
Safflower extract improves depression in mice by inhibiting the TLR4-NLRP3 inflammation signaling pathway
Introduction
Depression is a common and serious mental disorder affecting 16% of the world’s population. It is characterized by general depression, loss of interest in usual activities, and is accompanied by symptoms such as physical discomfort (1). Nowadays, there are many antidepressants, including selective serotonin reuptake inhibitors (SSRIs), monoamine oxidase inhibitors (MAOIs), and tricyclic antidepressants (TCA). Emerging evidence has shown that depression is associated with inflammation (2). Clinical studies have suggested that compared with healthy people, patients suffering from depression have higher circulating levels of pro-inflammatory cytokines (3). Animal experiments have also demonstrated that depressed mice produced large amounts of cytokines including interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) in the serum and brain tissue (4). In addition, some meta-analyses found that pro-inflammatory cytokines are positively correlated with depression. However, the mechanism involved has not yet been clearly investigated. IL-1β is an important neuroendocrine mediator, and its production and secretion are mainly regulated by the inflammasome. The nucleotide-binding oligomerization domain-like receptor family NLRP3 inflammasome is a cytoplasmic receptor containing NLRP3, ASC, and caspase-1 (5). Therefore, regulating the TLR4-NLRP3 signaling pathway and further inhibiting neuroinflammation may be a treatment strategy to improve depression. Safflower (Carthamus tinctorius L.) has long been a hot topic of research due to its diverse chemical components as well as extensive pharmacological effects. More and more studies have shown that safflower extract (SE) could inhibit inflammation and oxidation, protect the heart, and enhance immune function (6). Studies have shown that spermidine in safflower can alleviate depression symptoms, but the underlying mechanism is not yet clear (7). Whether SE can inhibit inflammation by inhibiting the TLR4 signaling pathway in depression remains unclear. In this study, we speculated that SE could treat depression through the inhibition of TLR4 activation and NLRP3 downregulation. To test this hypothesis, we established a mouse model of depression to investigate the effect of SE on the expression of the inflammatory cytokines TLR4 and NLRP3.
We present the following article in accordance with the ARRIVE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1728).
Methods
Animals
A total of 60 adult male ICR mice (18–22 g) were purchased from the Laboratory Animal Center, Zhongshan School of Medicine, Sun Yat-sen University. Mice were placed in cages alone for about 1 week to let them adapt to the environment under standard housing conditions: 12 h light/12 h dark cycle; abundant food and water supply; temperature maintained at 22±2 °C and humidity of 50%±10%. All animal experiments were approved by the Animal Experiment Ethics Committee (AEEC) of Zhongshan School of Medicine and were in compliance with the Guide for the Care and Use of Laboratory Animals.
Medications and treatment
Fluoxetine hydrochloride (FLU) was purchased from Changzhou Siyao Pharmaceutical Co., Ltd. (Changzhou, China); safflower extract was purchased from Nanning Shengyuan Chinese Medicine Co., Ltd. (Lot No. 140901, Nanning, China) and decocted in water to prepare SE solution. Cli-095 (TLR4 specific inhibitor) was purchased from Invivo Gen (San Diego, California, USA) and dissolved in 1% dimethyl sulfoxide (DMSO). Depression was induced with chronic unpredictable mild stress (CUMS). The mice were randomly assigned into 6 groups: control group, depression group (CUMS only), SE (10 mg/kg) + depression group (CUMS+SE, 10 mg/kg), SE (30 mg/kg) + depression group (CUMS+SE, 30 mg/kg), Cli-095 + depression group (CUMS+Cli-95), and FLU + depression group (CUMS+FLU). Both the control group and the CUMS group were given intragastric administration of medication dissolved in sterile 0.9% normal saline. The SE treatment group received different concentrations of SE (10 and 30 mg/kg). The concentration of Cli-095 for the Cli-095 treatment group was 3 mg/kg. The concentration of FLU for the FLU treatment group was 10 mg/kg. Treatments were given once daily for 4 consecutive weeks. In order to determine whether SE can alleviate depression, we used CUMS to establish a mouse model of depression. After 1 week of adaptation to the environment, the mice were exposed to CUMS for 2 weeks (week 1 and 2). Following the exposure, these mice were administered with either SE, Cli-095, or FLU for 4 weeks (week 3–6). CUMS continued during treatment (week 3–6).
