
Venous thromboembolism in patients with severe lung cancer: a narrative review
Introduction
Advanced severe lung cancer refers to patients with stages IIIB, IIIC, and IV lung cancer whose Eastern Cooperative Oncology Group (ECOG) performance status score is in the range of 2 to 4 due to causes related to the lung cancer itself or complications of anti-tumor drugs, at the same time these patients have a high probability of benefiting from the existing systemic anti-tumor treatments (1). Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is the third most frequent cardiovascular event after stroke and MI (myocardial infarction) in Western countries (2). Many studies have shown that the prevalence of VTE is significantly increased among patients with lung cancer compared to patients with other cancers. VTE can appear before cancer diagnosis, and before the start of lung cancer treatment, patients may be in a hypercoagulable state (3). Some investigations have shown that patients with lung cancer have the highest incidence of thrombotic events among all cancer patients (4). The related mechanisms of malignant tumor progression include direct invasion and external compression of blood vessels, injury of endothelial cells caused by chemotherapeutics, factor X activation and tissue factor expression from tumor cells, blood platelet activation and aggregation, and expression of inflammation factors caused by tumor, such as von Willebrand factor VIII (5). The occurrence of VTE often increases the severity of lung cancer patients’ conditions, leading to a poor prognosis. One study reported that among 94 cases of non-small cell lung cancer (NSCLC) after pneumonectomy, thrombosis was found in lung tumor tissue and nearby non-cancerous tissue in 56 cases (59.6%); there were24 cases (25.5%) of pulmonary artery thrombosis and 32 cases (34.0%) of pulmonary vein thrombosis. After 2 years of follow-up, the results showed that patients with thrombosis had a lower survival rate compared to patients without thrombosis (6). We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/apm-21-1281).
Status
Epidemiology
In 2018, GLOBOCAN data showed that lung tumor is the most common cause of cancer mortality in many places around the world (7). Between all cancer patients and the general population, the hazard-ratio of VTE is 4.7 (8). Thrombosis is the leading cause of death among all cancer patients (9). Lung tumorpatients are more likely to develop VTE than other cancer patients, with an incidence of 13.9% (10). Compared to that of the general population, the VTE risk ratio is elevated 20-foldfor patients with lung cancer. In particular, patients with lung adenocarcinoma have a higher risk of VTE than those with lung squamous cell carcinoma. Furthermore, for patients undergoing radiotherapy or chemotherapy, and for those with metastatic disease, the risk is also heightened (11). One study found that the incidence of VTE was remarkably higher among cancer patients than among non-cancer patients (12), especially in lung cancer patients during anticoagulant therapy, the occurrence rate of VTE could reached 27% (13). Patients with prolonged ICU length of stay, femoral central venous catheter, or a high Caprini score may have an increased risk of developing VTE, despite the use of guideline-recommended thromboprophylaxis, the cumulative incidence of VTE at 28 days of ICU stay was up to 9.55% (14). The proportion of COVID-19 patients with VTE was 6% in ward patients (15).
Clinical manifestations
Lung cancer patients with PE may present with symptoms including dyspnea, chest pain, hemoptysis, and syncope (16). In a study of 113 lung cancer patients, 88.5%of patients with VTE were symptomatic, and the rest were asymptomatic (17).
Risk factors
Lung cancer-related risk factors for VTE
Pathological types and stages
In a meta-analysis of 16 studies, the results showed that advanced tumor stage (TNM III–IV vs. I–II) and a history of adenocarcinoma were the clinical features of lung cancer patients with PE compared to those without PE (16). Another Cox regression analysis indicated that independent risk factors for VTE included metastatic disease and the adenocarcinoma subtype (18). The risk of VTE is higher for patients with adenocarcinoma than for patients with squamous cell carcinoma, especially for those with metastasis (11).
Gene mutation
A multicenter, retrospective cohort study investigated the incidence of VTE in patients with C-ros oncogene 1-receptor tyrosine kinase-positive (ROS1+), anaplastic lymphoma kinase-positive (ALK+), Kirsten rat sarcoma viral oncogene-positive (KRAS+), and epidermal growth factor receptor-positive (EGFR+) lung cancer. The study found that in comparison with patients with KRAS+ and EGFR+ lung cancer, patients with ROS1+ lung cancer had a significantly increased risk of VTE. The thromboembolic incidence among patients in the ALK+ NSCLC group was also found to be lower than that in the ROS1+group, although multivariable analysis showed there to be no significant differences between the two groups (19).
The METROS (Analysis From a Phase II, Prospective, Multicenter, Two-arms Trial) study showed that the incidence of VTE among patients with ROS1-rearranged NSCLC is 3 to 5 times higher than that in the general NSCLC population (20).
One study identified all patients with ALK-rearranged NSCLC diagnosed in Canada, and a confirmation cohort involving all eligible patients with ALK-rearranged NSCLC treated in Israel was enrolled. The overall VTE rate was 36%, and the incidence of VTE in this ALK-rearranged study population was 3 to 5 times larger than that in the general NSCLC population (21).
