
Intermittent fasting therapy promotes insulin sensitivity by inhibiting NLRP3 inflammasome in rat model
Introduction
Type 2 diabetes mellitus (T2DM) is the most common metabolic disease. To date, there are >400 million T2DM patients worldwide (1). Insulin resistance (IR) is one of the crucial features of T2DM (2), which is characterized by impaired glucose homeostasis, including hyperglycemia and dyslipidemia (3). Increasing evidence has shown that inflammation plays an important role in the development of T2DM; inflammation is considered the underlying mechanism of IR and a core characteristic of many other metabolic diseases (4). For example, some studies have detected prolific infiltration of inflammatory cells and aberrant releases of proinflammatory cytokines including interleukin (IL)-1, IL-6, IL-12, IL-18, and tumor necrosis factor alpha (TNFα) (5). Other studies have indicated that intermittent fasting therapy (FT) can down-regulate the expression of pro-inflammatory factors, such as IL-6 and C-reactive protein (CRP) (6).
Inflammasomes are cytosolic receptors and can be activated by metabolic stresses. Activation of inflammasomes will lead to over-production of proinflammatory cytokines, therefore, inflammasome activation is reported to be associated with T2DM (7). The inflammasome NLR pyrin domain containing 3 (NLRP3), a NLR family member, is constituted by several damage-associated molecular patterns (DAMPs) proteins. In recent studies, NLRP3 inflammasome has been found to participate in inflammatory responses contributing to the progression of IR. A critical role of NLRP3 has been found in the progression of IR in T2MD (8,9). Activation of NLRP3 inflammasome induces maturation of IL-1β and IL-18 and is highly associated with nutrient metabolism (7). In patients with obesity and/or IR, increased expression of the NLRP3 inflammasome was found in adipose tissue. Consistently, this change was also observed in the mice fed with a high fat diet, however, calorie-restriction decreased its expression (10). Inhibition of NLRP3 significantly ameliorates IR in the leptin-receptor knockout (KO) mice (11) and high-fat diet mice (9). These results have suggested an association between activation of the NLRP3 inflammasome and IR. Increasingly, evidence has shown that IF therapy can persistently improve physical function (12,13). Studies in both humans and animals have supported that IF therapy can significantly promote glucose metabolism. For example, IF therapy obviously reduced blood glucose and insulin levels, and promoted fatty acid metabolism in the IF mice, compared with those on calorie restricted diets (14-17). Besides, IF therapy has also been found to improve insulin sensitivity and glucose tolerance (18-20). Considering the complex mechanisms of T2DM, some researchers believe that IF therapy provides comprehensive effects and may be a promising option for the treatment of T2DM. Therefore, elucidating its underlying mechanisms would be very beneficial to patients suffering from metabolic diseases.
In the present study, we investigated the underlying molecular mechanisms of IF during the improvement of IR. We established an IR animal model using a high-fat diet (HFD) plus low dose of streptozotocin (STZ), and used the pre-adipocyte cell line 3T3-L1 as the in vitro IR cell model to explore the promising role of IF therapy. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2410).
Methods
Animal model and establishment
A total of 32 male Sprague-Dawley (SD) rats (weight, 150–190 g) were randomly grouped into four groups (n=8 in each group), namely, control group, IR group, IR+FT group, and IR+MET group. Rats in the IR, IR+FT, and IR+MET groups were fed with a high-fat diet (58% fat, 25% protein, and 17% carbohydrate by energy composition) for 1 month. The rats in the control group were fed normal chow, and administered with saline (10 mL/kg/day) via oral gavage in subsequent experiments. These rats were treated with intraperitoneal injection of STZ solution (35 mg/kg, dissolved in 0.1 M citrate-citrate solution), and fasted overnight. However, the animals were fed with a 5% glucose solution instead of water within 24 h after STZ injection to avoid hypoglycemia caused by fasting. Fasting blood glucose, fasting insulin levels, and IR levels were detected according to homeostasis model assessment of IR (HOMA-IR). The HOMA-IR was calculated according to the following formula: HOMA-IR = [FINS (pg/mL) × FBG (mmol/L)]/22.5 according the previous report (21). The experimental animals continued to be fed with the high fat diet throughout the entire experiment (22).
