
Independent risk factors of hypoxemia in patients after surgery with acute type A aortic dissection
Introduction
Acute type A aortic dissection (ATAAD) is a life-threatening condition associated with severe morbidity and mortality (1) and risk factors for AAD have been evaluated, such as gender, age, hypertension, smoking, aneurysm, congenital disorders, and inflammatory disease (2). Despite improved surgical technical and perioperative management, the incidence of postoperative complications remains high (3). Postoperative hypoxemia, one of the most common complications, is followed by prolonged mechanical ventilation duration, increased length of stay (LOS) of intensive care unit (ICU), even increased mortality. For postoperative severe postoperative hypoxemia (PaO2/FiO2 ratio <100 mmHg) of ATAAD, even rescue therapy is required, such as prone position ventilation (PPV) and extracorporeal membrane oxygenation (ECMO) (4,5). Therefore, it is crucial to identify patients who will develop such a critical condition, making medical resource assignments more reasonable and improving the prognosis for such a population.
Several risk factors of postoperative hypoxemia for ATAAD patients, such as obesity, female, preoperative PaO2/FiO2 ratio ≤300 mmHg, blood transfusion, have been investigated in some studies; however, the results remain controversial (6-12). Through retrospective analysis of patients’ data, to determine the risk factors of hypoxemia and its severity can help clinicians to adjust treatment strategies and avoid the occurrence of hypoxemia. As mentioned above, we conducted a retrospective study to assess the incidence, risk factors, as well as impact on clinical outcome of postoperative hypoxemia and severe postoperative hypoxemia of ATAAD. We present the following article in accordance with the SROBE reporting checklist (available at https://dx.doi.org/10.21037/apm-21-1428).
Methods
Study design
A single-center retrospective study was conducted in a 37 beds general ICU of the 1st affiliated hospital of Guangzhou Medical University. From January 2016 to May 2020, the patients diagnosed as ATAAD confirmed by enhanced computed tomographic scan and underwent urgent operation were included. However, patients were excluded if they died during operation or within 24 hours after surgery. The study was conducted as per the Declaration of Helsinki (as revised in 2013). The study was granted approval by the ethics committee at The 1st affiliated hospital of Guangzhou Medical University (Medical research ethics review: 2021NO. K-12), and individual consent for this retrospective analysis was waived.
Data collection
Overall, 75 subjects were enrolled, and data were extracted from our electronic hospital information system (HIS). The patient’s demography [age, gender, body mass index (BMI), and comorbidities], perioperative laboratory results, operative details (operation time, CPB time, clamping time, intraoperative blood and plasma transfusions), postoperative P/F rate (the worst value from blood gas analysis within 72 hours after ICU admission), and clinical outcomes [28 days ventilator-free days (VFD), mechanical ventilation time, LOS of ICU, LOS of hospital, occurrence of acute kidney injury (AKI) and mortality] were collected and analyzed. We divided the patients into three groups according to postoperative PaO2/FiO2 ratio, selecting the worst condition according to blood gas analysis within 72 hours after surgery: group severe (severe hypoxemia group, PaO2/FiO2 ratio ≤100 mmHg); group moderate (moderate hypoxemia group,100 mmHg < PaO2/FiO2 ratio ≤200 mmHg); and group mild (non-hypoxemia group, PaO2/FiO2 ratio >200 mmHg).