Depression model
After 1 week of adaptation to the new environment, the mice in the control group were kept in a separate room and the other 5 groups were modeled for depression. These animals were exposed to stress every week, including 12 h without food, 5 min cold swimming, 10 min cage shaking, 30 min behavior restraint, 5 min tail pinch, 8 h light/dark reversal, and 12 h cage tilting with a 45° angle. We applied stressors continuously during the day and night individually. In order to prevent the habituation of mice to the stressor and ensure the unpredictability of stressors, all stressors were randomly arranged within a week and repeated throughout the experiment.
Sucrose preference test (SPT)
In the first 24 h, 2 bottles (same bottle) of 1% (w/v) sucrose solution (SS) were placed in each cage in order to train mice to adapt to the SS. After 24 h of adaptation, we replaced 1 bottle of SS with cooled boiled-water (CBW) in the same bottle. The positions of the water bottle and the SS bottle were exchanged every 12 h to avoid any influence caused by the bottle location. When these mice adapted to the SS, we deprived them of water and food for 12 h. At 24 h later, the volumes of SS and CBW consumed were calculated. Sucrose preference (SP) = volume of SS consumption/(volume of CBW consumption and volume of SS consumption) ×100%.
Open field test (OFT)
Locomotor activity was measured by the OFT. The testing field consisted of a lighted glass-box (100 cm long, 100 cm wide, and 50 cm high) and a real time monitoring system. The test subject was initially placed right in the bottom center of the box, and was allowed to move freely in the box. Then, the total movement distance during 60 min was recorded automatically.
Forced swim test (FST)
The test subject was put in an open cylindrical glass water tank (diameter 20 cm, height 40 cm) filled with water of 25 cm depth at 22±1 °C. The environment around the tank was kept quiet when the test was conducted. The mice were placed in the cylinder individually for 6 min, and immobility time was recorded during the last 4 min of the test. The mouse was considered immobile only when it floated in the water without struggling. After each experiment, the used water was changed with fresh water.
Tail suspension test (TST)
The mouse was suspended by its tail in a box (25 cm long, 25 cm wide, and 30 cm high) with its head 5 cm above the bottom of the box. The test was also conducted in a relatively quiet environment as described before. The mouse was suspended for 6 min, and the immobility time was recorded in the last 4 min. The mouse was considered immobile when it was passive and completely immobile. The test process was recorded with a monitoring system and analyzed by a researcher.
ELISA
After the depression-related behavioral tests, all mice were sacrificed. Serum was obtained from the abdominal artery, and brain tissue was also collected from each mouse and stored in a freezer at −80 °C for further analysis. SOD (Superoxide dismutase), MDA (Malondialdehyde), and GSH-Px (Glutathione peroxidase) were tested using ELISA kits (Nanjing Jiancheng Institute of Bioengineering, Nanjing, China). Expression levels of 5-hydroxytryptamine (5-HT), norepinephrine (NE), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) in the hippocampal tissue and prefrontal cortex tissue were all measured by ELISA kits (R&D system, USA) according to the protocol provided in the kit. The concentration was set according to the manual of the kit and was calculated with reference to the standard curve.
Western blot
Mice were sacrificed after the behavioral tests. Under disinfected conditions, the hippocampal tissue and prefrontal cortex tissue were quickly removed from each mouse brain and kept in cold (4 °C) saline solution. Tissue samples were homogenized in radioimmunoprecipitation assay lysis buffer (RIPA lysis buffer, containing protease inhibitor), then centrifuged at 12,000 ×g for 15 min. The supernatant was then collected at 4 °C. The BCA protein assay and western blot were performed to determine protein concentration. The proteins were separated by SDS-PAGE and then transferred to a polyvinylidene fluoride membrane (Millipore Company, Bedford, Mass.). The membrane was blocked with 5% bovine serum albumin at room temperature for 1 h, and the primary antibody was incubated overnight at 4 °C. Then, the membrane was incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody. The main antibodies used included toll-like receptor type 4 (TLR4) (1:1,000), p-p38/p38 (1:1,000), p-NF-κb/NF-κb (1:1,000), NLRP3 (1:1,000), and caspase-1 (1:1,000). The membrane was then incubated with the enhanced chemiluminescence reagent (Perkinelmer) and secondary antibody (Chemicon). Data was obtained by a molecular imager.
Statistical analysis
GraphPad Instat 5.0 software was used for statistical analysis. Analysis of variance and Tukey’s multiple comparison test were used. The results are expressed as mean ± standard deviation. Statistical significance was considered if P<0.05.
Results
The effect of SE on the SPT
After 6 weeks of observation and intervention, CUMS significantly reduced the SP of treated mice compared with the control group. Mice who received SE treatment (10 or 30 mg/kg) significantly consumed more SS compared with mice in the CUMS only group. After 4 weeks of treatment, mice treated with FLU (10 mg/kg) and Cli-095 (3 mg/kg) also showed increased SP compared with the CUMS only group. The increase in SP indicates that SE has a strong antidepressant effect in CUMS-exposed mice (Table 1).