One retrospective case-control study included a total of 57 cases with VTE and 102 cases without VTE from 2000 to 2009. The odds ratio of thrombosis in patients with EGFR+ NSCLC was 0.99, compared with 2.67 in the KRAS+ group, thus showing a higher incidence of VTE in the latter group (22).
All of the abovementioned studies indicate that patients with ROS1+, ALK+, and KRAS+ NSCLC have an increased risk of VTE (19-22), while EGFR+ mutation may have no effect to the occurrence of VTE in NSCLC patients (22).
Treatment-related risk factors
Chemotherapy
VTE is a common disease in patients with lung tumors, especially among those receiving chemotherapeutic regimens. In most cases, VTE occurs within 6 months after the start of chemotherapeutic treatment, and the occurrence of VTE has been found to be closely associated with an increased risk of mortality in these patients (23).
Another retrospective study was performed of patients with lung cancer who underwent platinum-based chemotherapy from a national inpatient database in Japan. Using multivariable logistic regression analysis, the authors reported that compared with a carboplatin (CBDCA)-based or nedaplatin (CDGP)-based regimen, cisplatin-based chemotherapy carried a stronger risk of VTE (24). These two studies showed that chemotherapeutics increase the incidence of VTE for lung tumor patients, with cisplatin-based chemotherapy carrying a higher risk of VTE than other chemotherapeutic regimens.
Antiangiogenesis
Among advanced NSCLC patients, the use of anti-vascular endothelial growth factor (VEGF) drugs can significantly increase the risk of arterial thromboembolic events (ATEs), but not VTEs. Clinicians should be aware of the risk of severe ATEs with the use of these drugs (25). Several studies have shown that bevacizumab alone or in combination with chemotherapy increases the risk of VTE in cancer patients; however, the relationship between bevacizumab and VTE risk in lung cancer patients has rarely been reported (26,27).
Patient-related risk factors
Age
Lower age is associated with a higher VTE risk among lung cancer patients. For patients under the age of 45 years old, the incidence of VTE is about 3 times higher than that among patients aged over 75 years old (28).
Ethnicity
Race is also a risk factor for VTE in lung cancer patients. In the general population, the incidence of VTE varies among races, with African Americans and Asians having the highest and the lowest incidence, respectively (29).
Complications
Patients with atrial fibrillation and chronic kidney disease are more likely to suffer VTE (30). Incidence of recurrent VTE was seemingly not related to the development of chronic thromboembolic pulmonary hypertension in patients after a first episode of pulmonary embolism (31,32).
Prognosis
The Nationwide Inpatient Sample (NIS) was analyzed by one study and involved all lung tumor in patients from 2006 to 2010 in the United States. Among these inpatients, those with VTE had a higher death rate, higher medical costs, greater disability, and a longer length of stay than those without VTE (33). Acute symptomatic VTE deeply affects the living quality of patients with malignant tumors (34). Both PE and major bleeding are associated with an increased risk of death and could be indicators of lung cancer mortality (35).
Treatment of VTE in patients with severe lung cancer
At present, there are no specific guidelines for the treatment of lung cancer with VTE. However, lung cancer is regarded as an important factor in many prediction models for cancer with VTE. Therefore, the treatment of lung cancer with VTE may have its own characteristics. Here, we refer to the authoritative guidelines on cancer with VTE and discuss the treatment of lung tumor with VTE.
Low-molecular-weight heparin
Before 2002, vitamin K antagonists (VKAs) were the main anticoagulant therapy for tumor complicated with VTE. However, patients with lung cancer undergoing chemotherapy or targeted therapy often experience vomiting or diarrhea, which affects the absorption of VKAs, making the international normalized ratio (INR) value difficult to control. Moreover, there were interactions among VKAs and commonly used chemotherapeutic or targeted drugs for lung cancer, such as gemcitabine, paclitaxel, etoposide, carboplatin, and tyrosine kinase inhibitors. The 2002 Canthanox trial opened a new era of low-molecular-weight heparin (LMWH) in the treatment of cancer complicated with VTE. LMWH has many advantages, such as having a short half-life and less interaction with chemotherapeutic or targeted drugs, and being less affected by food. Subsequently, many clinical studies have been conducted to evaluate the effectiveness and safety of LMWH.
The CANTHANOX study was a stochastic, multicentric trial involving 146 tumor patients with VTE. The trial compared warfarin with subdermal enoxaparin (1.5 mg/kg once a day) over a period of 3 months. The results showed that warfarin is associated with a higher rate of bleeding in tumor patients with VTE, and prolonged treatment with enoxaparin sodium may be more effective and safer for these patients. This trial established the position of LMWH in the treatment of tumor complicated VTE (36).
In their meta-analysis of 1,169 cancer patients in 3 RCTs, Martínez-Zapata et al. aimed to evaluate the safety and efficacy of Tinzaparin in the treatment of VTE in cancer patients compared with other anticoagulants. The results of the meta-analysis suggested that short- and long-term treatment with Tinzaparin was superior to VKA treatment in preventing VTE recurrence (37).
Data from the CLOT study were analyzed for comparison of the efficacy and safety of dalteparin and VKAs for recurrent venous thromboembolism (rVTE) prevention in patients with cancer and kidney injury. The results confirmed that the incidence of rVTE could be decreased considerably by dalteparin compared with VKAs, and with better safety (38).
