-related dermatological changes and impaired capacity of renal function, as well as lower levels of exposure to sunlight and inadequate dietary vitamin D intake. Many studies have reported a correlation between a low vitamin D level and cognitive impairment. Our study set out to assess the relationship of vitamin D with cognitive function in older individuals. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-568).
Methods
Participants
A total of 299 patients aged 65 years or older (225 males and 74 females) were enrolled from the Geriatrics Ward of the Sixth People’s Hospital of Shanghai Jiaotong University between October 2010 and March 2011. The patients’ education levels were as follows: illiterate, 2 patients (0.6%); elementary school, 24 patients (8%); junior-high school, 43 patients (14.4%); high school, 36 patients (12%); and college education or beyond, 194 patients (64.8%).
Patients were excluded from the study if they had depression, Parkinson’s disease, severe hepatic or renal dysfunction, or primary hyperparathyroidism, or if they had experienced a transient ischemic attack in the previous 2 weeks, or a newly occurring cerebral infarction or hemorrhage in the previous 6 months. Those who had taken vitamin D supplement >400 IU per day or affected metabolic absorption of vitamin D, such as alpha-D3 or Rocaltrol® in the previous 3 months were also excluded.
Approval for this study was granted by the Ethics Committee of Shanghai Sixth People’s Hospital according to the principles of the Helsinki Declaration (as revised in 2013). All study participants gave their informed consent.
Blood parameters
Fasting blood samples were collected between at 6AM and 7AM and delivered to the laboratory within 1 hour. Serum 25-OH-D concentration was assayed with a radioimmunoassay kit (Roche Diagnostics GmbH, Germany). The minimum detectable level was 4 ng/mL. A competitive protein-binding assay with a vitamin B12 analysis kit (Siemens Healthcare Diagnostics Inc., USA) was used for measurement of the vitamin B12 level in the sera. Plasma glucose, cholesterol, aminotransferases, and creatinine were measured on an automatic analyzer (Hitachi 7080).
Dementia diagnosis
Cognitive function was assessed with the Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Clinical Dementia Rating (CDR), and Activities of Daily Living (ADL) scale.
Patients were diagnosed with dementia according to a standard protocol, with reference to the American Psychiatric Association’s criteria (DSM-IV). Mild cognitive impairment (MCI) was diagnosed based on the DSM-IV developed by Peterson but with some modifications (e.g., we defined individuals with an MMSE score of 28 to 30 and an MoCA score of less than 26 as MCI).
Statistical analysis
SPSS 13.0 (SPSS Inc., Chicago, Illinois, USA) was used for all statistical analyses. Numerical data were represented as mean ± standard deviation. The rank-sum test was applied for non-normally distributed data. The χ2 test was used for differences in proportion between groups. Spearman’s correlation test was applied to investigate the relationship of serum 25-OH-D level with MMSE/MOCA score. The association between plasma 25-OH-D concentration and cognitive function was examined through the construction of stepwise regression models. Statistical significance was set at P<0.05 (2-sided).
Results
25-OH-D status
Serum 25-OH-D concentrations among the 299 subjects ranged from 4.00 to 28.48 ng/mL (12.04±6.35 ng/mL). According to their 25-OH-D level, the patients were divided into 3 groups: group A (<10 ng/mL) 135 patients (45.15%); group B (10–19.99 ng/mL), 123 patients (41.13%); group C (>20 ng/mL), 41 patients (13.71%). The levels of 25-OH-D were 6.32±2.01 ng/mL in group A, 14.60±2.65 ng/mL in group B, and 23.23±2.57 ng/mL in group C.
Basic characteristics of patients in the 3 groups
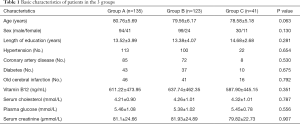
The characteristics of the study participants are shown in Table 1. The 3 groups did not differ significantly with respect to age, sex, years of education, hypertension, coronary artery disease, diabetes, old cerebral infarction, or blood chemistry test results (creatinine, cholesterol, blood sugar, and vitamin B12) (P>0.05).