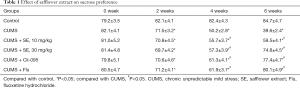
Full table
The effect of SE on the depression-like behaviors of mice
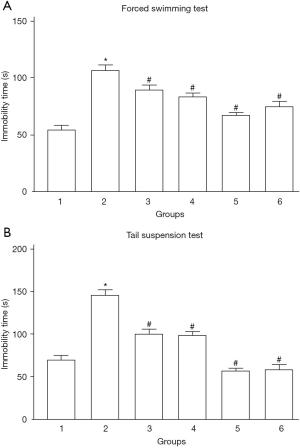
The results of the OFT showed no significant difference in total distance between the groups. Compared with the control group, CUMS significantly increased the immobility time in the other 5 groups, which is consistent with the results of the SPT (Figure 1A,B). Treatment with SE (10 or 30 mg/kg) could alleviate the stress induced by CUMS and reduce the immobility time in the FST (Figure 1A) and TST (Figure 1B). FLU (10 mg/kg, for example) and Cli-095 (3 mg/kg, for example) treatments also significantly alleviated CUMS-induced stress and reduced the immobility time in the FST and TST (Figure 1).
The effect of SE on 5-HT and NE levels and inflammatory cytokines in mice
Compared with the control group, the concentrations of 5-HT and NE in the prefrontal cortex tissue and hippocampal tissue in the CUMS group decreased significantly after 6 weeks of treatment, while the levels of 5-HT and NE in the prefrontal cortex and hippocampus increased significantly after treatment with SE (10 or 30 mg/kg). FLU and Cli-095 treatment also reversed the decrease in 5-HT and NE levels in the brain. In addition, serum 5-HT and NE levels in the CUMS group were lower than those in the control group. However, intervention with SE at a dose of 30 mg/kg significantly increased serum 5-HT and NE levels. TNF-α, IL-1β, and IL-6 in the prefrontal cortex and hippocampus of CUMS mice were significantly increased, and SE treatment effectively reduced the increase in pro-inflammatory cytokines. This indicates that the anti-inflammatory effects of SE may contribute to its antidepressant effect (Table 2).

Full table
The effect of SE on the TLR4/p38/NF-κB signaling pathway
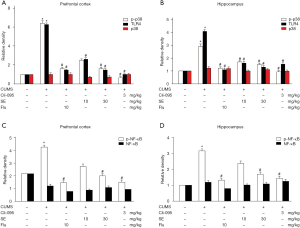
Our results showed that CUMS significantly upregulated the expression levels of TLR4 and p-p38 in the prefrontal cortex and hippocampus (Figure 2A,B). Administration of SE (10 or 30 mg/kg) or FLU (10 mg/kg) remarkably decreased the CUMS-induced increases in TLR4 and p-p38 (Figure 2A,B). SE could also significantly inhibit the CUMS-induced upregulation of p-NF-κB in the prefrontal cortex and hippocampus (Figure 2C,D).
The effect of SE on the expression of the NLRP3 inflammasome
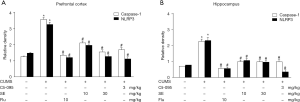
The NLRP3 inflammasome plays an important role in promoting the maturation of inflammatory cytokines. Evidence also shows that the NLRP3 inflammasome is involved in depression-like animal models. The potential effect of SE on the NLRP3 inflammasome in CUMS-treated mice was studied. CUMS induced the upregulation of NLRP3 and caspase-1 in the prefrontal cortex and hippocampus. Compared with the CUMS group, the decrease of NLRP3and caspase-1 were obviously reversed after administration of SE (10 or 30 mg/kg) or FLU (10 mg/kg). In addition, administration of Cli-095 (3 mg/kg) could significantly inhibit the activation of NLRP3 and caspase-1 in the prefrontal cortex and hippocampus induced by CUMS, as shown in Figure 3.
Discussion
In the present study, we showed that depression-like behaviors (such as impaired SP and prolonged immobility time induced by CUMS) could be improved by SE. In addition, SE decreased the CUMS-induced upregulation of 5-HT and NE expression in prefrontal cortex and hippocampal tissues. SE also reduced the increases in serum inflammatory cytokine levels (including TNF-α, IL-1β, and IL-6), and inhibited the upregulation of TLR4/p38/NF-κB/NLRP3 pathway-related proteins in prefrontal cortex and hippocampal tissues. Therefore, this study demonstrated that SE has a potential antidepressant effect on CUMS-induced depressive mice.