Direct oral anticoagulants
The Hokusai-VTE Cancer Clinical Trial was a non-inferiority, open-label study of 1,046 patients to compare the composite outcomes of patients with recurrent VTE or primary bleeding treated with edoxaban or dalteparin. Patients were split into two groups: the first group was given 60 mg of edoxaban every day after receiving LMWH for at least 5 days, and the second group was given 200 IU/kgsubdermal dalteparin every day for 1 month followed by 150 IU/kg subdermal dalteparin every day. The treatment was given for at least 6 months and for up to 12 months in both groups. The trial results confirmed that oral edoxaban was non-inferior to dalteparin in the recurrent composite outcome of VTE of main bleeding (39).
A randomized, multicenter, open-label pilot study, the SELECT-D trial, aimed to evaluate rivaroxaban as a selective therapy for VTE in cancer patients. In total, 203 sick people were randomly divided into two groups to receive dalteparin (150 IU/kg every day for 1–5 months after 1 month of 200 IU/kg daily) or rivaroxaban (20 mg once daily for 6 months after 15 mg twice a day for 3 weeks).The trial showed that rivaroxaban was associated with a lower rate of VTE than dalteparin; however, the incidence of primary bleeding and clinical relative secondary bleeding in the rivaroxaban group was increased (40).
Two multicenter, randomized trials (41,42) were conducted to compare the risk of VTE recurrence and main bleeding between apixaban and dalteparin. The ADAM VTE trial was a superiority trial that included 287 patients, and the Caravaggio clinical trial was a non-inferiority trial involving 1,155 patients. Patients in both trials were given subdermal dalteparin (150 IU/kg daily after 200 IU/kg of body weight daily for the first month) or oral apixaban (5 mg twice a day after 10 mg twice a day for 7 days) for at least half a year. The results of the two trials showed that compared with subdermal dalteparin, oral apixaban could reduce the recurrence rate of cancer-related VTE, without increasing the risk of massive hemorrhage.
Management of thrombocytopenia after antitumor therapy
The International Society on Thrombosis (ISTH) (43) and the British Committee for Standards in Haematology (BCSH) (44) recommend a full-dose anticoagulant therapy for patients with cancer-related VTE who have a platelet count over 50×109/L. For patients whose platelet count is between 25×109 and 50×109/L, the ISTH recommends full-dose anticoagulant therapy plus platelet transfusion until the platelet count reaches the empirical threshold, while the BCSH recommends half-dose anticoagulant therapy. However, the American Society of Clinical Oncology (ASCO) (45), BCSH, and ISTH all state that anticoagulation is contraindicated for severe thrombocytopenia patients [those with a platelet count below 25×109/L (BCSH and ISTH) or 20×109/L (ASCO)].
Inferior vena cava filter
In a prospective randomized trial, patients with PE and/or DVT were randomly divided into two groups. All patients received anticoagulant therapy with fondaparinux sodium; one group received a vena cava filter placement, while the other group did not. Neither group showed advantages in terms of safety, recurrence, or survival (46). As an international prospective, multicenter, single-arm clinical trial, the Crux Inferior Vena Cava Filter System study involved 125 patients implanted with the Crux filter. Follow-up lasted for 180 days after filter placement and for 30 days after filter retrieval, the data showed that treatment was successful in 96.0% of patients, with high rates of technical, clinical, and retrieval success, the Crux vena cava filter performed well (47). Patients with acute PE and DVT who have absolute contraindications for anticoagulants have an extremely poor clinical prognosis, but there is no high-quality prospective studies to support the use of an inferior vena cava (IVC) filter (44). One study analyzed the CENTRAL, MEDLINE, LILACS, and EMBASE databases, and used the Cochrane Collaboration’s instrument and a modified version for cohort research to assess the risk of bias. A fixed-effects analysis of 7 studies involving 35,333 patients was conducted. The majority of research items indicated a low risk of bias. Among cancer patients, vena cava filter implantation has no benefits for preventing recurrent VTE (48). The absence of random clinical experiments, as well as conflicting results from retrospective studies, preclude evidence-based judgment on the efficacy and safety of IVC filters for patients who have an absolute contraindication to anticoagulants. Moreover, emerging data on IVC filter-related complications emphasize that IVC filters should be used with caution (49). To conclude, the benefits of IVC filters in the treatment of VTE are not clear, and more high-quality studies still need to be conducted.
Prevention of VTE in patients with advanced severe lung cancer
Risk assessment tool
Khorana risk score
The Khorana score can be used to predict the risk of VTE for symptomatic cancer patients in hospital and is an assistive model for tailoring anticoagulant thromboprophylaxis in such hospitalized patients (50). One study used this simple model to assess the risk of VTE in tumor patients beginning chemotherapy and found it to be a reliable model based on 5 clinical and laboratory parameters. It could identify patients with an almost 7% short-term risk of VTE, and may be used to tailor anticoagulant thromboprophylaxis in outpatients (51).