Surgical procedure
After standard anaesthetic management, patients were provided a total arch replacement and descending aorta stent implantation which was conducted according to the Sun’s produce (13). Briefly, a median sternotomy procedure was used in all the operations. For the arterial perfusion, routine exposure of the right axillary artery was done, and for venous drainage, cannulation of the inferior of superior vena cava or the right atrium was performed. The patients were then heparinized, followed by establishment of the cardiopulmonary bypass (CPB). When the CPB started, we initiated systemic cooling. Clamping of the ascending aorta was done, followed by longitudinal opening, and subsequent infusion of cardioplegic solution consisting of cold-blood into the right and left coronary artery separately. Surgery of the aortic root, entailing David or Bentall surgery or replacement of the ascending aorta was done based on the lesions of the aortic root during the cooling process. At a temperature of between 17 °C and 26 °C in the nasopharynx, and 19 °C and 29 °C in the rectum, the brachiocephalic artery, left common carotid artery, as well as left subclavian artery were sequentially clamped, followed by initiation of the DHCA (deep hypothermic circulatory arrest) along with SCP (selective cerebral perfusion) via the right axillary artery (5–10 mL/kg). Transection of the aortic arch was done between the left subclavian artery and the left common carotid artery. After that, a stented elephant graft (MicroPort Medical Co. Ltd., China) was first released in the descending aorta. Direct saturation of the left subclavian artery residual in the arch was performed. Thereafter, the three parts consisting of the stented graft, four-branch prosthetic graft, and the descending aorta were anastomosised, followed by immediate lower body reperfusion via perfusion with half of the normal flow in one limb of the four-branch prosthetic graft. Bilateral perfusion of the brain was done at the end of the anastomosis action on the left common carotid artery. Thereafter, normal flow was resumed in the CPB gradually and began to rewarm, then saturation of the bleeding was done on the distal anastomosis. Resuscitation of the heart was done after the area between the proximal aortic arch and the ascending aorta was anastomosed. Afterwards, we separately anastomosed the brachiocephalic artery along with the left subclavian artery and the respective branch of the four-branch prosthetic graft. Finally, drainage was made to the right atrium with the arterial wall residual or the pericardium or biological patch about the artificial graft. After completion of the repair and adequate rewarming, the patient was weaned from CBP.
Management after surgery
After surgery, the patients were placed in ICU and received comprehensive care according to our protocol: volume-controlled ventilation was performed in the ICU with a minimum FiO2 and a PEEP (positive end expiratory pressure) of 6–10 cmH2O to achieve SpO2 95–97%. For patients who suffered from respiratory distress or moderate to severe hypoxemia (P/F <150 mmHg, deep sedation or even neuromuscular block (Richmond agitation sedation scale, RASS -5) and protective ventilatory strategy were performed. If the condition was not improved, prone positioning ventilation, even ECMO was applied to the patients. Furthermore, renal replacement therapy (RRT) was used to the patients who suffered from oliguria caused by AKI and required liquid management.
Statistical analysis
Descriptive data are given as mean, ranges, standard deviation, and median for continuous variables and as percentages and counts for categorical variables. A χ2 test with Fisher’s exact test or ANOVA test was used to compare groups as appropriate. A two-tailed P value less than 0.05 was considered statistically significant. Variables with a P value less than 0.1 or less at univariate analysis were included in a logistic regression multivariate analysis with a stepwise forward conditional approach, estimates of odds ratio (OR), 95% CI and P values are displayed. All analyses were implemented in SPSS V. 20 (IBM Corp, Armonk, NY). All P values are based on 2-sided tests and considered statistically significant at P<0.05.
Results
Baseline characteristics
A total of 75 enrolled patients, comprising 61 males (81.3%), with a mean age of (53.53±13.98) years, were divided into three groups: group severe (24 patients), group moderate (39 patients), and group mild (12 patients). The incidence of postoperative severe hypoxemia and hypoxemia was 32% and 52%, respectively, and only 16% of patients did not develop such complications. Among the three groups, BMI and preoperative white blood cell (WBC) were remarkably higher in group severe (26.20±3.40 vs. 22.98±4.94 vs. 20.75±4.03 kg/m2, P=0.001, and 12.48±3.97 vs. 10.5±3.80 vs. 8.69±2.48 kg/m2, P=0.014, respectively). Hypertension was mainly distributed in group severe (91.67% vs. 82.05% vs. 50%, P=0.015). Meanwhile, Marfan syndrome was mainly distributed in group mild (0% vs. 2.56% vs. 25%, P=0.01). The other variables, such as age, gender, medical history, and preoperative laboratory test results, were not remarkably different. More details are shown in Table 1.