The rats in IR+FT group were treated with fasting every other day, while the rats in IR+MET group were treated with gavages of 300 mg/kg metformin (MET) every day for 6 weeks. All rats were weighted every 5 days. The dose of MET was adjusted according to body weight change. Food intake was calculated by subtracting the amount of residual food from the amount of supply food.
Experiments were performed under a project license (NO.: 2021-519) granted by committee board of Medical Center of Hong Kong University of Shenzhen Peking University, in compliance with Medical Center of Hong Kong University of Shenzhen Peking University institutional guidelines for the care and use of animals.
Detection of markers
After 6 weeks of FT or MET treatment, rats were anesthetized with 10% urethane (1.0 g/kg) after 12 h fasting. Blood samples were collected from the abdominal aorta and centrifuged immediately at 3,000 rpm for 10 min at 4 °C. Free blood glucose and blood glucose were determined using a Cobas 6000 analyzer (Roche Diagnostics Ltd., Basel, Switzerland). The free insulin (FINS) was measured by enzyme-linked immunosorbent assay (ELISA) kits (EMD Millipore, Burlington, MA, USA). Measurement of CRP, IL-1β, and IL-18 were measured by Rat CRP ELISA Kit (PTX1) (Abcam, Cambridge, UK, cat lot.: ab108827), Rat IL-1 beta ELISA Kit (Abcam, Cambridge, UK, cat lot.: ab100768), and anti-IL-18 antibody (Abcam, Cambridge, UK, cat lot.: ab191860), respectively. Livers in each group were collected to evaluate the levels of caspase-1 (Abcam, Cambridge, UK, cat lot.: ab1872), p-caspase-1 (Abcam, Cambridge, UK, cat lot.: ab179515), IL-18/p-IL-18 (Abcam, Cambridge, UK, cat lot.: ab191860), L-1β/p-IL-1β (Abcam, Cambridge, UK, cat lot.: ab9722), as well as the expressions of GLUT (Abcam, Cambridge, UK, cat lot.: ab115730), IRS (Abcam, Cambridge, UK, cat lot.: ab134101), and IRS2 (Abcam, Cambridge, UK, cat lot.: ab134101). Serum insulin levels were measured by an insulin radioimmunoassay kit (Linco, St. Charles, MO, USA). All experimental procedures were conducted following the Regulations for Laboratory Animal Management approved by the Animal Ethics Committee.
3T3-L1 cell culture and induced differentiation
The 3T3-L1 cells, a mouse pre-adipocyte cell line, were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). Pre-adipocyte 3T3-L1 cells were cultured in Dulbecco’s Modified Eagle Medium DMEM with high glucose [4.5 mM glucose, 10% fetal calf serum (FCS)] at 37 °C in a 5% CO2 atmosphere, and induced into adipocytes as previously described (9). Briefly, after a 2-day incubation, the medium was replaced with 10% FCS DMEM in which 0.5 mM isobutyl methylxanthine, 1 M dexamethasone, and 101 g/mL insulin were added. The cells were incubated with 10 mg/mL insulin in 10% FCS DMEM for 24 hours and then cultured with 10% FCS media every other day for 8 days.
Establishment of insulin resistant in 3T3-L1 adipocyte model
The 3T3-L1 cells that were well-differentiated into adipocytes were pretreated with DMEM (high glucose) and supplemented with 0.5% (w/v) FBS for 3 h. The cells were then exposed to 10 ng/mL TNF-α in DMEM (high glucose) containing 10% (w/v) fetal bovine serum (FBS) for 24 h. Cell medium was replaced with DMEM (high glucose) containing 100 nM insulin and 10% FBS and incubated for 30 min. Well-differentiated 3T3-L1 adipocytes without TNF-α and/or insulin treatment were used as control. Glucose uptake tests were performed to confirm the establishment of IR. An uptake test for 2-Deoxy-D-(3H) glucose (3H-2-DG) (GE healthcare, Waukesha, WI, USA) was performed, and 3H-2-DG radioactivity absorbed by the cells was also calculated by liquid scintillation using a scintillation counter.