Vienna score (expanded risk model of the Khorana score)
A study used the Vienna score, which is an expanded risk model based on the Khorana score, and found a statistically significant difference in the cumulative incidence of VTE between patients with the highest risk score and those with an intermediate risk score. The hazard ratio of the patients with the highest grade to those with the lowest grade was 25.9. Five clinical and standard laboratory parameters of the Khorana score with the addition of biomarkers (soluble P-selectin, and D-Dimer) can predict VTE and identify cancer patients who are at high or low risk of VTE (52).
Protecht score
The Protecht model adds 1 point for therapy with carboplatin or cisplatin-based chemotherapy or gemcitabine, and 2 points for association with the score based on the 5 predictive variables of the Khorana score. Compared to the Khorana score, this score model has been reported to have an improved capacity to distinguish patients who are at high risk of VTE (53).
The ONKOTEV study
The ONKOTEV study, which was based on Khorana score >2, took various clinical parameters into account, including a history of previous VTE, the compression of vascular/lymphatic structures by the tumor, and the presence of metastatic disease. It offered a promising model to improve primary prophylaxis for cancer outpatients (54).
COMPASS-CAT
Compared with the Khorana score, the COMPASS-CAT risk assessment model contains easily collected and reliable VTE risk parameters. The variables included in this risk assessment model are shown in Table 1. The COMPASS-CAT model is more suitable for patients with common solid tumors after the initiation of anticancer therapy (55).
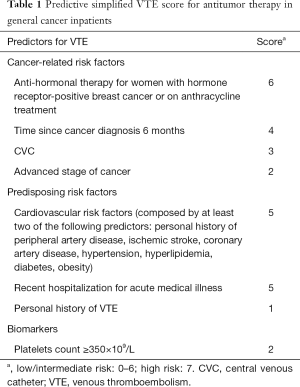
Full table
One retrospective external validation study of cancer patients undergoing active therapy indicated that, while the discrimination of the COMPASS-CAT risk assessment model for VTE was moderate and the model calibration was poor, the model had a good negative predictive value (56).
TiC-Onco risk score
The TiC-Onco risk assessment tool takes into account two new parameters, patient genetics and clinical information, in its examination of risk factors for VTE. One study showed that this model can perform better in identifying cancer patients who have a high risk of VTE compared to the Khorana score (57).
Two variables
Data from the prospective Vienna Cancer and Thrombosis Study cohort was used to select predictive variables for a clinical prediction model. Finally, two variables (tumor-site risk category and continuous D-dimer concentration) were chosen for the predictive tool. Compared to previous predictive tools for cancer-related VTE, this simple model represents an important improvement, and it also makes it easier for doctors to identify patients who could benefit from thromboprophylaxis (58).
Performance of risk assessment tools in lung cancer
In both multivariable and univariate analyses, the patients were predominantly older males with NSCLC, a high-risk Khorana score (a score of >2) was not associated with risk of VTE compared to an intermediate score (a score of 1–2), as high-risk Khorana risk score could not distinguish patients who were at the highest risk of VTE, but a high-risk Khorana score was a predictor of lung cancer mortality (59). CANTARISK is a prospective, global, non-interventional cohort study, clinical data were selected before the end of the 6-month follow-up. Univariable and multivariable Cox regression analyses showed that the Khorana score was an important predictor of early mortality, but it was not suitable for predicting the risk of VTE inpatients with lung malignancies in the era of targeted therapy (60). One study assessed several models: the PROTECHT score, COMPASS-CAT, CONKO score, and Khorana risk score. Only the COMPASS-CAT score had the ability to distinguish VTE accurately and was characterized by good differentiation among high- and low-risk patients with VTE. Comparing all VTE risk assessment models, this study indicated that COMPASS-CAT had the best performance n precisely predicting VTE in lung cancer patients (30).
The prevention of VTE in patients with severe lung cancer
At present, there is no specific guideline for the prevention of VTE in lung cancer patients. Here, we refer to the major guidelines for the treatment of cancer complicated with VTE and the relevant literature on lung cancer with VTE, and discuss the drug-based prevention of VTE in hospitalized and outpatient lung cancer patients undergoing chemotherapy.
Hospitalized patients
Critically ill patients are at an increased risk of VTE due to unique risk factors: prolonged immobilization, invasive lines and devices, certain medications, and acquired thrombophilia. Furthermore, VTE in the critically ill is associated with increased duration of mechanical ventilation, increased length of intensive care unit and hospital stay, and a trend toward increased mortality. Clinical practice guidelines therefore recommend VTE prophylaxis with either subcutaneous heparin or low-molecular-weight heparin for all critically ill patients without contraindication (61). However, no trials have assessed inpatient thromboprophylaxis in patients with lung malignancies. Three randomized controlled trials (RCTs) involving cancer inpatients showed that compared with the placebo, thromboprophylaxis could reduce the incidence of VTE without increasing the risk of massive hemorrhage. However, a meta-analysis of these three RCTs, which covered VTE incidents as a main outcome and analyzed it on the basis of cancer patient subgroups, found that the risk ratio of VTE events in hospitalized cancer patients undergoing thromboprophylaxis was 0.91, and thromboprophylaxis was found to lack efficacy in these patients (62).