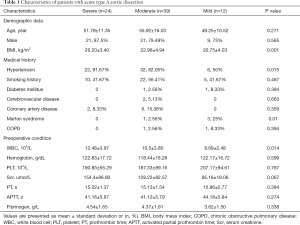
Full table
Intraoperative and postoperative characteristics of patients
The intraoperative details were similar among the three groups. The Acute Physiology and Chronic Health Evaluation (APACHE II) score of postoperative patients on ICU admission was remarkably greater in group severe (21.75±6.27 vs. 18.18±7.38 vs. 15.75±6.00, P=0.033), and more patients would present shock on ICU admission in group severe (37.5% vs. 28.2% vs. 0%, P=0.042). The other postoperative patient’s characteristics and laboratory test results were similar among the three groups. More details are presented in Table 2.
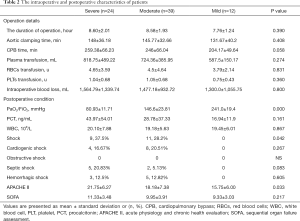
Full table
Clinical outcomes
The subjects in group severe had a higher incidence of postoperative AKI and usage of renal replacement therapy, longer LOS of ICU, and shorter 28 days VFDs (P=0.002, 0.027, and 0.043, respectively). Notably, though there was no remarkable difference in mortality among the three groups, group mild patients were all survived. More details are displayed in Table 3.
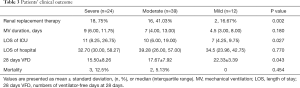
Full table
Risk factors for postoperative hypoxemia, severe hypoxemia and the prediction models
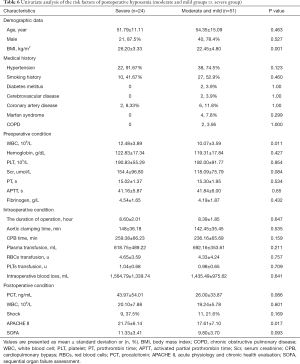
In the first step, we compared group (severe and moderate) with group mild to evaluate the risk factor for postoperative hypoxemia (PaO2/FiO2 ratio ≤200 mmHg). In the univariate analysis, BMI, preoperative WBC, preoperative serum creatinine, and intraoperative CPB time were remarkably higher in group (severe and moderate) (P=0.019, 0.035, 0.047, and 0.024, respectively). Hypertension and postoperative shock were mainly distributed in group (severe and moderate) (P=0.011 and 0.029, respectively); meanwhile, Marfan syndrome was mainly distributed in group mild (P=0.012). Although postoperative APACHE II score was not remarkably higher in group (severe and moderate) (P=0.089), we included this variable into the logistic regression according to our predefined statistical analysis protocol. We found that only hypertension was (OR 0.117, 95% CI: 0.023–0.589, P=0.009) independent risk factors using logistic regression. More details are presented in Tables 4 and 5.
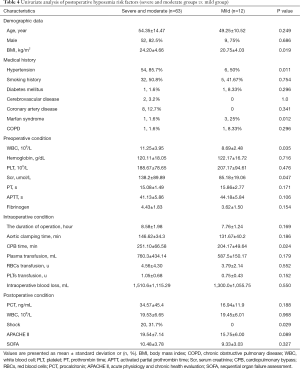
Full table

Full table
In the second step, we used the same strategy to compare group severe with group (moderate and mild) to evaluate the risk factor for severe postoperative hypoxemia (PaO2/FiO2 ratio ≤100 mmHg). In the univariate analysis, BMI, preoperative WBC, and postoperative APACHE II score were remarkably higher in group severe (P=0.001, 0.011 and 0.017, respectively). Although preoperative serum creatinine, postoperative PCT, and postoperative SOFA score were not remarkably higher in group severe (P=0.084, 0.086, and 0.093, respectively), we included these variables in the logistic regression as mentioned above. Using logistic regression, we found that BMI (OR 0.758, 95% CI: 0.640–0.897, P=0.001) and postoperative APACHE II score (OR 0.900, 95% CI: 0.828–0.978, P=0.013) were independent risk factors. More details are shown in Tables 6 and 7.