Full table
MMSE and MoCA scores of the 3 groups (Table 2)

Full table
The MMSE and MoCA scores in group A were 26.02±3.99 and 21.56±5.59, respectively, compared with 27.34±2.79 and 23.94±4.74, respectively, in group B, and 27.65±2.54 and 24.95±4.45, respectively, in group C. The scores in Group A were statistically significantly different from those in groups B and C (both P<0.05). Group C had higher MMSE and MoCA scores than group B, but the scores did not differ significantly (P>0.05).
Prevalence of cognitive impairment (Table 3)

Full table
Among the 299 subjects for analysis, there were 42 (14%) patients with dementia, 140 (46.8%) with MCI, and 117 (39.1%) with normal cognitive function. Group A had an increased proportion of patients with cognitive impairment compared to groups B and C. The odds ratios were respectively 2.065 [confidence interval (CI): 1.231–3.61, P=0.004] group A compared to group B and 3.262 (CI: 1.585–6.714, P=0.001) group B compared to group C. Group B did not differ significantly from group C in relation to the prevalence of cognitive impairment.
Serum 25-OH-D levels in patients with dementia, MCI, and normal cognitive function (Table 4)

Full table
The serum 25-OH-D concentrations in individuals with dementia, MCI, and normal cognitive function were 9.11±1.01, 11.26±0.48, and 14.03±0.59 ng/mL, respectively. In each pairwise comparison, we found that the differences were significant (P<0.05).
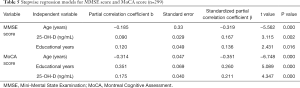
The association of 25-OH-D level with MMSE score and MoCA score (Table 5)
Full table
Positive associations of serum 25-OH-D level with the MMSE and MoCA scores were shown by a correlation coefficient r of 0.251 and 0.736, respectively (both P<0.01). Following adjustments for possible confounding variables such as sex, age, and years of education, both associations retained their significance (P<0.05). In the stepwise regression analysis, MMSE score and MoCA score were defined as the dependent variable individually, and age, sex, educational years, vitamin B12, vitamin D, plasma glucose, serum cholesterol, hypertension, diabetes, coronary artery disease, and old cerebral infarction were taken as independent variables. Among them, only 25-OH-D level (MMSE β score at 0.167 and MoCA β score at 0.211), age (MMSE β score at −0.319 and MoCA β score at −0.351), and educational years (MMSE β score at 0.136 and MoCA β score at 0.26) were kept in the final multiple regression model (P<0.05).
Discussion
In vitro and in vitro investigations have evidenced the effect vitamin D has on cognitive function (1,6,7). Vitamin D has been shown to play a role in protecting the brain from the damage inflicted by age-associated neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, by inhibiting the inducible nitric oxide synthase enzyme, the upregulation of which is seen with the occurrence of ischemic events (8). Vitamin D is also an upregulator of gamma glutamyl transpeptidase activity, through which it elevates the concentration of glutathione in glial cells. These cells then exert protective effects on oligodendrocytes and the integrity of the nerve conduction pathway, which is crucial to mental processing (9,10). There is evidence to suggest that vitamin D could protect the structure of the brain by modulating neuronal calcium homeostasis (11). The result as well as indicates that vitamin D not only maintains calcium and phosphorus homeostasis, but also protects the brain from cognitive damage by preventing cardiovascular and cerebrovascular disease .
The relationship between serum 25-OH-D concentration and cognition is still unclear. In this paper, we found that MMSE and MoCA scores were evidently lower in patients who have severe 25-OH-D deficiency than in those who have vitamin D deficiency. Serum 25-OH-D level and MMSE score were positively correlated, and their association remained significant even after adjustment for sex, age, and education.
As a screening tool for cognitive impairment, MMSE possesses many beneficial features such as high sensitivity and operability. But it may sometimes be confounded by educational level or linguistic competence, resulting in a false positive result. The MoCA scale is a screening tool to identify MCI promptly, with a sensitivity of 92.4% and a specificity of 88.4%. Moreover, the MoCA is more sensitive to cognitive impairment-related diseases causing major damage in operational function and concentration (12). We applied both the MMSE and MoCA to assess the cognitive function of older individuals. Through the complementarity of the two scales to reduce bias of outcomes, thus improving the reliability of the conclusion.
