
Analysis of the physiological and pathological factors of hospitalized patients taking warfarin and the correlation between drug interactions and warfarin efficacy
Introduction
Warfarin is currently the most widely used clinical anticoagulant drug. It is commonly used for both treatment and prevention in patients with mechanical heart valves, deep vein thrombosis, pulmonary embolism, stroke, and other diseases, and has been associated with a reduction in the recurrence of myocardial infarction and thromboembolic death (1). The treatment window of warfarin is very narrow and is affected by individual differences, low treatment index, susceptibility to a variety of factors, and susceptibility to adverse reactions. There are many factors that affect the efficacy of warfarin anticoagulation in clinical practice, including sex, age, pathology, combination therapy, diet, and genetic polymorphisms, among others. In this study, we collected the medical records of hospitalized patients who used warfarin in a third-grade class a hospital from 2018 to 2019, and analyzed the relationships between sex, age, pathology, and combination therapy with anticoagulation in patients who used warfarin. Our results can be used to promote the rational use of warfarin in clinical practice.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-830).
Methods
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved of exemptions by ethics board of Shenzhen Hospital of Southern Medical University. Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
Research participants
A total of 125 hospitalized patients taking warfarin in the Department of Cardiology of Shenzhen Hospital of Southern Medical University between 2018 and 2019 were selected.
Analysis of patient characteristics
A total of 125 patients taking warfarin were analyzed, and the following indexes were collected: (I) gender; (II) age; (III) total number of days of hospitalization; (IV) the total number of days taking warfarin during hospitalization; (V) whether international normalized ratio (INR) >3 occurred; (VI) INR values at discharge; (VII) dose of warfarin at discharge; (VIII) whether adverse effects of warfarin-related bleeding occurred.
The above indicators were evaluated in terms of relevance to hospitalization factors, such as infection, liver dysfunction, hyperthyroidism, hypothyroidism, and other complications. Drug combinations that may affect the efficacy of warfarin were also evaluated.
Statistical analysis
SPSS 17.0 statistical software (IBM Corp.) was used for statistical analysis in this study. The specific approach we’re using is that Continuous variables were analyzed using the t-test, while categorical variables were analyzed using the chi-square test. The Levene variance homogeneity test was used: if the variance was homogeneous, a 2-sample t-test was used; if the variance was missing, a t test was used. The chi-square test was implemented with an analysis of variance.
Results
The basic characteristics of patients taking warfarin
Of the 125 patients, 68 were male (54.4%) and 57 were female (45.6%). There were 13 cases (10.4%) aged 20–40 years old, 43 cases (34.4%) aged 40–60 years old, and 69 cases (55.2%) aged 60–80 years old. The minimum dose of warfarin was 0.75 mg and the maximum dose was 7.50 mg. The minimum INR value measured was 0.83 and the maximum INR value was 7.45. The medications used included aspirin, clopidogrel, spironolactone, amiodarone, amlodipine, trimetazidine, bisoprolol, isosorbide, atorvastatin, furosemide, digoxin, metoprolol, acarbose, levamlodipine, enoxaparin, moxifloxacin, levofloxacin, and other drugs. Complications included hypertension, diabetes, hyperlipidemia, hyperthyroidism, hypothyroidism, hepatitis, renal insufficiency, and nephritis, among others.
The impact of gender on efficacy indicators
The effect of gender on the efficacy indexes is shown in Table 1.

Full table
In Table 1, the total number of days of hospitalization, the number of days taking warfarin, the dose at discharge, and the INR values at discharge were analyzed using the t-test, and the number of cases with INR >3 and warfarin-related bleeding adverse drug reactions (ADRs) were analyzed using the chi-square test. The results showed that the ADR of warfarin-related bleeding in males was much higher than that in females, and the difference was statistically significant (P<0.05). Additionally, the dose of warfarin in male and female discharged patients was similar, and the difference was statistically significant (P<0.05). There were no significant differences in the number of days of hospitalization, the number of days taking warfarin, and the number cases with INR >3 (P>0.05).
The data suggest that the dose of warfarin between the different sexes is comparable, but men are more likely to have warfarin-related bleeding during hospitalization. However, gender had little effect on the rest of the relevant indicators.
