
A prediction score model and survival analysis of acute kidney injury following orthotopic liver transplantation in adults
Introduction
Acute kidney injury (AKI) is one of the postoperative complications following orthotopic liver transplantation (OLT), and is also associated with other complications such as metabolic acidosis, excessive volume load, electrolyte disturbance, etc. Previous studies have shown that AKI is related to the development of chronic kidney disease (CKD), end-stage kidney disease (ESRD), and even impaired survival rates of liver transplant recipients (1).
However, due to the different criteria in diagnosing AKI, reports on the incidence of post-liver transplantation (post-LT) AKI varies widely, ranging from 11% to 95% (2). In 2004, the Acute Dialysis Quality Initiative (ADQI) group developed the Risk, Injury, Failure, Loss of renal function and End-stage renal disease (RIFLE) criteria to standardize the definition and classification of AKI (3). In 2007, a multidisciplinary working team composed of nephrologists and critically ill physicians—Acute Kidney Injury Network (AKIN) group, proposed the AKIN criteria based on the RIFLE criteria, which is more sensitive and strict in defining AKI (4). In 2012, the International Kidney Disease Improving Global Outcomes (KDIGO) workgroup developed the KDIGO criteria integrating the previous work results, which is currently widely recognized by the scientific community (5).
Given the severe impacts on short- and long-term survival of post-operative AKI for OLT recipients, diagnosing AKI after OLT as soon as possible become particularly important. However, the etiology of AKI after OLT is multifactor, including recipient, donor, intra-, and post-OLT factors. Moreover, the proportion of various factors’ contribution to the AKI varies greatly. Thus, if we can develop a score model using these risk factors, according to their contributions to the development of AKI, it will help clinicians identify recipients at high risk for postoperative AKI intuitively, just like the model of end-stage liver disease (MELD), which can effectively predict the risk of death in patients with end-stage liver disease by calculating the score of this model (6). There have been several studies concerning the AKI prediction score model. For example, Min et al. developed a score model using modified-prognostic nutritional index (mPNI) to predict AKI (7). And Sujan et al. proposed an AKI prediction model for inpatients with cirrhosis and validated this model’s prognostic value in a multicenter study (8). Cheng et al. suggested that MELD score and MELD-Na score could be used to predict the occurrence of AKI directly and compared the prognostic value of them (9). Tan et al., Kalisvaart et al., etc. also have done some efforts. Therefore, our purpose was also to construct a score model to predict AKI and to evaluate the impact of AKI on the long-term survival of patients.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-842).
Methods
Patients
This study retrospectively reviewed all 116 liver transplantation patients in Union Hospital, Tongji Medical College, Huazhong University of Science and Technology from October 2014 to July 2020. Exclusion criteria: (I) Under 18 years old. (II) Death within 48 hours after liver transplantation. (III) Combined liver/kidney transplantation. (IV) Living donor liver transplantation (LDLT). (V) Re-transplantation. (VI) A history of nephrectomy before liver transplantation. (VII) Data missing. The patients were dichotomized into non-AKI group and AKI group according to the KDIGO criteria. And we defined stage-1 AKI as mild AKI, stage-2 AKI and stage-3 AKI as severe AKI. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Tongji Medical College, Huazhong University of Science and Technology (No. 2021-S117) and individual consent for this retrospective analysis was waived.
Preoperative and postoperative data
- Demographic data: gender, age, body mass index (BMI), history of hepatitis B virus infection, history of hypertension, and diabetes mellitus.
- Preoperative data: blood urea nitrogen (BUN), serum creatine (SCr), serum sodium, international normalized ratio (INR), total bilirubin (TBiL), albumin (ALB), lymphocyte count.
- Intraoperative data: operative time, non-hepatic period, the volume of intraoperative blood transfusion, the volume of intraoperative liquids transfusion, postoperative SCr concentration, urine volume change.
- Postoperative intensive care unit (ICU) stay.
- Survival time: from the first day after surgery to July 31, 2020.