Treatment and transfection in the IR adipocytes
The IR 3T3-L1 adipocytes were seeded on 96-well plates when the cells were growing with a 50% confluent. To investigate the effects of FT on inflammatory response and IR, free-serum medium or medium containing serum and MET (10 nM) was added into the IR 3T3-L1 adipocytes every other day for 7 days, respectively. Finally, 100 nM insulin was added into each group for 30 min, followed by determination of 3H-2-DG radioactivity.
Alum crystal (200 nM) was used as the NLRP3 inflammasome agonist, and the cells were incubated with alum crystal (200 nM) in an incubator with 5% CO2, 95% humidity, at 37 °C for 4 h.
Western blotting assay
Western blot was performed as previously reported (23). In brief, protein quantification was conducted by bicinchoninic acid (BCA) protein assay with Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: 23225). Each lane was loaded with 120 µL, and the samples were separated by 5% compressing gel and 10% sodium dodecyl sulphate (SDS)-polyacrylamide separating gels. Then, the proteins were transferred onto polyvinylidene fluoride (PVDF) membranes. We then used 10% skim milk diluted by phosphate buffered saline with Tween 20 (PBST) as a blocking buffer. The blocked membranes were incubated with primary antibodies against caspase-1 (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: 14-9832-82), p-caspase-1 (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: PA5-38565), IL-18/p-IL-18 (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: PA5-79481), p-IL-1β/IL-1β (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: M421B), GLUT (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: MA5-31960), insulin receptor substrate 1 (IRS1) (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: PA5-56267), IRS2 (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: PA5-17056), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Cell Signaling Technology, Danvers, TX, USA) overnight at 4 °C. The membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: A-11059; and Thermo Fisher Scientific, cat lot.: A-21247). Signals were visualized using enhanced chemi-luminescence detection (GE Healthcare, Waukesha, WI, USA).
ELISA
Levels of FIN, CRP, IL-1β, and IL-18 were measured by Glucose Assay Kit (Abcam, Cambridge, UK, cat lot.: ab65333), Mouse C Reactive Protein ELISA Kit (Abcam, Cambridge, UK, cat lot.: PTX1), Monkey IL-1beta ELISA Kit (Abcam, Cambridge, UK, cat lot.: ab242234), and Mouse IL-18 ELISA Kit (Abcam, Cambridge, UK, cat lot.: ab216165), respectively. Triglyceride Assay Kit (Abcam, Cambridge, UK, cat lot.: ab65336) and non-esterified fatty acid (NEFA) ELISA Kit (Jianglai Biotech., Shanghai, China, 0-018344) were used, and the absorbance was then measured at 450 nm using a microplate spectrophotometer (Multiskan GO, Thermo, Waltham, MA, USA).
Immunofluorescence assay
Immunofluorescence staining of glucose transporter 1 (GLUT1) was performed as previously described (24). Briefly, 3T3-L1 adipocytes were washed with PBS three times, fixed with 4% paraformaldehyde solution for 15 min, and blocked with 10% normal goat serum at 37 °C for 2 h. Then, the cells were washed and incubated with anti-GLUT1 antibody (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: MA5-31960) at 4 °C overnight, and with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody (Thermo Fisher Scientific, Waltham, MA, USA, cat lot.: F-2765). We then used 4',6-diamidino-2-phenylindole (DAPI; Beyotime, Shanghai, China) for 20 min at room temperature. Immunofluorescence was examined by an inverted fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Oil Red O staining
After being washed with PBS, the cells were fixed in 10% formaldehyde solution at 25 °C for 15 min. The cells were incubated with Oil red O working solution at 25 °C for 30 min. Then, the cells were washed with 75% ethanol and PBS. Observation was performed by a Leica DMI4000B inverted microscope with a 510 nm spectrophotometer (Leica, Wetzlar, Germany) to quantify the intracellular lipid content.