Ambulatory patients with cancer undergoing systemic chemotherapy
The PROTECHT and TOPIC-2 studies were two randomized, double-blind, placebo-controlled studies which evaluated the clinical benefits of VTE prophylaxis in advanced cancer patients undergoing chemotherapy. A combined subgroup analysis of the two studies included 811 lung cancer patients (532 patients in the TOPIC-2 study and 279 patients in the PROTECHT study). All patients accepted active prophylaxis: certoparin 3,000 IU once daily subcutaneously for 6 months (TOPIC-2 study) or nadroparin 3,800 IU once daily subcutaneously for up to 4 months (PROTECHT study) (63). According to these studies, preventive treatment with LMWH could reduce the risk of VTE in cancer patients, including those with locally advanced lung cancer who were undergoing chemotherapy (59).
The SAVE-ONCO study was a double-blind, multicenter trial comparing the safety and efficacy of subcutaneous semuloparin (20 mg once daily) to that of a placebo in cancer patients receiving chemotherapy. A total of 1,608 patients received semuloparin, and 1,604 received the placebo. The results showed that semuloparin lowered the risk of VTE occurrence in malignant tumor patients undergoing chemotherapy, without obviously raising the risk of major bleeding (64).
AVERT was a double-blind, controlled, randomized clinical trial that evaluated the safety and efficacy of apixaban (at a dose of 2.5 mg twice a day) for thromboprophylaxis in outpatients with malignant tumors who were undergoing chemotherapy and had a Khorana score of ≥2. Altogether, 563 patients were included in the modified intention-to-treat analysis. The results showed that apixaban can significantly reduce the risk of VTE; however, it can also increase the incidence of main bleeding (61).
CASSINI was a double-blind, randomized trial which compared the safety and effectiveness of rivaroxaban (10 mg once daily) with that of a placebo for up to 180 days in high-risk ambulatory cancer patients with a Khorana score ≥2. A total of 1,080 patients were enrolled. Rivaroxaban resulted in an observably reduced risk of VTE and did not increase the risk of major bleeding (65).
Central venous catheters (CVCs)
A meta-analysis of 13 RCTs assessed the harms and benefits of LMWH, VKAs, unfractionated heparin (UFH), or fondaparinux, and evaluated the outcomes produced by medicines among cancer patients with CVCs. The results showed that the effect of LMWH and VKA on patients with catheters was not conclusive; however, moderate-certainty evidence that LMWH reduced catheter-related VTE compared to no LMWH was found. Therefore, we should balance the possible benefit and harms when considering anticoagulation in patients with cancer with CVCs (66). Another study randomly assessed intervention with warfarin (at a low dose)for cancer patients carrying CVCs and showed that warfarin (at a dose of 1 mg daily) did not decrease the risk of VTE for these patients (67). Another study found that treatment with primary thromboprophylaxis in cancer patients carrying CVCs was neither beneficial nor harmful (68). The Good Clinical Practices Guidelines (GCPG) suggest that anticoagulant treatment for routine prophylaxis is not recommended for CVC-related thrombosis (CRT), and the best places for CVC placement are the right jugular vein and the junction of the right atrium and the superior vena cava (69).
Conclusions
Lung cancer combined with VTE has adverse effects on patients, but its diagnosis can easily be missed due to the symptoms and signs of lung cancer itself. Many risk factors can lead to VTE in lung cancer patients, including advanced disease and adenocarcinoma, chemotherapy and antiangiogenic therapy, and KRAS+, ROS+, or ALK+ NSCLC. In terms of chemotherapy, cisplatin-based regimens carry a higher incidence of VTE than carboplatin/nedaplatin-based regimens. VEGF drugs can also significantly increase the risk of advanced arterial thromboembolic (ATE). Finally, among lung cancer patients, younger age, certain races (e.g., African Americans), and comorbidities are also high-risk factors for VTE. At present, there are no specific guidelines for the treatment and prevention of VTE in lung cancer, and large-scale, prospective, multicenter studies are also lacking in this area. Therefore, herein, we referred to the recommendations of the relevant guidelines for cancer with VTE and the related research on lung cancer with VTE, and discussed the prevention and treatment of VTE in lung cancer.
Compared with warfarin, enoxaparin can reduce thrombosis-related mortality. Long-term use of enoxaparin may be safer for cancer patients. Dalteparin sodium significantly reduces the risk of rVTE in patients with cancer and kidney damage, and shows comparable safety. In terms of the prevention of VTE recurrence, tinzaparin is superior to VKAs as a short- and long-term treatment. Compared with dalteparin sodium, edoxaban had a lower rate of VTE recurrence but a higher incidence of massive hemorrhage. With rivaroxaban, the recurrence rate of VTE was reported to be lower than that with dalteparin, while the incidence of clinically relevant non-main bleeding was increased. Apixaban shows better efficacy in the treatment of malignant tumor-associated VTE and does not increase the risk of massive hemorrhage. These 3 new oral anticoagulants can be used as alternatives to LMWH in patients with tumor with VTE. In addition, the guidelines point out that when the platelet count exceeds 50×109/L, there is no effect on the anticoagulation of LMWH. However, when the platelet count reaches (25–50)×109/L, anticoagulation therapy should be the half-value dose and be stopped when the platelet count is ≤25×109/L. Furthermore, there is no high-quality evidence that IVC filters are beneficial in the treatment of acute VTE. The Khorana score is the main prediction model used to select cancer outpatients for thromboprophylaxis. This model has been improved many times. It is suitable for use as a predictive tool for thromboprophylaxis in lung cancer outpatients; however, the COMPASS-CAT model may be more suitable for thromboprophylaxis in these patients.