The etiology of orthotopic liver transplantation was determined by postoperative pathological examination. And preoperative laboratory data were obtained from the latest laboratory examination before surgery.
Immunosuppressive regimen
1 g methylprednisolone was given before reperfusion of the allograft followed by tacrolimus. The tacrolimus was taken orally on the first day after OLT, and the dose was 2 mg twice a day for recipients. We regularly tested patients’ SCr concentrations and blood concentration of tacrolimus, if the SCr levels rose, the dose of tacrolimus would be reduced, which aimed for maintaining the blood concentration of tacrolimus at 8–12 ng/mL at the 1st month, 7–10 ng/mL at the 2nd–6th month, and 5–8 ng/mL after 6th month.
Definition and calculation formula
The prognostic nutritional index (PNI) is used to quantified the patients nutritional and immunological status:
PNI =10× albumin (g/dL)+0.005× lymphocyte count (/mm3) (7,10);
MELD =3.78× ln [TBiL(mg/dL)] +11.2× ln [PT−INR] +9.57× ln[SCr (mg/dL)] +6.43;
MELD−Na =MELD +1.32× (137−Na) − [0.033× MELD × (137−Na)]. (Sodium values ≤125 mmol/L will be set to 125 mmol/L, and values ≥137 mmol/L will be set to 137 mmol/L).
KDIGO criteria: an increase in SCr by ≥26.5 µmoI/L within 48 h or SCr increase to ≥1.5 times baseline within the first 7 after surgery. AKI was dichotomized into 3 stages: stage-1 AKI, an increase in SCr by ≥26.5 µmoI/L or SCr increase to 1.5–1.9 times baseline, or urine volume <0.5 mL·kg-1·h-1 for 6–12 hours; stage-2 AKI, SCr increase to 2–2.9 times baseline, or urine volume <0.5 mL·kg-1·h-1 for more than 12 hours; stage-3, SCr increase to >3 times baseline or increase in SCr to 354 µmoI/L or the initiation of renal replace therapy (RRT), or urine volume <0.3 mL·kg-1·h-1 for more than 24 hours or anuria for more than 12 hours (5).
Although patients with end-stage liver disease (ESLD) often have oliguria and avid sodium and water retention, they can still maintain a relatively normal glomerular filtration rate (GFR); moreover, some patients may also have a normal urine volume output for the use of diuretic (11). So, we preferred SCr concentration as the major criteria to identify AKI.
Statistical analysis
According to the continuous variables whether confirmed to the normality, they were presented by mean (standard deviation, SD) or median (interquartile range, IQR). The Kolmogorov-Smirnov test was executed to evaluate the normality. And Student’s t-test or Mann-Whitney U test was executed to compare the difference between continuous variables when appropriate. Categorical variables were presented by counts (proportion). And Chi-square test or Fisher’s exact test were executed to assess the difference between categorical variables.
The odds rations (ORs) for the association and the significant statistical variables were obtained from logistic regression (univariate analysis), and P values for these variables were also corrected according to logistic regression analysis. Variables that were statistically significant (P<0.05), and that were not statistically significant on univariate analysis but may contribute to the AKI (diabetes mellitus, etc.) were also entered to the multivariate logistic regression analysis. Moreover, we executed the Hosmer-Lemeshow test to evaluate the overall significance of the models and to decide which model had a better fit. Three receiver operating characteristic (ROC) curves of the MELD, the MELD-Na and the novel AKI prediction model were used to calculate the area under the receiver operating characteristic curve (AUC) for each, to compare their prognostic value for AKI. The Youden index was calculated to obtain the best cutoff value, sensitivity, and specificity. Kaplan-Meier method and log-rank test were executed to estimate the long-term survival of AKI group and non-AKI group. Statistical analyses were performed by SPSS software, version 24.0 (IBM Corp, USA). P<0.05 was considered as the standard of statistical significance.