Statistical analysis
All statistical analyses were performed using statistical software SPSS version 20.0 (IBM, Armonk, NY, USA); the tests were two-sided, with a significance level of P<0.05. One-way analysis of variance (ANOVA) followed with Tukey’s post-hoc test was used.
Results
The FT significantly improved IR induced by a high-fat diet and STZ in rats
To evaluate the effect of FT on IR, we established an IR model induced by high-fat diet and low-dose STZ treatment in rats. The rats were grouped into the IR group treated with vehicle, IR+FT group with FT, and IR+MET group treated by MET.
We first measured the levels of fasting blood insulin and glucose, and further calculated the HOME-IR. As shown in Figure 1A,B,C, we found that the levels of fasting blood insulin, glucose, and HOMA-IR were significantly increased in the rats treated with a high-fat diet and STZ injection, compared with the control group (P<0.05). Treatment with FT down-regulated the levels of fasting blood insulin and glucose, as well as HOMA-IR (P<0.05), which were comparable to that of the IR+MET group, suggesting that FT significantly improved the insulin sensitivity. On the other hand, all levels of inflammatory markers including CRP (Figure 1D), IL-1β (Figure 1E) and IL-18 (Figure 1F) were increased in the IR rats compared with the control group (P<0.05), and they were reversed by both FT and metformin.
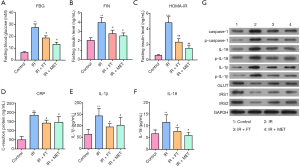
Western blotting assay further confirmed the potential of FT to improve IR. As demonstrated in Figure 1G, FT reversed the expression of GLUT1, IRS, and IRS2 induced by the high-fat diet and low-dose STZ treatment in the IR group. Besides, IR up-regulated the expression of inflammatory cytokines including IL-18, p-IL-18, p-IL-1β, IL-1β, caspase-1, and p-caspase-1 compared with the control (P<0.05), all of which were down-regulated by FT and MET. These results indicate that the underlying mechanisms of FT may be associated with its anti-inflammatory activities.
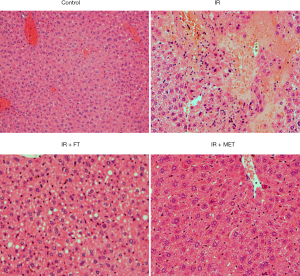
Hepatocyte involvement is critical in the development of IR. Some reports have shown that lipid accumulation was increased in the liver of rats treated by high fat-diet and STZ, further contributing to the deterioration of IR (25). Consistently, we also found that lipid accumulation in the liver of rats was significantly greater in the IR group than control (Figure 2), and FT or MET dramatically reduced this accumulation.
FT reversed the IR of 3T3-L1 adipocytes in vitro
To further investigate the underlying mechanisms of FT, well-differentiated 3T3-L1 adipocytes were adopted as an in vitro model of IR (26-28). As shown in Figure 3A, significant lipid accumulation was observed in the 3T3-L1 treated by DMEM and high glucose, indicating differentiation of pre-adipocytes into differentiated adipocytes. Liquid scintillation was used to evaluate the insulin-stimulated 3H-2-DG uptake (Figure 3B). We found that significantly decreased glucose uptake existed in the 3T3-L1 adipocytes in IR group, compared with the control (P<0.05), and the uptake was promoted by FT and MET (Figure 3B).
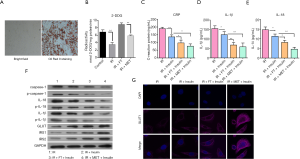
The above results have revealed that FT significantly decreased inflammation in rats. Next, we evaluated the effects of FT on the level of CRP, IL-1β, and IL-18. Our results showed that FT or MET also significantly decreased the secretion of CRP (Figure 3C), IL-1β (Figure 3D), and IL-18 (Figure 3E) in the 3T3-L1 adipocytes. High expressions of inflammatory markers induced by IR, including caspase-1, IL-18, and, IL-1β were also reversed by FT (Figure 3F). Immunofluorescence and western blotting assays further demonstrated that FT or MET dramatically elevated the expression of GLUT1 in whole cells (Figure 3F) and in cytoplasm (Figure 3G), as well as the levels of IRS1 and IRS2. These findings suggest that FT can also effectively improve IR in the 3T3-L1 adipocytes in vitro.