Previous related studies have shown that CUMS is widely used in establishing animal behavioral models of depression with satisfying effectiveness and reliability (8). In our present study, we used CUMS to establish a mouse model of depression successfully. Using behavioral tests, mice treated with CUMS all showed depression-like behaviors. Compared with the controls, these mice drank less SS in the SPT and had longer immobility time in the FST and TST. According to previous studies, these manifestations suggested that these mice had depression-like symptoms. Furthermore, our experiments showed that treatment with SE for 4 weeks could significantly improve these depression-like behaviors through increasing SP and reducing the immobility time. Our study is the first, to the best of our knowledge, to provide new experimental evidence for the beneficial effect of SE in a CUMS-induced depression animal model. Excessive secretion of pro-inflammatory cytokines can lead to the downregulation of neurotrophic factors and inhibition of hippocampal neurogenesis, which can lead to memory and cognitive impairment (9). Inhibition of inflammation can improve the depressive behaviors of mice, which is consistent with our research results. The results showed that CUMS could significantly increase the expression levels of TNF-α, IL-1β, and IL-6 in circulating blood and the prefrontal cortex and hippocampal tissues. In SE-treated mice, the increases in the expression levels of TNF-α, IL-1β, and IL-6 were inhibited, suggesting that the beneficial effect of SE on depression may be attributed to its role in the inhibition of inflammation.
Some previous studies have demonstrated that TLR4 is significantly involved in the occurrence and progression of depression (10-12). Stress could significantly increase the expression levels of TLR4 and NF-κB in the hippocampus, which could be alleviated by gene knockout of TLR4. In this study, we also found that SE could significantly prevent the increase of TLR4 and p-p38 in the prefrontal cortex and hippocampal tissue induced by CUMS. In addition, Cli-095 could also significantly inhibit the increase of TLR4 and p-p38 in the prefrontal cortex and hippocampal tissues, and improve the depressive behaviors induced by CUMS. NF-κB is an important transcription factor which plays a key role in the regulation of immune responses. Neuroscientific studies have also shown that after activation, NF-κB plays an important role in cognitive dysfunction, which is a manifestation of depression. In clinical studies, it has been found that somatic and social stress can both activate the NF-κB signaling pathway in depressive patients. In animal studies, investigators also found that the activation of NF-κB signals induced by chronic stress contributes to depression-like behaviors and reduces the proliferation of neural stem cells in rodents (13). Since lipopolysaccharide (LPS) strongly stimulates the expression of IL-1β and the activation of NF-κB, these depression-related behaviors have also been found in animal models of acute depression induced by LPS (14). In addition, inhibition of NF-κB signals could alleviate the negative effects of chronic stress on the SP of animals, which indicates that inhibiting NF-κB signals may treat depression effectively (15). At the same time, our results showed that the effect of CUMS on the activation of the TLR4-p38-NF-κB signaling pathway was significantly decreased by SE.
The NLRP3 inflammasome, as many studies have demonstrated, is an important downstream signaling molecule of TLR4 (16). Previous studies have shown that the NLRP3 inflammasome plays a key role in the process of inflammation (17). In molecular experiments and animal model experiments, the NLRP3 inflammasome can be easily activated by various stimuli, including different types of stressors and inflammatory cytokines (18). Recently, clinical and preclinical studies have revealed that the NLRP3 inflammasome can be activated in depressive patients and in animal models of depression (5). This study found that CUMS induced the activation of the NLRP3 inflammasome in the prefrontal cortex and hippocampal tissues, indicating that chronic stress-induced depression is related to the NLRP3 inflammasome-dependent inflammatory response (19). Continuous treatment with SE for 4 weeks decreased the expression level of NLRP3 upregulated by CUMS. In addition, Cli-095 also inhibited the activation of the NLRP3 inflammasome induced by CUMS. In short, these findings demonstrated that SE could treat depression effectively, possibly through the inhibition of the TLR4-NK-κB-NLRP3 signaling pathway.