Results
Study population
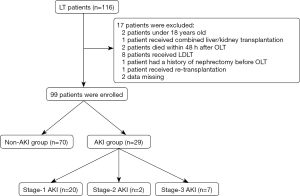
A total of 116 patients received liver transplantation in our center, 17 patients were excluded (2 patients under 18 years old, 2 patients died within 48 h after OLT, 1 patient received combined liver/kidney transplantation, 8 patients received LDLT, 1 patient received re-transplantation, 1 patient had a history of nephrectomy before OLT, and 2 data missing) and 99 patients were eventually enrolled in our study (Figure 1); of these, 91 patients (91.92%) were male and 8 patients (8.08%) were female, the mean age was 48.56±9.8 years old, the median BMI was 23.18 kg/m2. Patients with the history of diabetes mellitus, hypertension and hepatitis B virus infection account for 9.09%, 15.15%, 73.74%, respectively. The median of Child-Pugh score, PNI score, MELD score, MELD-Na score was 7 points, 42.1 points, 11 points, 12 points, respectively. The median preoperative SCr concentration was 68.3 µmol/L, and there were 4 patients with high preoperative SCr concentration (>133 µmol/L) (Table 1).

Full table
Development of AKI after OLT
Among 99 patients, there were 29 patients (29.29%) developed AKI within 7 days after OLT, stage-1 AKI, stage-2 AKI and stage-3 AKI account for 20.20% (20 of 29 patients), 2.02% (2 of 29 patients), 7.07% (7 of 29 patients), respectively, and eventually 13.79% of patients received RRT (4 of 29 patients) (Figure 1).
Patients with AKI were more likely to have the presence of hypertension (27.59% vs. 10%, P=0.035) than those without AKI. Patients who developed AKI were also more likely to had a high score of Child-Pugh (9 vs. 7, P=0.006), MELD (13 vs. 10, P=0.072) and MELD-Na (13 vs. 11, P=0.093). Comparing the surgical methods used for patients with and without AKI separately, standard orthotopic liver transplantation (SOLT) and modified piggy-back orthotopic liver transplantation (MPOLT) were more frequently than piggy-back orthotopic liver transplantation (POLT) (75.86% vs. 62.86%), however, the application of these two category surgical methods did not show any statistical difference (P=0.248). There were also no differences in the volume of blood and fluids transfusion among the AKI group and the non-AKI group during the perioperative. In addition, we found that the length of ICU stay after OLT in the non-AKI group was significantly shorter than that in the AKI group (40.30 vs. 68.00 h, P<0.001) (Table 1).
Predictors of AKI after OLT
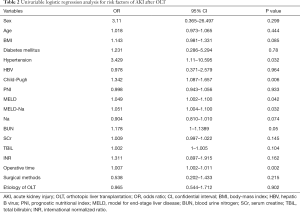
On univariate analysis, there were 5 variables—hypertension (OR =3.429, 95% CI, 1.11–10.595; P=0.032), Child-Pugh score (OR =1.342, 95% CI, 1.087–1.657; P=0.006), MELD score (OR =1.049, 95% CI, 1.002–1.100; P=0.042), MELD-Na score (OR =1.051, 95% CI, 1.004–1.100; P=0.032) and operative time (OR =1.007, 95% CI, 1.002–1.011; P=0.002) that predicted the development of AKI after OLT (Table 2). There were two models revealed by multivariate logistic regression analysis related to the postoperative AKI. Model 1 included operative time (OR =1.006, 95% CI, 1.002–1.010, P=0.003) and MELD-Na score (OR =1.336, 95% CI, 1.063–1.679, P=0.013). Model 2, which included Child-Pugh score (OR =1.336, 95% CI, 1.063–1.679, P=0.013) instead of MELD-Na score. However, according to the Hosmer-Lemeshow test, we found that model 1 had a better fit (Hosmer-Lemeshow P value 0.966 vs. 0.759) than model 2, and the AKI prediction score model was generated as follows: −5.594+0.007× operative time +0.060× MELD−Na (Table 3).