NLRP3 inflammasome agonist inhibited the effects of FT on reversion of IR
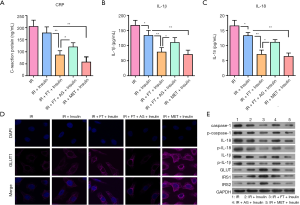
The above rat model and 3T3-L1 adipocyte results have indicated that activation of the inflammatory response may play a key role in the development of IR; therefore, inhibition of inflammation would be the one of the underlying mechanisms of FT. To further confirm, we used an inflammatory agonist and FT to treat the 3T3-L1 adipocytes. Our observations found that increased expression of inflammatory markers including CRP (Figure 4A), IL-1β (Figure 4B), and IL-18 (Figure 4C) induced by IR in 3T3-L1 adipocytes was inhibited by FT; however, this inhibition was antagonized by the inflammatory agonist. Besides, fluorescence and western blotting results showed that the agonist also reduced the expression of GLUT1 in cytoplasm (Figure 4D) and whole cell (Figure 4E), as well as other key molecules in insulin pathways such as IRS1 and IRS2 (Figure 4E). However, the level of inflammatory markers including caspase-1, CRP, IL-1β, and IL-18 was elevated in the cells treated with the inflammatory agonist (Figure 4E). These results provide an understanding that inhibition of the NLRP3 inflammasome may contribute to the effects of FT on improving IR.
Discussion
A crucial role is played by IR in the pathogenesis of obesity and T2DM. Aberrant activation of inflammation is reported to contribute to the development of IR. Obesity can lead to insulin resistance. The functional abnormalities of adipose decomposition products, free fatty acids, tumor necrosis factor-α, leptin, resistin, adiponectin and PPAR γ may be related to insulin resistance (29). Insulin resistance is typically defined as reduced sensitivity and responsiveness to insulin-mediated glucose disposal and inhibition of glucose production in the liver. To make up for the dysfunction of insulin, the compensatory insulin secretion of islet cells increases, leading to hyperinsulinemia, but the patient is still in the normal stage of glucose tolerance. Insulin secreted by islet cells fails compensate completely, resulting in an abnormal increase in blood glucose levels and entering the period of abnormal glucose tolerance. Finally, the islet cells failed due to long-term compensation, the glucose metabolism was disordered, and the blood glucose further increased, leading to clinical diabetes (30). It is extremely important to explore the underlying mechanisms of IR and effective methods of management.
Researchers have extensively reported that IR can be significantly improved by FT. In our present study, we established an in vivo IR model in rats using a high-fat diet combined with STZ injection. Our results suggest that FT indeed effectively reduces the high-fat diet- and STZ-induced elevation of blood insulin, glucose, and HOMA-IR, as well as increases the expression of GLUT1, IRS1, and IRS2. On the other hand, FT can also inhibit the levels of CRP, IL-1β, IL-18, and caspase-1, revealing the inhibitory effect of FT on activation of inflammation. Consistently, similar results were also observed in the 3T3-L1 adipocytes with IR. We have found that the activation of NLRP3 inflammasomes by its agonist antagonized or weakened the effect of FT on the amelioration of IR in the 3T3-L1 adipocytes.
The many benefits of FT have already been extensively reported. For example, IR was developed in the rats or mice model of type I diabetes, and FT exerted significant anti-diabetic effects, with improvements to IR (31). Other studies have also provided potent evidence that FT can reverse or improve impaired glucose tolerance, hyperinsulinemia, and systemic inflammation (32-34). Consistent with the results of these previous studies, we observed that FT can significantly decrease the levels of blood insulin and glucose, and improve IR. Considering that inflammation is an important element during the development of IR and that FT obviously decreases the expression of IL-18 and CRP, we suppose that FT exerts the anti-IR effect by suppressing the pro-inflammatory factors. Some previous studies have demonstrated that FT significantly reduced the concentration of pro-inflammatory factors in obese humans (35). Our present observations offer direct evidence in support of the mechanism that FT inhibits the release of systemic inflammation.