In summary, SE has antidepressant effects in the CUMS-induced depression mouse model. The results of this study showed that SE can inhibit the TLR4-NF-κB pathway induced by CUMS and regulate the activation of the NLRP3 inflammasome in CUMS mice. In addition, SE also showed effects similar to FLU, which provides a theoretical basis for SE in the treatment of depression. Based on these results, we assume that safflower extract can be treated to improve the effects of chronic mild stress on emotional learning and long-term potentiation in the hippocampal accumbens which will be verified in future study. This study has some limitations. First, this is an animal experiment which is different from the condition in human beings. Second, this study has no positive control. The protocol of this study was prepared before the study without registration.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1728
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1728
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1728). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were approved by the Animal Experiment Ethics Committee (AEEC) of Zhongshan School of Medicine and were in compliance with the Guide for the Care and Use of Laboratory Animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Abd El-Fattah AA, Fahim AT, Sadik NAH, et al. Resveratrol and dimethyl fumarate ameliorate depression-like behaviour in a rat model of chronic unpredictable mild stress. Brain Res 2018;1701:227-36. [Crossref] [PubMed]
- Alvarez K, Vasquez G. Damage-associated molecular patterns and their role as initiators of inflammatory and auto-immune signals in systemic lupus erythematosus. Int Rev Immunol 2017;36:259-70. [Crossref] [PubMed]
- Cheng J, Dong S, Yi L, et al. Magnolol abrogates chronic mild stress-induced depressive-like behaviors by inhibiting neuroinflammation and oxidative stress in the prefrontal cortex of mice. Int Immunopharmacol 2018;59:61-7. [Crossref] [PubMed]
- Fleshner M, Frank M, Maier SF. Danger Signals and Inflammasomes: Stress-Evoked Sterile Inflammation in Mood Disorders. Neuropsychopharmacology 2017;42:36-45. [Crossref] [PubMed]
- Kaufmann FN, Costa AP, Ghisleni G, et al. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav Immun 2017;64:367-83. [Crossref] [PubMed]
- Zhou XY, Zhang F, Hu XT, et al. Depression can be prevented by astaxanthin through inhibition of hippocampal inflammation in diabetic mice. Brain Res 2017;1657:262-8. [Crossref] [PubMed]
- Li H, Lin S, Qin T, et al. Senegenin exerts anti-depression effect in mice induced by chronic un-predictable mild stress via inhibition of NF-kappaB regulating NLRP3 signal pathway. Int Immunopharmacol 2017;53:24-32. [Crossref] [PubMed]
- Du Y, Ruan J, Zhang L, et al. Jieyu Anshen Granule, a Chinese Herbal Formulation, Exerts Effects on Poststroke Depression in Rats. Evid Based Complement Alternat Med 2020;2020:7469068 [Crossref] [PubMed]
- Alam A, Hana Z, Jin Z, et al. Surgery, neuroinflammation and cognitive impairment. EBioMedicine 2018;37:547-56. [Crossref] [PubMed]
- Liu Y, Li X, Jiang S, et al. Tetramethylpyrazine protects against high glucose-induced vascular smooth muscle cell injury through inhibiting the phosphorylation of JNK, p38MAPK, and ERK. J Int Med Res 2018;46:3318-26. [Crossref] [PubMed]
- Porcu M, Urbano MR, Verri WA Jr, et al. Effects of adjunctive N-acetylcysteine on depressive symptoms: Modulation by baseline high-sensitivity C-reactive protein. Psychiatry Res 2018;263:268-74. [Crossref] [PubMed]
- Eliwa H, Brizard B, Le Guisquet AM, et al. Adult neurogenesis augmentation attenuates anhedonia and HPA axis dysregulation in a mouse model of chronic stress and depression. Psychoneuroendocrinology 2021;124:105097 [Crossref] [PubMed]
- Shao Z, Wu P, Wang X, et al. Tetramethylpyrazine Protects Against Early Brain Injury and Inhibits the PERK/Akt Pathway in a Rat Model of Subarachnoid Hemorrhage. Neurochem Res 2018;43:1650-9. [Crossref] [PubMed]
- Zhao X, Cao F, Liu Q, et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav Brain Res 2019;364:494-502. [Crossref] [PubMed]
- Luo L, Sun T, Yang L, et al. Scopoletin ameliorates anxiety-like behaviors in complete Freund's adjuvant-induced mouse model. Mol Brain 2020;13:15. [Crossref] [PubMed]
- Yang J, Wise L, Fukuchi KI. TLR4 Cross-Talk With NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer's Disease. Front Immunol 2020;11:724. [Crossref] [PubMed]
- Tan S, Wang Y, Chen K, et al. Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice. Biol Pharm Bull 2017;40:1260-7. [Crossref] [PubMed]
- Kelley N, Jeltema D, Duan Y, et al. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int J Mol Sci 2019;20:3328. [Crossref] [PubMed]
- Zhang HX, Xu YQ, Li YY, et al. Difference in proinflammatory cytokines produced by monocytes between patients with major depressive disorder and healthy controls. J Affect Disord 2018;234:305-10. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