Full table

Full table
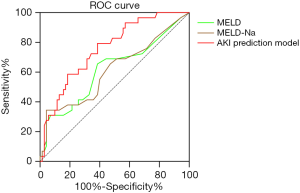
The AKI prediction score model showed an AUC of 0.762, and the sensitivity and specificity were 79.3% and 61.4%, respectively. And the MELD score, MELD-Na score showed an AUC of 0.617 (95% CI, 0.488–0.746, P=0.068), 0.61 (95% CI, 0.481–0.740, P=0.085), respectively (Figure 2, Table 4).

Full table
Impact of AKI on patient survival
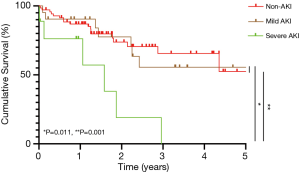
All patients were followed up from the first day after OLT to July 31, 2020. Cumulative survival curves of recipients are depicted in Figure 3 and Figure 4, respectively. The cumulative survival time of patients with severe AKI was significantly lower than that of patients with mild AKI (P=0.001). Although there was no difference in cumulative survival time comparing mild AKI group and non-AKI group (P=0.751). Moreover, we also analyzed the survival outcome of patients with liver cancer and cirrhosis who received OLT, separately, and the findings were similar and consistent with those in the original cohort (Figure 4).

Discussion
A total of 99 patients who received OLT were enrolled in our study. The incidence of post-OLT AKI was 29.29%, which was similar to the multicenter study of Kalisvaart et al. (12), but lower than the studies of Cheng et al. and Hilmi et al. (9,13). RRT was required in 13.79% of AKI patients, which was closed to the study of Sanchez et al. (12%) (14), but lower than the study of Kalisvaart (18%) (12).
Previous researches have suggested that preoperative nutritional status, BMI, gender, diabetes mellitus, hypertension, and volume of intraoperative blood transfusion were risk factors associated with the development of AKI after liver transplantation (7,12,13,15). However, in our research, except the variable of hypertension showed a difference between the AKI group and the non-AKI group on univariate analysis, diabetes mellitus, BMI, age, gender, and volume of intraoperative blood transfusion, etc. did not show any differences comparing AKI and non-AKI groups. Malnutrition is associated with decreased immune system function, poor wound healing, etc. (16), as well as the increased risks of morbidity and mortality after operation and prolonged hospital stays (17). Min et al. believed that the preoperative nutritional status of the recipient was associated with postoperative AKI, and they designed a score model based on modified-prognostic nutritional index (mPNI) to predict AKI after LDLT (7). We also introduced the PNI to assess the preoperative nutritional status of the OLT recipients. However, there was no difference between the two groups. Tan et al. proposed BMI >25 kg/m2 was an independent risk factor of AKI (15), and Hilmi et al. also pointed out that weight >100 kg was a predictor of AKI (13). Obesity could contribute to hyperfiltration syndrome, glomerular hypertrophy, and mesangial hyperplasia, and finally resulted in renal dysfunction. Besides, obese patients may had a higher rate of postoperative infection following liver transplantation (18), and infection could also contribute to the development of AKI in turn (8,19). A retrospective study believed that female patients were more likely to develop post-OLT AKI (13). Considering the protective effects of estrogen on cardiovascular and kidney diseases, it seemed unexpected that female patients were more susceptible to AKI, however, they thought that these women were in premenopausal or menopausal period, so the protective effect of estrogen on cardiovascular and kidney diseases was weakened or lost. Since there were only a small number of female patients in our study, we were unable to investigate this phenomenon. With regard to perioperative blood transfusion, we believe that it is associated with the development of AKI, for numerous studies had proven that perioperative blood transfusion would aggravate ischemia-reperfusion injury (IRI). On the one hand, IRI could directly lead to distal organ dysfunction directly and also result in IRI-related AKI through hemodynamic disorders (20). On the other hand, systemic inflammatory response syndrome (SIRS) can secondary to ischemia-reperfusion, which was also associated with the development of post-OLT AKI (8,19). But it is regrettable that we did not find any evidence to support this point in our study.