The impact of age on efficacy indicators
The effect of different ages on the efficacy indexes is shown in Table 2.

Full table
In Table 2, the total number of days of hospitalization, the number of days taking warfarin, the dose at discharge, and the INR values at discharge were analyzed for variance, while the number of cases with INR >3 and warfarin-related bleeding ADR were analyzed using the chi-square test. The results showed that dose at discharge and warfarin-related bleeding ADR were similar among different aged patients (P<0.05), indicating that the 2 indicators were not age dependent. The total number of days of hospitalization, the number of days taking warfarin, the number of cases with INR >3, and the INR values at discharge all increased with increasing age, and the increase in age therefore decreased the efficacy of warfarin (P>0.05).
The influence of pathological factors on efficacy indices during hospitalization
Among the 125 patients, 23 (18.4%) were infected during hospitalization, 15 (12%) had abnormal liver function, 2 (1.6%) had hyperthyroidism, and 27 (21.6%) had hypothyroidism.
Table 3 indicates that the total days of hospitalization of infected patients, number of days taking warfarin, and the incidence of warfarin-related bleeding ADR were significantly higher than those in noninfected patients (P<0.05). The proportion of cases with INR >3 in infected patients was also much higher than that in noninfected patients, while the prevalence of INR was similar between the infected patients and noninfected patients, with neither of these factors showing a statistically significant difference (P>0.05).
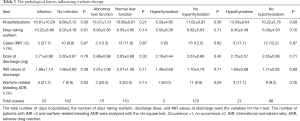
Full table
The number of days of hospitalization and the number of days taking warfarin in patients with abnormal liver function were higher than those of normal participants. The other indices between the groups were similar (P>0.05).
Table 3 also indicates that in patients with hyperthyroidism, the incidence of warfarin-related bleeding ADRs was significantly higher than that of normal patients, and the difference was statistically significant. The number of days of hospitalization in patients with hyperthyroidism and hypothyroidism were higher than that of normal participants. The INR values were similar at discharge, and the difference was not statistically significant (P>0.05).
In addition, the diseases that may increase the efficacy of warfarin include high blood pressure (44%), hepatitis (4.8%), and renal insufficiency (12.8%). Diseases that may impair warfarin efficacy include diabetes (23/125, 18.4%) and hyperlipidemia (38/125, 30.4%), among others.
The effect of drugs on efficacy indicators
Among the 125 patients, drugs that might have enhanced warfarin efficacy included aspirin (20%), clopidogrel (19.2%), amiodarone (28%), atorvastatin (37.6%), digoxin (18.4%), acarbose (7.2%), moxifloxacin (2.4%), levofloxacin (3.2%), antibiotics (24%), and enoxaparin (28.8%). Drugs that might have reduced the efficacy of warfarin included furosemide (18.4%) and spironolactone (31.2%).
Significant drugs such as furosemide, moxifloxacin, digoxin, and especially moxifloxacin increased the number of days of hospitalization and the number of days of warfarin administration. Of which the occurrence cases of INR >3 fewer drugs are: enoxaparin, acarbose; combined with the occurrence of INR >3 cases of more drugs are: moxifloxacin.
A low INR value at discharge was associated with the combination of enoxaparin and acarbose.
A high dose of warfarin at discharge was associated with the use of enoxaparin and acarbose, and a low dose of warfarin at discharge was associated with the use of furosemide.
Analysis of adverse reactions and related factors
Among the 125 hospitalized patients, 13 patients (10.4%) experienced the adverse effect of hemorrhagic bleeding on warfarin and the number of days of bleeding was significantly prolonged (P<0.05). There was also a significant extension of the total number of days of hospitalization (see Table 3 for details).
Discussion
Warfarin is a coumarin anticoagulant that acts by inhibiting the liver vitamin K epoxide reductase, so that inactive oxidized vitamin K cannot be converted into active reduced vitamin K, blocking the recycling of vitamin K. Interference of vitamin K-dependent coagulation factor II, VII, IX, X, protein C, and protein S synthesis hinders the coagulation factor amino-terminal glutamate residues of γ carboxylation, so that coagulation factors remain inactive at the precursor phase to achieve the purpose of anticoagulation (2).