On the basis of the in vitro and in vivo results, we speculate that FT might provide some protection for the β-cells. Other studies have demonstrated the protective effects of FT on β-cells in rat models with type I diabetes (31,36), however, the exact mechanisms are unclear. Our study suggests that FT is likely able to protect the β-cells by its anti-inflammatory activities, owing to its significant inhibitory actions on the proinflammatory cytokines and CRP. To verify this speculation, we used 3T3-L1 adipocytes model and the NLRP3 inflammasomes involved in the process of inflammation. The NLRP3 can interact with caspase-1 and induce caspase-1 auto-activation to exert pro-inflammatory effects, including promotion of pro-IL-18 into mature IL-18 (37) and release of IL-1β (38). Therefore, the association between NLRP3 inflammasomes and metabolic diseases have been reported, revealing the pivotal role of NLRP3 inflammasome in the pathophysiology of T2DM (39). Our results have found that FT can improve IR by inhibiting inflammation due to its regulatory effect on the secretion of proinflammatory cytokines which can be antagonized by the NLRP3 inflammasomes. Pedersen et al. review that physical exercise has anti-inflammatory effects, such as inhibiting TNF-α and causing the up-regulation of IL-6/10, and limiting the preference transmission of IL-1β. This direct anti-inflammatory effect can improve the symptoms of type 2 diabetes and is also beneficial to cardiovascular and other chronic diseases (40). The traditional anti-diabetic drug metformin has also been proved to have anti-inflammatory effects in diabetes (41). In addition to caloric therapy, changing the nutritional structure may also contribute to the improvement of insulin resistance (42).
The short-term regulation of energy metabolism during hunger/refeeding depends to a large extent on the interaction between hormones (leptin, insulin and corticosteroids) and energy substrates (glucose, free fatty acids, triglycerides) (43). It is well known that fasting leads to adaptive hormonal and metabolic responses, including a shift to dependence on fatty acids and ketones to produce energy, accompanied by lower blood sugar, triglycerides, leptin and insulin (44). Therefore, this treatment may be functioned due to the regulation of blood glucose, insulin levels, leptin and so on.
In conclusion, as a promising supplement in treating T2DM, FT and its molecular mechanisms for improving IR were investigated in high-fat diet-induced obese rats and the IR 3T3-L1 adipocytes model. Our results have demonstrated that FT can increase insulin-sensitivity through down-regulation of proinflammatory cytokines and activation of the insulin signaling cascade, which is inhibited by the activation of NLRP3 inflammasomes. Up-to-data, there is no drugs targeting NLRP3 for treatment of T2DM. Our findings indicate that FT may be a promising therapeutic method for T2DM by exerting anti-inflammatory effects and targeting NLRP3 inflammasome signaling. However, patients undergoing a fasting must have a full knowledge of their condition. For example, patients with adrenal insufficiency should be treated with Chronocort, Plenadren or others for preventing eventual complications (45).