Full table

Full table
Discussion
Hypoxemia is a common postoperative complication in ATAAD patients. Our study illustrated that the incidence of such complication was 84% (63/75), much higher than previous studies (30–50%) (6-11). However, the incidence of severe hypoxemia (P/F <100 mmHg) was 32% (24/75) in our study, slightly lower than a recent study (36.6%) (12). We reasoned that since most of our patients transported from other hospitals takes a long time from symptom onset to entering the operating room, which would put the patients at a higher risk to develop the postoperative hypoxemia.
Herein, we illustrated that higher BMI was linked to developing postoperative hypoxemia and severe hypoxemia in line with previous studies (8-12). Obesity, defined as BMI >25 kg/m2, had significant adverse effects on cardiopulmonary physiology. Obese patients often showed a decreased functional residual capacity (FRC), total lung capacity (TLC), expiratory reserve volume (ERV), as well as vital capacity (VC), and severity was inversely proportional to BMI. In addition, obesity could lead to abdominal distention, resulting in decreased chest wall compliance, increased respiratory resistance, pleural pressure, and upper and lower airway resistance (14). Besides, obesity was associated with chronic increased proinflammatory cytokines and mediators, leading to cell membrane damage (15). Consequently, obesity was considered as an independent high-risk factor of postoperative pulmonary complications, and improved intra and postoperative ventilation strategies should be crucial in such populations (16,17).
The mechanical and physical effects, such as inflammatory cascade reaction and pulmonary ischemia-reperfusion injury, caused by CPB were considered a significant cause of postoperative hypoxemia (10,12). Our result showed that CPB time was longer in severe and moderate groups (P/F ratio ≤200 mmHg) in contrast with the mild group (P/F ratio >200 mmHg).
Elevated APACHE II score and occurrence of postoperative shock were identified to contribute to postoperative hypoxemia development in our study. Given that these indexes were developed to represent disease’s seriousness, it was unsurprising to discover this connection. It worth noting that both hypertension and Marfan syndrome were recognized as risk factors of AAD. Our study illustrated that hypertension was associated with postoperative hypoxemia development; however, Marfan syndrome seemed to be a protective factor. Also, hypertension was mainly distributed in groups severe and group moderate, and Marfan syndrome was all in group mild. It seemed that if AAD was induced by Marfan syndrome rather than hypertension, it might develop postoperative hypoxemia.
Our study showed that severe postoperative hypoxemia would negatively impact clinical outcomes, including higher RRT usage, longer LOS of ICU, and shorter 28 days VFDs. The incidence of AKI, which manifests through rapid loss of renal function with high morbidity and mortality (18), remained high in such a population (19,20). The postoperative low oxygen supply and high oxygen might play a vital role in this progression (21). Interestingly, the linear relationship between obesity and AKI was illustrated (22) as an essential contributor to postoperative hypoxemia.
There were two limitations in our study. First, it was a single-center retrospective observational study, and the sample size was limited. Secondly, the impact of factors, including individual surgeon’s experience along with institutional philosophy with regards to treatment decision was not considered in this analysis.
Conclusions
Herein, we established that postoperative moderate and severe hypoxemia were high in ATAAD patients. The high-risk factors had been evaluated. Meanwhile, such postoperative complications negatively impacted the clinical outcomes.