Anticoagulant effect occurred after about 2–7 days of warfarin administration. The initial dose of warfarin was 10 mg, and 2 days later, according to the INR dose, there were significant differences in warfarin liver metabolic enzymes in Asian patients. Antithrombotic study of atrial fibrillation in patients of China indicates maintaining a dose of about 3 mg of warfarin (1). In order to lower the risk of applying excessive anticoagulant, a load dose is not usually recommended. If treatment is not urgent (such as in chronic atrial fibrillation) a load dose is not recommended in outpatient service, as monitoring can be inconvenient outside of hospital. It is recommended that the initial dose for patients of China be 1–3 mg (2.5 and 3 mg), as this can reach the target range in 2–4 weeks. For those patients who are at high-risk due older people, impaired liver function, congestive heart failure, or bleeding, the initial dose can be reduced appropriately. If rapid anticoagulation is needed, for example in acute venous thromboembolism (VTE) treatment, (low molecular weight heparin can be applied with warfarin for more than 5 days; i.e., warfarin on the first or second day of heparin administration, with discontinuation of heparin or low molecular weight heparin should the INR reach the target and or Oral administration time exceed 2 days of administration.
Dose adjustment should be performed carefully during treatment, as frequent dose adjustment can cause fluctuations in INR. If the continuous INR measures are outside the target range, dose adjustment can commence, but minor increases or decreases of INR value should not incite a sudden dose adjustment. If the warfarin dose adjustment is small, it can be used to calculate the weekly dose, which is more accurate than adjusting the daily dose. If the INR exceeds the target range, we should increase or decrease the original dose in 5–20 mg. Should the INR [2–3] remain stable or fluctuate occasionally and not exceed 0.5 INR target range, no adjustment is required. We should to adjust the dose as appropriate, and based on review of the INR every several days or every 1 or 2 weeks.
For the patients with vascular calcification, the liver metabolism rate and coagulation factor synthesis may be decreased by warfarin sodium, which can easily lead to the enhancement of the anticoagulant effect of warfarin sodium. Accordingly, the dose should be reduced to a level appropriate to the INR value.
Animal liver, egg yolk, seaweed, green vegetables, green tea, low sugar, high protein foods, soy milk, and other soy products can reduce the anticoagulant effect of warfarin. Additionally, spinach, cabbage, yogurt, lettuce, cod liver oil, carrots, and cilantro may affect the anticoagulation effect of warfarin. These foods can change the pharmacokinetic processes related to warfarin absorption, metabolism, and excretion (3-5). Angelica root, safflower, fennel, ferulic, celery, licorice, rue, pineapple, cloves, and onions etc. can enhance the anticoagulant effect of warfarin, and pose an additional risk of bleeding.
Excessive drinking can increase the activity of warfarin metabolic enzymes, accelerating metabolism and lowering efficacy. Hence, there is a need to increase the dose of warfarin to achieve the purpose of anticoagulation. Habits such as smoking and drinking alcohol will also affect warfarin’s anticoagulant effect, and patients taking warfarin should thus cease smoking and limit alcohol intake.
In this study, our analysis of physiological factors and warfarin anticoagulant effects found that the number of days of hospitalization, the number of days taking warfarin, and warfarin discharge dose had little effect, but men who took warfarin did show significantly higher warfarin-related bleeding than did women, which is cause for clinical concern. Previous literature has shown that with age, warfarin dose has a tendency to decrease (1,2). After 6 years of age, the warfarin dose should be reduced by 8% at each increase of 10 years. This age effect may be related to decreased liver metabolism of warfarin along with the reduced capability of the liver to synthesize coagulation factors.
Our study also found that patients with infection, liver dysfunction, and hypothyroidism had significantly longer hospitalization and warfarin treatment, which is consistent with the literature (6-8). Vitamin K–dependent coagulation factors are mainly synthesized in the liver, and abnormal liver function may affect the synthesis of vitamin K. Thus, abnormal liver function may affect the anticoagulant effect of warfarin. In the group of patients with hyperthyroidism and normal hospital stay days, the number of warfarin-administration days was not increased. This is inconsistent with literature, which may be due to the number of cases selected in this study or other factors, warranting further research. In our study, hepatitis, infection, fever, and hyperthyroidism enhanced the anticoagulant effect of warfarin, while diabetes, hypothyroidism, and hyperlipidemia reduced the anticoagulant effect of warfarin. This indicates that complications have an effect on the anticoagulation of warfarin, which is consistent with the reported literature (9).