Acknowledgments
Funding: This work was supported by the Shenzhen Science and Technology Plan Project (2016-148).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2410
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2410
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2410). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (NO.: 2021-519) granted by committee board of Medical Center of Hong Kong University of Shenzhen Peking University, in compliance with Medical Center of Hong Kong University of Shenzhen Peking University institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu L, Zhu X, Sun G, et al. Dai-Zong-Fang, A Traditional Chinese Herbal Formula, Ameliorates Insulin Resistance in db/db Mice. Front Physiol 2018;9:224. [Crossref] [PubMed]
- DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 1988;37:667-87. [Crossref] [PubMed]
- Zhu S, Sun F, Li W, et al. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol Cell Biochem 2011;353:305-13. [Crossref] [PubMed]
- Xu L, Nagata N, Ota T. Glucoraphanin: a broccoli sprout extract that ameliorates obesity-induced inflammation and insulin resistance. Adipocyte 2018;7:218-25. [Crossref] [PubMed]
- Kim F, Pham M, Luttrell I, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 2007;100:1589-96. [Crossref] [PubMed]
- Aksungar FB, Topkaya AE, Akyildiz M. Interleukin-6, C-reactive protein and biochemical parameters during prolonged intermittent fasting. Ann Nutr Metab 2007;51:88-95. [Crossref] [PubMed]
- Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res 2015;8:15-27. [PubMed]
- Stienstra R, Tack CJ, Kanneganti TD, et al. The inflammasome puts obesity in the danger zone. Cell Metab 2012;15:10-8. [Crossref] [PubMed]
- Stienstra R, van Diepen JA, Tack CJ, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A 2011;108:15324-9. [Crossref] [PubMed]
- Ye T, Meng X, Wang R, et al. Gastrodin Alleviates Cognitive Dysfunction and Depressive-Like Behaviors by Inhibiting ER Stress and NLRP3 Inflammasome Activation in db/db Mice. Int J Mol Sci 2018;19:3977. [Crossref] [PubMed]
- Kim Y, Wang W, Okla M, et al. Suppression of NLRP3 inflammasome by γ-tocotrienol ameliorates type 2 diabetes. J Lipid Res 2016;57:66-76. [Crossref] [PubMed]
- Goodrick CL, Ingram DK, Reynolds MA, et al. Effects of intermittent feeding upon growth and life span in rats. Gerontology 1982;28:233-41. [Crossref] [PubMed]
- Singh R, Manchanda S, Kaur T, et al. Middle age onset short-term intermittent fasting dietary restriction prevents brain function impairments in male Wistar rats. Biogerontology 2015;16:775-88. [Crossref] [PubMed]
- Anson RM, Guo Z, de Cabo R, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A 2003;100:6216-20. [Crossref] [PubMed]
- Harvie MN, Pegington M, Mattson MP, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond) 2011;35:714-27. [Crossref] [PubMed]
- Harvie M, Howell A. Potential Benefits and Harms of Intermittent Energy Restriction and Intermittent Fasting Amongst Obese, Overweight and Normal Weight Subjects-A Narrative Review of Human and Animal Evidence. Behav Sci (Basel) 2017;7:4. [Crossref] [PubMed]
- Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev 2017;39:46-58. [Crossref] [PubMed]
- Gotthardt JD, Verpeut JL, Yeomans BL, et al. Intermittent Fasting Promotes Fat Loss With Lean Mass Retention, Increased Hypothalamic Norepinephrine Content, and Increased Neuropeptide Y Gene Expression in Diet-Induced Obese Male Mice. Endocrinology 2016;157:679-91. [Crossref] [PubMed]
- Arum O, Saleh JK, Boparai RK, et al. Preservation of blood glucose homeostasis in slow-senescing somatotrophism-deficient mice subjected to intermittent fasting begun at middle or old age. Age (Dordr) 2014;36:9651. [Crossref] [PubMed]
- Westbrook R, Bonkowski MS, Arum O, et al. Metabolic alterations due to caloric restriction and every other day feeding in normal and growth hormone receptor knockout mice. J Gerontol A Biol Sci Med Sci 2014;69:25-33. [Crossref] [PubMed]
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-9. [Crossref] [PubMed]