Acknowledgments
Funding: This study was supported by Emergency Key Program of Guangzhou Laboratory (Grant No. EKPG21-17), The Special Project of Guangdong Science and Technology Department (2020B1111340016) and Natural Science Foundation of Guangdong Province (Grant No. 2020A1515011459).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/apm-21-1428
Data Sharing Statement: Available at https://dx.doi.org/10.21037/apm-21-1428
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/apm-21-1428). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee at the First Affiliated Hospital of Guangzhou Medical University (Medical research ethics review: 2021NO. K-12), and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jassar AS, Sundt TM 3rd. How should we manage type A aortic dissection? Gen Thorac Cardiovasc Surg 2019;67:137-45. [Crossref] [PubMed]
- Gawinecka J, Schönrath F, von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly 2017;147:w14489 [PubMed]
- Gudbjartsson T, Ahlsson A, Geirsson A, et al. Acute type A aortic dissection - a review. Scand Cardiovasc J 2020;54:1-13. [Crossref] [PubMed]
- Girdauskas E, Kuntze T, Borger MA, et al. Acute respiratory dysfunction after surgery for acute type A aortic dissection. Eur J Cardiothorac Surg 2010;37:691-6. [Crossref] [PubMed]
- Yokota K, Fujii T, Kimura K, et al. Life-threatening hypoxemic respiratory failure after repair of acute type a aortic dissection: successful treatment with venoarterial extracorporeal life support using a prosthetic graft attached to the right axillary artery. Anesth Analg 2001;92:872-6. [Crossref] [PubMed]
- Shen Y, Liu C, Fang C, et al. Oxygenation impairment after total arch replacement with a stented elephant trunk for type-A dissection. J Thorac Cardiovasc Surg 2018;155:2267-74. [Crossref] [PubMed]
- Gao Z, Pei X, He C, et al. Oxygenation impairment in patients with acute aortic dissection is associated with disorders of coagulation and fibrinolysis: a prospective observational study. J Thorac Dis 2019;11:1190-201. [Crossref] [PubMed]
- Liu N, Zhang W, Ma W, et al. Risk factors for hypoxemia following surgical repair of acute type A aortic dissection. Interact Cardiovasc Thorac Surg 2017;24:251-6. [PubMed]
- Wang Y, Xue S, Zhu H. Risk factors for postoperative hypoxemia in patients undergoing Stanford A aortic dissection surgery. J Cardiothorac Surg 2013;8:118. [Crossref] [PubMed]
- Nakajima T, Kawazoe K, Izumoto H, et al. Risk factors for hypoxemia after surgery for acute type A aortic dissection. Surg Today 2006;36:680-5. [Crossref] [PubMed]
- Ge H, Jiang Y, Jin Q, et al. Nomogram for the prediction of postoperative hypoxemia in patients with acute aortic dissection. BMC Anesthesiol 2018;18:146. [Crossref] [PubMed]
- Gong M, Wu Z, Xu S, et al. Increased risk for the development of postoperative severe hypoxemia in obese women with acute type a aortic dissection. J Cardiothorac Surg 2019;14:81. [Crossref] [PubMed]
- Sun LZ, Ma WG, Zhu JM, et al. Sun’s procedure for chronic type A aortic dissection: total arch replacement using a tetrafurcate graft with stented elephant trunk implantation. Ann Cardiothorac Surg 2013;2:665-6. [PubMed]
- Hibbert K, Rice M, Malhotra A. Obesity and ARDS. Chest 2012;142:785-90. [Crossref] [PubMed]
- Sheng W, Yang HQ, Chi YF, et al. Independent risk factors for hypoxemia after surgery for acute aortic dissection. Saudi Med J 2015;36:940-6. [Crossref] [PubMed]
- Ball L, Hemmes SNT, Serpa Neto A, et al. Intraoperative ventilation settings and their associations with postoperative pulmonary complications in obese patients. Br J Anaesth 2018;121:899-908. [Crossref] [PubMed]
- Pirrone M, Fisher D, Chipman D, et al. Recruitment Maneuvers and Positive End-Expiratory Pressure Titration in Morbidly Obese ICU Patients. Crit Care Med 2016;44:300-7. [Crossref] [PubMed]
- Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2012;2:1303-53. [Crossref] [PubMed]
- Liu J, Xue Y, Jiang W, et al. Thyroid Hormone Is Related to Postoperative AKI in Acute Type A Aortic Dissection. Front Endocrinol (Lausanne) 2020;11:588149 [Crossref] [PubMed]
- Helgason D, Helgadottir S, Ahlsson A, et al. Acute Kidney Injury After Acute Repair of Type A Aortic Dissection. Ann Thorac Surg 2021;111:1292-8. [Crossref] [PubMed]
- Shu S, Wang Y, Zheng M, et al. Hypoxia and Hypoxia-Inducible Factors in Kidney Injury and Repair. Cells 2019;8:207. [Crossref] [PubMed]
- Schetz M, De Jong A, Deane AM, et al. Obesity in the critically ill: a narrative review. Intensive Care Med 2019;45:757-69. [Crossref] [PubMed]