In terms of VTE prevention, no trial has assessed thromboprophylaxis in hospitalized lung cancer patients. Patients receiving chemotherapy regimens (gemcitabine and cisplatin or carboplatin) may benefit more from the use of nadroparin in the prevention of thrombosis. Ultra-LMWH sodium semuloparin is also effective and safe for preventing VTE in cancer patients undergoing outpatient chemotherapy. For high-risk outpatients receiving chemotherapy (Khorana score ≥2), apixaban can significantly reduce the risk of VTE at the start of chemotherapy, although it carries a high incidence of massive hemorrhage. Rivaroxaban significantly reduces the incidence of such events during the intervention period. In addition, anticoagulant therapy is not recommended for routine prevention of CRT in cancer patients with CVCs.
Acknowledgments
Thanks my wife, Li-Juan Pi, and my daughter, Yu-Han Zheng, for their support in my work, and happy birthday to my daughter.
Funding: This study was supported by grants from Guangzhou Science and Technology Project (No. 202102080518).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-1281
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-1281). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xie Z, Zhou C, Qin Y, et al. Diagnosis and treatment strategy for advanced severe lung cancer. Chinese Journal of Practical Internal Medicine 2019;39:416-9.
- Wendelboe AM, McCumber M, Hylek EM, et al. Global public awareness of venous thromboembolism. J Thromb Haemost 2015;13:1365-71. [Crossref] [PubMed]
- Zhang Y, Yang Y, Chen W, et al. Prevalence and associations of VTE in patients with newly diagnosed lung cancer. Chest 2014;146:650-8. [Crossref] [PubMed]
- Corrales-Rodriguez L, Blais N. Lung cancer associated venous thromboembolic disease: a comprehensive review. Lung Cancer 2012;75:1-8. [Crossref] [PubMed]
- Hall IE, Andersen MS, Krumholz HM, et al. Predictors of venous thromboembolism in patients with advanced common solid cancers. J Cancer Epidemiol 2009;2009:182521 [Crossref] [PubMed]
- Chen W, Zhang Y, Yang Y, et al. Prognostic significance of arterial and venous thrombosis in resected specimens for non-small cell lung cancer. Thromb Res 2015;136:451-5. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Walker AJ, Card TR, West J, et al. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer 2013;49:1404-13. [Crossref] [PubMed]
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007;5:632-4. [Crossref] [PubMed]
- Connolly GC, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory patients with lung cancer. Lung Cancer 2012;78:253-8. [Crossref] [PubMed]
- Blom JW, Osanto S, Rosendaal FR. The risk of a venous thrombotic event in lung cancer patients: higher risk for adenocarcinoma than squamous cell carcinoma. J Thromb Haemost 2004;2:1760-5. [Crossref] [PubMed]
- Jeong J, Jeong MJ, Choi K, et al. Clinical outcomes of comorbid cancer patients with venous thromboembolism: A retrospective, single-center study in Korea. Medicine (Baltimore) 2019;98:e17181 [Crossref] [PubMed]
- Mahé I, Chidiac J, Bertoletti L, et al. The Clinical Course of Venous Thromboembolism May Differ According to Cancer Site. Am J Med 2017;130:337-47. [Crossref] [PubMed]
- Zhang C, Zhang Z, Mi J, et al. The cumulative venous thromboembolism incidence and risk factors in intensive care patients receiving the guideline-recommended thromboprophylaxis. Medicine (Baltimore) 2019;98:e15833 [Crossref] [PubMed]
- Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost 2020;18:1995-2002. [Crossref] [PubMed]
- Ruiz-Artacho P, Trujillo-Santos J, Lopez-Jimenez L, et al. Clinical Characteristics and Outcomes of Patients with Lung Cancer and Venous Thromboembolism. TH Open 2018;2:e210-e217. [Crossref] [PubMed]
- Maia R, Neves I, Morais A, et al. Venous and lung thromboembolism in the context of lung cancer: clinical manifestations, risk factors and prognosis. Acta Med Port 2019;32:647-53. [Crossref] [PubMed]
- Walker AJ, Baldwin DR, Card TR, et al. Risk of venous thromboembolism in people with lung cancer: a cohort study using linked UK healthcare data. Br J Cancer 2016;115:115-21. [Crossref] [PubMed]
- Ng TL, Smith DE, Mushtaq R, et al. ROS1 Gene Rearrangements Are Associated With an Elevated Risk of Peridiagnosis Thromboembolic Events. J Thorac Oncol 2019;14:596-605. [Crossref] [PubMed]
- Chiari R, Ricciuti B, Landi L, et al. ROS1-rearranged Non-small-cell Lung Cancer is Associated With a High Rate of Venous Thromboembolism: Analysis From a Phase II, Prospective, Multicenter, Two-arms Trial (METROS). Clin Lung Cancer 2020;21:15-20. [Crossref] [PubMed]