The current investigation set out to explore the association of vitamin D with cognitive function in a cohort of older individuals (≥65 years). The findings indicate that the level of serum 25-OH-D is usually decreased in older individuals compared to younger people. The World Health Organization once considered a 25-OH-D level of less than 20 ng per milliliter to indicate vitamin D deficiency and a level of less than 10 ng per milliliter to indicate vitamin D insufficiency. Moreover, laboratory reference ranges have recently changed, with a serum 25-OH-D level in the range of 30 to 76 ng per milliliter (75 to 190 nmol per liter) now considered to be normal for adults. Using this range, as many as 50 to 80% of people in the general population are estimated to be vitamin D insufficient (13,14). Data from studies in the United States and Europe indicate that 40% to 100% of older people living in the community (i.e., not in nursing homes) have insufficient levels of vitamin D, and among postmenstrual women, over 50% have 25-OH-D levels below the optimal line (30 ng/mL, or 75 nmol/L) (15). Similar conclusions have been reached by some Chinese investigations (16,17).
All participants (100%) in our study had vitamin D insufficiency or deficiency, with 25-OH-D levels of less than 30 ng/mL. These findings are in agreement with recent studies. A study by Przybelski et al. reported serum 25-OH-D3 level to be positively correlated with MMSE score (r=0.23; P=0.006) (18). Similarly, Oudshoorn analyzed serum 25-OH-D and vitamin D levels, and explored their relationships with MMSE score in 225 outpatients diagnosed with probable Alzheimer’s disease. The results showed there to be a significant correlation between 25-OH-D concentrations and MMSE scores but no association between vitamin B levels and MMSE scores (19). A cross-sectional study of 318 geriatric patients receiving home care also produced similar results (20). Recently, Annweiler found that high vitamin D dietary intake was linked to a reduced risk of Alzheimer’s disease among 498 older females living in the community with researching for 7 years (21). They also found that a decreased level of 25-OH-D was related to MCI status in older people living in the community who did not have dementia but had subjective memory complaints (22).
In contrast to the findings above, Evatt reported no correlation between vitamin D insufficiency and Alzheimer’s disease (23). McGrath failed to find a relationship between 25-OH-D and psychometric test performance in teenagers (16–19 years) and adults (20–59 years) in the National Health and Nutrition Examination Survey (NHANES) data. However, they did identify a significant, albeit extremely small, inverse association between 25-OH-D level and Learning and memory ability in individuals aged 60 to 90 years (20,24). Limitations existed in McGrath’s research including the male-only population as well as the study design. To date, studies examining the relationship between 25-OH-D status and cognitive function have reported conflicting results. In addition, there has also been limited by small sample sizes, a lack of control for potential confounding factors and a lack of a standard test to measure cognition in different populations.
The present investigation also has some limitations. Firstly, due to the cross-sectional design, no causality can be concluded from vitamin D deficiency and cognitive impairment. Second, it was a small-cohort study, and a prospective study involving a larger cohort is needed in order to conduct a more powerful analysis of the causality of vitamin D deficiency and cognitive impairment. Moreover, there are several intractable confounding factors to serum vitamin D level, such as dressing style, sunscreen use, skin pigment type, and diet, which may have affected our results.
In conclusion, vitamin D deficiency is prevalent in older individuals. Our study found that severely 25-OH-D-deficient (<10 ng/mL) patients tend to score lower in MMSE and MoCA tests and have an increased risk of developing cognitive impairment. The results suggest an association between vitamin D status and cognition. Further investigations are needed to establish whether vitamin D supplements can provide a cost-efficient and safe means of reducing the risk of developing cognitive impairment for the world’s aging population.
Acknowledgments
The authors would like to thank Geng Hui, Xingliang Zhang, and Zhen Zhang for their support in the data management of this study, and Dr. Zhenlin Zhang for his advice on experimental design.