As expected, the severity of liver disease was associated with AKI after OLT (13,21), as assessed by the Child-Pugh score and MELD/MELD-Na score in our research. Due to the significant overlap existed between the etiologies of liver and kidney diseases (22), some factors that caused liver disease could cause simultaneous damage to kidney, such as autoimmune disease, various drugs and toxins, and hepatitis C virus, etc. (22). Due to the systemic and hemodynamic changes (low systemic vascular resistance, low effective circulation volume, etc.), combined with the sensitivity of the kidney to injury, simultaneous liver and kidney injury could also occur in patients with ESLD. Given the close relationship between the severity of liver disease and post-OLT AKI, some researchers believed that MELD/MELD-Na score had the ability in predicting the occurrence of postoperative AKI (9,23). The main use of the MELD score is regarded as liver graft allocation, for it can identify patients who are in greater immediate needing for liver transplantation and can also predict outcomes and survival of post-LT (24,25). But Romano et al. pointed out that the MELD score still needed to be improved in predicting the development of postoperative AKI (23). Other researches also believed that the MELD score was defective in predicting the development of AKI (7,12). For this reason, researchers such as Sujan et al. tried to take the MELD/MELD-Na score or the Child-Pugh score into the AKI prediction model, and achieved good performance (8,13,21). In our research, the novel AKI prediction score model was designed based on the variables of MELD-Na score and operative time, and also achieved good performance in predicting the development of AKI after OLT, compared with the MELD score and MELD-Na score.
Previous studies have confirmed that renal dysfunction is an independent risk factor for AKI in many patients, in which it enrolled OLT patients, acute-chronic liver failure patients, cirrhosis patients, etc. (26). We also noted that the proportion of patients with elevated SCr concentration before OLT was higher in the AKI group than the non-AKI group (6.90% vs. 2.86%), and the level of SCr in the AKI group was also higher than the non-AKI group (70.10 vs. 65.95 µmol/L). Regretfully, there were no differences between them. Other studies also did not confirm the association between preoperative renal dysfunction and AKI in LT patients (13,23).
Interestingly, we found that prolonged operative time was a predisposing factor for AKI in our study, which has not been found in previous literature. Prolonged operative time implies prolonged cold ischemia time and portal vein occlusion time, and increased the intraoperative hemodynamic disorder. Prolonged ischemia time aggravated hepatic IRI, and led the allografts to release pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor (TNF), etc., and then triggering an inflammatory response and subsequent cell damage, especially renal tubular damage, and further increasing the risk of development of AKI (27). Intraoperative portal vein occlusion and reperfusion after occlusion were resulted in hemodynamic disorder, such as intraoperative hypotension, multitude studies have proved that prolonged intraoperative hypotension will increase the incidence of postoperative AKI. Some studies believed that 70% of the patients with mean artery pressure (MAP) decreased 30% during the reperfusion period would experience severe postoperative renal dysfunction (27); even transient MAP <55 mmHg would also increase the incidence of AKI, and the longer the duration, the higher the risk of AKI (28). Moreover, because piggyback liver transplantation does not need to clamp the inferior vena cava during perioperative, so this method of surgery can reduce the hemodynamic disorder and also decrease the development of reperfusion syndrome, compared with other surgical methods (29). In our study, we put SOLT and MPOLT as one group, which both need surgeons to clamp the inferior vena cava during perioperative, while POLT as the other group, which did not require clamping the inferior vena cava during perioperative, and we found that the higher proportion of POLT methods were adopted in the AKI group than that in the non-AKI group.
Researches have shown that mild, even transient AKI following OLT also could lead to serious complications, including the prolonged the length of ICU stay or hospital stay and the increased patient mortality (30). In our research, the length of ICU stay in the AKI group was significantly longer than that in the non-AKI group; The long-term outcome of OLT patients with severe AKI was significantly different from that of OLT patients with mild or non-AKI, which was somewhat different from previous studies (31). In fact, there were some studies which pointed out that patients who developed stage-1AKI could be dichotomized into two groups: one group was those whose peak SCr concentration did not exceed 1.5 mg/dL (<133 µmol/L), the short-term mortality of these patients might be similar to those without AKI; another group was those whose peak SCr concentration exceeded 1.5 mg/dL (>133 µmol/L), the short-term mortality of these patients might be higher than those without AKI (32,33). This maybe can explain why the effect of stage-1 AKI on patients’ survival was limited in our study. We worried that the liver malignancy could affect patients’ cumulative survival, so we analyzed the cumulative survival time of patients with liver cancer and patients with cirrhosis separately, and we still found that the cumulative survival time of these patients with severe AKI was significantly lower than that of patients with mild AKI or non-AKI.