- Zer A, Moskovitz M, Hwang DM, et al. ALK-Rearranged Non-Small-Cell Lung Cancer Is Associated With a High Rate of Venous Thromboembolism. Clin Lung Cancer 2017;18:156-61. [Crossref] [PubMed]
- Corrales-Rodriguez L, Soulieres D, Weng X, et al. Mutations in NSCLC and their link with lung cancer-associated thrombosis: a case-control study. Thromb Res 2014;133:48-51. [Crossref] [PubMed]
- Huang H, Korn JR, Mallick R, et al. Incidence of venous thromboembolism among chemotherapy-treated patients with lung cancer and its association with mortality: a retrospective database study. J Thromb Thrombolysis 2012;34:446-56. [Crossref] [PubMed]
- Mitani A, Jo T, Yasunaga H, et al. Venous thromboembolic events in patients with lung cancer treated with cisplatin-based versus carboplatin/nedaplatin-based chemotherapy. Anticancer Drugs 2018;29:560-4. [Crossref] [PubMed]
- Zhang D, Zhang X, Zhao C. Risk of venous and arterial thromboembolic events associated with anti-VEGF agents in advanced non-small-cell lung cancer: a meta-analysis and systematic review. Onco Targets Ther 2016;9:3695-704. [Crossref] [PubMed]
- Chen N, Ren M, Li R, et al. Bevacizumab promotes venous thromboembolism through the induction of PAI-1 in a mouse xenograft model of human lung carcinoma. Mol Cancer 2015;14:140. [Crossref] [PubMed]
- Nalluri SR, Chu D, Keresztes R, et al. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA 2008;300:2277-85. [Crossref] [PubMed]
- Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006;166:458-64. [Crossref] [PubMed]
- Roberts LN, Patel RK, Arya R. Venous thromboembolism and ethnicity. Br J Haematol 2009;146:369-83. [Crossref] [PubMed]
- Rupa-Matysek J, Lembicz M, Rogowska EK, et al. Evaluation of risk factors and assessment models for predicting venous thromboembolism in lung cancer patients. Med Oncol 2018;35:63. [Crossref] [PubMed]
- Becattini C, Agnelli G, Pesavento R, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest 2006;130:172-5. [Crossref] [PubMed]
- Poli D, Grifoni E, Antonucci E, et al. Incidence of recurrent venous thromboembolism and of chronic thromboembolic pulmonary hypertension in patients after a first episode of pulmonary embolism. J Thromb Thrombolysis 2010;30:294-9. [Crossref] [PubMed]
- Steuer CE, Behera M, Kim S, et al. Predictors and outcomes of venous thromboembolism in hospitalized lung cancer patients: A Nationwide Inpatient Sample database analysis. Lung Cancer 2015;88:80-4. [Crossref] [PubMed]
- Marin-Barrera L, Munoz-Martin AJ, Rios-Herranz E, et al. A Case-Control Analysis of the Impact of Venous Thromboembolic Disease on Quality of Life of Patients with Cancer: Quality of Life in Cancer (Qca) Study. Cancers (Basel) 2019;12:75. [Crossref] [PubMed]
- Howlett J, Benzenine E, Cottenet J, et al. Could venous thromboembolism and major bleeding be indicators of lung cancer mortality? A nationwide database study. BMC Cancer 2020;20:461. [Crossref] [PubMed]
- Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med 2002;162:1729-35. [Crossref] [PubMed]
- Martínez-Zapata MJ, Mathioudakis AG, Mousa SA, et al. Tinzaparin for long-term treatment of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. Clin Appl Thromb Hemost 2018;24:226-34. [Crossref] [PubMed]
- Woodruff S, Feugere G, Abreu P, et al. A post hoc analysis of dalteparin versus oral anticoagulant (VKA) therapy for the prevention of recurrent venous thromboembolism (rVTE) in patients with cancer and renal impairment. J Thromb Thrombolysis 2016;42:494-504. [Crossref] [PubMed]
- Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med 2018;378:615-24. [Crossref] [PubMed]
- Young AM, Marshall A, Thirlwall J, et al. Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial (SELECT-D). J Clin Oncol 2018;36:2017-23. [Crossref] [PubMed]
- Agnelli G, Becattini C, Meyer G, et al. Apixaban for the Treatment of Venous Thromboembolism Associated with Cancer. N Engl J Med 2020;382:1599-607. [Crossref] [PubMed]
- McBane RD 2nd, Wysokinski WE, Le-Rademacher JG, et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J Thromb Haemost 2020;18:411-21. [Crossref] [PubMed]
- Khorana AA, Noble S, Lee AYY, et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost 2018;16:1891-4. [Crossref] [PubMed]
- Watson HG, Keeling DM, Laffan M, et al. Guideline on aspects of cancer-related venous thrombosis. Br J Haematol 2015;170:640-8. [Crossref] [PubMed]
- Key NS, Khorana AA, Kuderer NM, et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2020;38:496-520. [Crossref] [PubMed]