In our analysis of the association between clinical characteristics and drugs used, for patients who used acarbose alone, only 1 patient had an INR value greater than 3 and had higher doses of medication at discharge, along with a lower INR value at discharge, suggesting that the combination of warfarin and acarbose can reduce the efficacy of warfarin. Of the patients who received heparin therapy, only 2 had an INR greater than 3, a relatively high dose at discharge, and a relatively low INR value at discharge (10).
This study found that warfarin-related bleeding ADRs and anticoagulant effects have a significant association. Bleeding not only affected the total number of days hospitalized, but also extended the number of days of warfarin administration. However, in terms of patients’ pathological states, INR values combined with drugs used were not associated with disease and may be useful as a reference for bleeding risk.
The US Food and Drug Administration (FDA) amended the warfarin labeling in August 2007 to suggest that patients should be genotyped before using warfarin. Warfarin gene polymorphism studies have shown that individual differences in the cytochrome P450 2C9 (CYP2C9) gene and the vitamin K epoxide reductase complex 1 (VKORC1) may be relevant to warfarin dosing. Warfarin metabolism occurs mainly through liver enzymes, and enzyme gene polymorphisms can lead to individuals having different warfarin metabolism, thus affecting the dose–effect relationship.
Warfarin, a long-acting, oral anticoagulant drug, is commonly used in patients with heart valve replacements, nonvalvular atrial fibrillation, and thrombotic diseases. However, its anticoagulant effects depend on several factors. In summary, this study has investigated the demographic factors (sex, age), pathological factors, combined medications, and comorbidities of patients taking warfarin, and has discussed certain factors that affect the efficacy of warfarin, including genetic polymorphisms, Chinese herbal medicines, diet, and living habits. In addition to bleeding, warfarin may involve other more rare adverse reactions, including acute thrombosis, including skin necrosis and limb gangrene. These usually occurs on days 3–8 of medication and may be associated with protein c and protein S deficiency. Warfarin also interferes with bone protein synthesis, leading to osteoporosis and vascular calcification. Therefore, it is hoped that physicians and pharmacists pay close attention to these factors in the application of warfarin. Avoiding certain factors that have been described in this study will enhance the anticoagulant effect of warfarin and reduce the incidence of warfarin-related bleeding complications.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-830
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-830
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-830). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved of exemptions by ethics board of Shenzhen Hospital of Southern Medical University. Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zheng C. 95 cases of patients with warfarin anticoagulation analysis. Chinese Pharmacy 2009;20:2527-9.
- Zheng B, Liu J. Anti-coagulation effect of warfarin and its influencing factors. Anhui Medicine and Pharmaceutical Journal 2013;17:1975-7.
- Zhong W, Zhou S, Peng J. Comprehensive analysis of factors affecting the efficacy of warfarin. Chinese Journal of Modern Drug Application 2010;4:29-31.
- Jin H. Study on influencing factors of anticoagulant effect of warfarin. Medical Recapitulate 2011;17:449-51.
- Zhang B, Li Y. Interaction of warfarin and its safety application. Adverse Drug Reactions Journal 2007;9:112.
- He K. Analysis of influencing factors of hospital stay and medical expenses. Hospital Administration Journal of Chinese People’s Liberation Army 2009;16:1131-3.
- Xiang Q, Su H, Mu G, et al. Retrospective analysis of the combined use of drugs and pathological factors on the efficacy of warfarin anticoagulant patients. The Chinese Journal of Clinical Pharmacology 2014;30:822-4.
- Bird J, Carmona C. Probable interaction between warfarin and torsemide. Ann Pharmacother 2008;42:1893-8. [Crossref] [PubMed]
- Hu Y. A case of deep vein thrombosis in patients with multiple recurrence of anticoagulant therapy in pharmacological care. Chinese Pharmacy 2010;21:4029-31.
- Gage BF, Eby C, Milligan PE, et al. Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thromb Haemost 2004;91:87-94. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