Shortcomings of this study: (I) This study was a single-center retrospective study, so there exists deviation. (II) Due to too little research data, the novel AKI prediction score model cannot be independently validated. (III) Absence of donor data, the influence of donor factors on postoperative AKI cannot be evaluated.
In conclusion, a high incidence of postoperative AKI existed in our center. The development of postoperative AKI posed a threat to the long-term survival of patients. In particular, severe AKI has a significant impact on the patients’ long-term survival, while mild AKI has a relatively limited impact on the patients’ prognosis. The AKI prediction score model developed in this study based on the operative time and the MELD-Na score had potentially prognostic value for the patients who would develop AKI following OLT. However, developing an accurate prediction score model for AKI still needs to be carried out through multicenter, prospective studies, which will provide better healthcare strategies and decision-making information for potential transplant recipients and their relatives.
Acknowledgments
Funding: This work was supported by grants from the National Nature Science Foundation of China (grant numbers 81570657, 81572985).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-842
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-842
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-842). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Tongji Medical College, Huazhong University of Science and Technology (No. 2021-S117) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017;13:241-57. [Crossref] [PubMed]
- de Haan JE, Hoorn EJ, de Geus HRH. Acute kidney injury after liver transplantation: Recent insights and future perspectives. Best Pract Res Clin Gastroenterol 2017;31:161-9. [Crossref] [PubMed]
- Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004;8:R204-12. [Crossref] [PubMed]
- Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007;11:R31. [Crossref] [PubMed]
- Kellum JA, Lameire N, Group KAGW. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. [Crossref] [PubMed]
- Kamath PS. A model to predict survival in patients with end-stage liver disease. Hepatology 2001;33:464-70. [Crossref] [PubMed]
- Min JY, Woo A, Chae MS, et al. Predictive Impact of Modified-Prognostic Nutritional Index for Acute Kidney Injury within 1-week after Living Donor Liver Transplantation. Int J Med Sci 2020;17:82-8. [Crossref] [PubMed]
- Sujan R, Cruz-Lemini M, Altamirano J, et al. A Validated Score Predicts Acute Kidney Injury and Survival in Patients With Alcoholic Hepatitis. Liver Transpl 2018;24:1655-64. [Crossref] [PubMed]
- Cheng Y, Wei GQ, Cai QC, et al. Prognostic Value of Model for End-Stage Liver Disease Incorporating with Serum Sodium Score for Development of Acute Kidney Injury after Liver Transplantation. Chinese Medical Journal 2018;131:1314-20. [Crossref] [PubMed]
- Okadome K, Baba Y, Yagi T, et al. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann Surg 2020;271:693-700. [Crossref] [PubMed]
- Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol 2015;62:968-74. [Crossref] [PubMed]
- Kalisvaart M, Schlegel A, Umbro I, et al. The AKI Prediction Score: a new prediction model for acute kidney injury after liver transplantation. HPB (Oxford) 2019;21:1707-17. [Crossref] [PubMed]
- Hilmi IA, Damian D, Al-Khafaji A, et al. Acute kidney injury following orthotopic liver transplantation: incidence, risk factors, and effects on patient and graft outcomes. Br J Anaesth 2015;114:919-26. [Crossref] [PubMed]