- Barginear MF, Gralla RJ, Bradley TP, et al. Investigating the benefit of adding a vena cava filter to anticoagulation with fondaparinux sodium in patients with cancer and venous thromboembolism in a prospective randomized clinical trial. Support Care Cancer 2012;20:2865-72. [Crossref] [PubMed]
- Smouse HB, Mendes R, Bosiers M, et al. The RETRIEVE trial: safety and effectiveness of the retrievable crux vena cava filter. J Vasc Interv Radiol 2013;24:609-21. [Crossref] [PubMed]
- Rojas-Hernandez CM, Zapata-Copete JA, Garcia-Perdomo HA. Role of vena cava filters for the management of cancer-related venous thromboembolism: Systematic review and meta-analysis. Crit Rev Oncol Hematol 2018;130:44-50. [Crossref] [PubMed]
- Hoffer EK. Inferior Vena Cava Filters in the Management of Venous Thromboembolism. JAMA 2019;321:1006. [Crossref] [PubMed]
- Parker A, Peterson E, Lee AYY, et al. Risk stratification for the development of venous thromboembolism in hospitalized patients with cancer. J Thromb Haemost 2018;16:1321-6. [Crossref] [PubMed]
- Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111:4902-7. [Crossref] [PubMed]
- Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood 2010;116:5377-82. [Crossref] [PubMed]
- Verso M, Agnelli G, Barni S, et al. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med 2012;7:291-2. [Crossref] [PubMed]
- Cella CA, Di Minno G, Carlomagno C, et al. Preventing Venous Thromboembolism in Ambulatory Cancer Patients: The ONKOTEV Study. Oncologist 2017;22:601-8. [Crossref] [PubMed]
- Gerotziafas GT, Taher A, Abdel-Razeq H, et al. A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS-Cancer-Associated Thrombosis Study. Oncologist 2017;22:1222-31. [Crossref] [PubMed]
- Spyropoulos AC, Eldredge JB, Anand LN, et al. External Validation of a Venous Thromboembolic Risk Score for Cancer Outpatients with Solid Tumors: The COMPASS-CAT Venous Thromboembolism Risk Assessment Model. Oncologist 2020;25:e1083-e1090. [Crossref] [PubMed]
- Muñoz Martín AJ, Ortega I, Font C, et al. Multivariable clinical-genetic risk model for predicting venous thromboembolic events in patients with cancer. Br J Cancer 2018;118:1056-61. [Crossref] [PubMed]
- Pabinger I, van Es N, Heinze G, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol 2018;5:e289-e298. [Crossref] [PubMed]
- Mansfield AS, Tafur AJ, Wang CE, et al. Predictors of active cancer thromboembolic outcomes: validation of the Khorana score among patients with lung cancer. J Thromb Haemost 2016;14:1773-8. [Crossref] [PubMed]
- Kuderer NM, Poniewierski MS, Culakova E, et al. Predictors of Venous Thromboembolism and Early Mortality in Lung Cancer: Results from a Global Prospective Study (CANTARISK). Oncologist 2018;23:247-55. [Crossref] [PubMed]
- Lewis TC, Cortes J, Altshuler D, et al. Venous Thromboembolism Prophylaxis: A Narrative Review With a Focus on the High-Risk Critically Ill Patient. J Intensive Care Med 2019;34:877-88. [Crossref] [PubMed]
- Carrier M, Khorana AA, Moretto P, et al. Lack of evidence to support thromboprophylaxis in hospitalized medical patients with cancer. Am J Med 2014;127:82-86.e1. [Crossref] [PubMed]
- Verso M, Gussoni G, Agnelli G. Prevention of venous thromboembolism in patients with advanced lung cancer receiving chemotherapy: a combined analysis of the PROTECHT and TOPIC-2 studies. J Thromb Haemost 2010;8:1649-51. [Crossref] [PubMed]
- Chen YW, Khorana AA. The association between race and venous thromboembolism risk after initiation of chemotherapy: An analysis of the SAVE-ONCO trial control arm. Am J Hematol 2017;92:E101-E103. [Crossref] [PubMed]
- Khorana AA, Soff GA, Kakkar AK, et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med 2019;380:720-8. [Crossref] [PubMed]
- Kahale LA, Tsolakian IG, Hakoum MB, et al. Anticoagulation for people with cancer and central venous catheters. Cochrane Database Syst Rev 2018;6:CD006468 [Crossref] [PubMed]
- Couban S, Goodyear M, Burnell M, et al. Randomized placebo-controlled study of low-dose warfarin for the prevention of central venous catheter-associated thrombosis in patients with cancer. J Clin Oncol 2005;23:4063-9. [Crossref] [PubMed]
- Navaneethan S, Singh S, Radhakrishnan S, et al. Thromboprophylaxis in cancer patients with central venous catheters. Thromb Haemost 2008;99:38-43. [Crossref] [PubMed]
- Debourdeau P, Farge D, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost 2013;11:71-80. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