Funding: This study was supported by the Research Fund of Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University (0757).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-568
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-568
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-568). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval for this study was granted by the Ethics Committee of Shanghai Sixth People’s Hospital according to the principles of the Helsinki Declaration (as revised in 2013). All study participants gave their informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Garcion E, Wion-Barbot N, Montero-Menei CN, et al. New clues about vitamin D functions in the nevous system. Trends Endocrinol Metab 2002;13:100-5. [Crossref] [PubMed]
- Leung PS. Does vitamin D supplementation reduce type 2 diabetes risk? Ann Transl Med 2019;7:614. [Crossref] [PubMed]
- Becker A, Eyles DW, McGrath J, et al. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res 2005;161:306-12. [Crossref] [PubMed]
- Wang Q, Yu D, Wang J, Lin S. Association between vitamin D deficiency and fragility fractures in Chinese elderly patients: a cross-sectional study. Ann Palliat Med 2020;9:1660-5. [Crossref] [PubMed]
- Institute of Medicine (U. S.) Standing Committee on the cientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC:National Academy Press, 1997.
- McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J 2008;22:982-1001. [Crossref] [PubMed]
- Bourre JM. Effects of nutrients (in food) on the structure and function of the nervous system: update on dietary requirements for brain. Part 1:micronutrients. J Nutr Health Aging 2006;10:377-85. [PubMed]
- Garcion E, Nataf S, Betod A, et al. 1, 25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res 1997;45:255-67. [Crossref] [PubMed]
- Baas D, Prufer K, Ittle ME, et al. Rat oligodendrocytes express the vitamin D3 receptor and respond to 1, 25-dihydroxyvitamin D3. Glia 2000;31:59-68. [Crossref] [PubMed]
- Garcion E, Sindji L, Leblondel G, et al. 1, 25-Dihydroxyvitamin D3 regulates the synthesis of gamma-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J Neurochem 1999;73:859-66. [Crossref] [PubMed]
- Brewer LD, Thibault V, Chen KC, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci 2001;21:98-108. [Crossref] [PubMed]
- Zadikoff C, Fox SH, Tang-Wai DF, et al. A comparison of the minimental state exam to the montreal congnitive assessment in identifying congnitive deficits in Parkinson’s disease. Mov Disord 2008;23:297-9. [Crossref] [PubMed]
- Holick MF, Siris ES, Binkley N, et al. Prevalence of vitamin D inadequacy among North American postmenopausal women receiving osteoporosis therapy. J Clin Endocrinol Metab 2005;90:3215-24. [Crossref] [PubMed]
- Ginde AA, Liu MC, Camargo CA Jr. Eemographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med 2009;169:626-32. [Crossref] [PubMed]
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. [Crossref] [PubMed]
- Lu L, Yu Z, Pan A, et al. Plasma 25-hydroxyvitamin D Concentration and metabolic syndrom Among Middle-Aged and Eldly Chinese Individuals. Diabetes Care 2009;32:1278-83. [Crossref] [PubMed]
- Zhang H, Huang Q, Zhang Z, et al. Vitamin D status in winter of postmenopausal women in Shanghai. China Journal of Osteoporosia 2011;1:43-46.
- Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys 2007;460:202-5. [Crossref] [PubMed]
- Oudshoorn C, Mattace-Raso FU, van der Velde N, et al. Higher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 2008;25:539-43. [Crossref] [PubMed]
- Buell JS, Dawson-Hughes B, Scott TM, et al. 25-hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology 2010;74:18-26. [Crossref] [PubMed]
- Annweiler C, Rolland Y, Schott AM, et al. Higher Vitamin D Dietary Intake Is Associated With Lower Risk of Alzheimer's Disease: A 7-Year Follow-up. Gerontol A Bio Sci 2012;67:1205-11.
- Annweiler C, Schott AM, Fantino B, et al. Vitamin D insufficiency and mild cognitive impairment: cross-sectional association. Eur J Neurol 2012;19:1023-9. [Crossref] [PubMed]
- Evatt ML, Delong MR, Khazai N, et al. Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch Neurol 2008;65:1348-52. [Crossref] [PubMed]
- McGrath J, Scragg R, Chant D, et al. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology 2007;29:49-54. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