- Sanchez EQ, Gonwa TA, Levy MF, et al. Preoperative and perioperative predictors of the need for renal replacement therapy after orthotopic liver transplantation. Transplantation 2004;78:1048-54. [Crossref] [PubMed]
- Tan L, Yang Y, Ma G, et al. Early acute kidney injury after liver transplantation in patients with normal preoperative renal function. Clin Res Hepatol Gastroenterol 2019;43:475-82. [Crossref] [PubMed]
- Chow O, Barbul A. Immunonutrition: Role in Wound Healing and Tissue Regeneration. Adv Wound Care (New Rochelle) 2014;3:46-53. [Crossref] [PubMed]
- Jeon HG, Choi DK, Sung HH, et al. Preoperative Prognostic Nutritional Index is a Significant Predictor of Survival in Renal Cell Carcinoma Patients Undergoing Nephrectomy. Ann Surg Oncol 2016;23:321-7. [Crossref] [PubMed]
- van Hoek B, de Rooij BJ, Verspaget HW. Risk factors for infection after liver transplantation. Best Pract Res Clin Gastroenterol 2012;26:61-72. [Crossref] [PubMed]
- Michelena J, Altamirano J, Abraldes JG, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology 2015;62:762-72. [Crossref] [PubMed]
- Park SW, Kim M, Brown KM, et al. Paneth Cell-Derived Interleukin-17A Causes Multiorgan Dysfunction After Hepatic Ischemia and Reperfusion Injury. Hepatology 2011;53:1662-75. [Crossref] [PubMed]
- Gomes RM Junior, Cezar LC, Meneses GC, et al. Preoperative Risk Factors for Acute Kidney Injury after Liver Transplantation: Results from a Cross-Sectional Study in Northeast of Brazil. Arq Gastroenterol 2018;55:18-22. [Crossref] [PubMed]
- DellaVolpe J, Al-Khafaji A. Acute Kidney Injury Before and After Liver Transplant. J Intensive Care Med 2019;34:687-95. [Crossref] [PubMed]
- Romano TG, Schmidtbauer I, Silva FMD, et al. Role of MELD Score and Serum Creatinine as Prognostic Tools for the Development of Acute Kidney Injury after Liver Transplantation. PLoS One 2013;8:e64089 [Crossref] [PubMed]
- Luo X, Leanza J, Massie AB, et al. MELD as a metric for survival benefit of liver transplantation. Am J Transplant 2018;18:1231-7. [Crossref] [PubMed]
- Saab S, Wang V, Ibrahim AB, et al. MELD score predicts 1-year patient survival post-orthotopic liver transplantation. Liver Transpl 2003;9:473-6. [Crossref] [PubMed]
- Xue FS, Liu GP, Li RP. Acute kidney injury after orthotopic liver transplantation. Br J Anaesth 2016;116:144. [Crossref] [PubMed]
- Paugam-Burtz C, Kavafyan J, Merckx P, et al. Postreperfusion Syndrome During Liver Transplantation for Cirrhosis: Outcome and Predictors. Liver Transpl 2009;15:522-9. [Crossref] [PubMed]
- Walsh M, Devereaux PJ, Garg AX, et al. Relationship between Intraoperative Mean Arterial Pressure and Clinical Outcomes after Noncardiac Surgery: Toward an Empirical Definition of Hypotension. Anesthesiology 2013;119:507-15. [Crossref] [PubMed]
- Bukowicka B, Akar RA, Olszewska A, et al. The occurrence of postreperfusion syndrome in orthotopic liver transplantation and its significance in terms of complications and short-term survival. Ann Transplant 2011;16:26-30. [Crossref] [PubMed]
- Durand F, Francoz C, Asrani SK, et al. Acute Kidney Injury After Liver Transplantation. Transplantation 2018;102:1636-49. [Crossref] [PubMed]
- Kalisvaart M, de Haan JE, Hesselink DA, et al. The postreperfusion syndrome is associated with acute kidney injury following donation after brain death liver transplantation. Transpl Int 2017;30:660-9. [Crossref] [PubMed]
- Piano S, Rosi S, Maresio G, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol 2013;59:482-9. [Crossref] [PubMed]
- Fagundes C, Barreto R, Guevara M, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol 2013;59:474-81. [Crossref] [PubMed]