- Srinivasan K, Viswanad B, Asrat L, et al. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res 2005;52:313-20. [Crossref] [PubMed]
- Kong P, Chi R, Zhang L, et al. Effects of paeoniflorin on tumor necrosis factor-α-induced insulin resistance and changes of adipokines in 3T3-L1 adipocytes. Fitoterapia 2013;91:44-50. [Crossref] [PubMed]
- Prasad CN, Anjana T, Banerji A, et al. Gallic acid induces GLUT4 translocation and glucose uptake activity in 3T3-L1 cells. FEBS Lett 2010;584:531-6. [Crossref] [PubMed]
- Nie XQ, Chen HH, Zhang JY, et al. Rutaecarpine ameliorates hyperlipidemia and hyperglycemia in fat-fed, streptozotocin-treated rats via regulating the IRS-1/PI3K/Akt and AMPK/ACC2 signaling pathways. Acta Pharmacol Sin 2016;37:483-96. [Crossref] [PubMed]
- Li H, Peng S, Li S, et al. Chronic olanzapine administration causes metabolic syndrome through inflammatory cytokines in rodent models of insulin resistance. Sci Rep 2019;9:1582. [Crossref] [PubMed]
- Sim SY, Shin YE, Kim HK. Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes. Nutr Res 2019;65:54-62. [Crossref] [PubMed]
- Zhang XH, Wang Z, Kang BG, et al. Antiobesity Effect of Astilbe chinensis Franch. et Savet. Extract through Regulation of Adipogenesis and AMP-Activated Protein Kinase Pathways in 3T3-L1 Adipocyte and High-Fat Diet-Induced C57BL/6N Obese Mice. Evid Based Complement Alternat Med 2018;2018:1347612 [Crossref] [PubMed]
- Barazzoni R, Gortan Cappellari G, Ragni M, et al. Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord 2018;23:149-57. [Crossref] [PubMed]
- Yaribeygi H, Farrokhi FR, Butler AE, et al. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol 2019;234:8152-61. [Crossref] [PubMed]
- Belkacemi L, Selselet-Attou G, Hupkens E, et al. Intermittent fasting modulation of the diabetic syndrome in streptozotocin-injected rats. Int J Endocrinol 2012;2012:962012 [Crossref] [PubMed]
- de Cabo R, Mattson MP. Effects of Intermittent Fasting on Health, Aging, and Disease. N Engl J Med 2019;381:2541-51. [Crossref] [PubMed]
- Antoni R, Johnston KL, Collins AL, et al. Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc 2017;76:361-8. [Crossref] [PubMed]
- Rangan P, Choi I, Wei M, et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep 2019;26:2704-19.e6. [Crossref] [PubMed]
- Aksungar FB, Sarıkaya M, Coskun A, et al. Comparison of Intermittent Fasting Versus Caloric Restriction in Obese Subjects: A Two Year Follow-Up. J Nutr Health Aging 2017;21:681-5. [Crossref] [PubMed]
- Rojas-Morales P, Tapia E, León-Contreras JC, et al. Mechanisms of Fasting-Mediated Protection against Renal Injury and Fibrosis Development after Ischemic Acute Kidney Injury. Biomolecules 2019;9:404. [Crossref] [PubMed]
- Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 2015;526:666-71. [Crossref] [PubMed]
- Martín-Sánchez F, Diamond C, Zeitler M, et al. Inflammasome-dependent IL-1β release depends upon membrane permeabilisation. Cell Death Differ 2016;23:1219-31. [Crossref] [PubMed]
- Wen H, Gris D, Lei Y, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol 2011;12:408-15. [Crossref] [PubMed]
- Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest 2017;47:600-11. [Crossref] [PubMed]
- Wu MC, Ye WR, Zheng YJ, et al. Oxamate Enhances the Anti-Inflammatory and Insulin-Sensitizing Effects of Metformin in Diabetic Mice. Pharmacology 2017;100:218-28. [Crossref] [PubMed]
- Mirabelli M, Chiefari E, Arcidiacono B, et al. Mediterranean Diet Nutrients to Turn the Tide against Insulin Resistance and Related Diseases. Nutrients 2020;12:1066. [Crossref] [PubMed]
- Kmiec Z, Pokrywka L, Kotlarz G, et al. Effects of fasting and refeeding on serum leptin, adiponectin and free fatty acid concentrations in young and old male rats. Gerontology 2005;51:357-62. [Crossref] [PubMed]
- Perry RJ, Wang Y, Cline GW, et al. Leptin Mediates a Glucose-Fatty Acid Cycle to Maintain Glucose Homeostasis in Starvation. Cell 2018;172:234-48.e17. [Crossref] [PubMed]
- Chihaoui M, Grira W, Bettaieb J, et al. The risk for hypoglycemia during Ramadan fasting in patients with adrenal insufficiency. Nutrition 2018;45:99-103. [Crossref] [PubMed]
(English Language Editor: J. Jones)


