
Preoperative pectoralis muscle radiodensity as a risk factor for postoperative complications after thoracoscopic lobectomy for non-small cell lung cancer
Introduction
Lung cancer is one of the most common cancers worldwide and a leading cause of cancer-related mortality (1,2). While complete surgical resection is a potentially curative treatment for resectable non-small cell lung cancer (NSCLC), lobectomy by video-assisted thoracoscopic surgery (VATS), also known as thoracoscopic lobectomy, is an effective minimally invasive surgical approach for NSCLC, although the incidence of complications following the latter is 10.3–31.0% (3). Postoperative morbidity is a major clinical challenge in the surgical treatment of cancer, and the decision to perform surgery is made after weighing the benefits versus the potential complications. Thus, preoperative clinical evaluation is critical for the optimal management of cancer patients undergoing surgery.
Sarcopenia is characterized by low skeletal muscle quantity or quality (4), and has been the focus of increasing attention in surgical oncology. Accurately measuring muscle mass and quality is technically difficult, and computed tomography (CT), which is used to image tumors, has been applied to the evaluation of muscle composition and distribution (4). Data on the association between preoperative CT-based measures of muscle mass and morbidity following surgery for NSCLC are inconsistent. The measurements of the pectoralis muscle are readily obtained from CT scans, which are performed as part of standard preoperative assessments and do not require specific research protocols. However, CT-based measurements of pectoralis muscle mass have seldom been used in studies investigating the relationship between muscle quantity and post-treatment outcomes in patients with NSCLC. Furthermore, the contribution of preoperative skeletal muscle radiodensity (quality) to postoperative complication risk in NSCLC patients has never been reported. This study thus investigated the relationship between preoperative pectoralis muscle radiodensity and postoperative complication risk in patients with NSCLC treated by complete resection with the VATS approach. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-902).
Methods
Patients
We retrospectively reviewed the data of all consecutive patients who underwent thoracoscopic lobectomy for NSCLC at the Affiliated Cancer Hospital of Guangzhou Medical University between March 2016 and October 2019. Patients who had preoperative treatments, a history of malignancy in other organs, primary tumor >7 cm, regional lymph node status ≥ N2 for the tumor, incomplete VATS, sleeve lobectomy, incomplete resection, and no CT image of pectoralis muscles before surgery were excluded. Clinical staging was based on chest CT scans with contrast, upper abdomen CT or ultrasound, brain CT or magnetic resonance imaging, radionuclide bone scan, and/or positron emission tomography with fluorine-18 fluorodeoxyglucose. Mediastinal and hilar lymph node status was defined as positive if the chest CT showed that the short axis of any node was >1 cm. Mediastinoscopy and endobronchial ultrasound-guided biopsy were not routinely performed. Histopathologic diagnosis of the tumor was based on the World Health Organization (WHO) histologic classification of lung tumors (5) and staging was performed according to the TNM Classification of Malignant Tumors (eighth edition) of the Union for International Cancer Control (6). The ethics committee of the Affiliated Cancer Hospital of Guangzhou Medical University approved this study (ethics approval number 2020-2). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Data collection
The following data on patients were obtained from medical records (maximum of 30 days before surgery): age; sex; smoking history; body mass index (BMI); Charlson Comorbidity Index score; Eastern Cooperative Oncology Group performance status (PS); preoperative spirometry test values, including percent forced vital capacity (%FVC), percent forced expiratory volume in 1 s (%FEV1.0), and forced expiratory volume in 1 s as a percentage of forced vital capacity (FEV1.0%); preoperative blood examination values, including hemoglobin, neutrophil, lymphocyte, platelet, triglyceride, cholesterol, albumin, creatinine, and C-reactive protein (CRP); tumor site; and operative time. We also recorded the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio. Postoperative complications were graded according to the Clavien-Dindo classification (7) with grade ≥ III within 30 days of the operation considered as a major complication.
CT scan analysis and study groups
Preoperative CT scans were obtained within 30 days before surgery, and the investigator who performed the measurements was blinded to the postoperative outcome. Quantitative and qualitative measurements of the pectoralis muscle were performed in the picture archive and communication system on a single axial slice of the precontrast phase CT scan above the aortic arch by manual tracing, and the slice used for evaluation was selected by scrolling through images toward the apex of the lungs and identifying the first axial image above the arch (Figure 1) (8,9).
The total pectoralis muscle area, presented as the aggregate of the right and left pectoralis major and minor muscles and pectoralis muscle mass index (PMI), was calculated as the total pectoralis muscle area/height2 (cm2/m2). The pectoralis muscle radiodensity (PMD) was assessed as mean radiodensity [Hounsfield unit (HU)] and was calculated as follows: PMD = [(right pectoralis major area × right pectoralis major HU) + (right pectoralis minor area × right pectoralis minor HU) + (left pectoralis major area × left pectoralis major HU) + (left pectoralis minor area × left pectoralis minor HU)]/total pectoralis muscle area (HU) (10).
As measures such as muscle mass and quality depend on stature and vary within patient populations, the cutoff values for PMI and PMD in our cohort were set at the lowest tertile, and as body composition is highly influenced by sex, separate cutoff values were set for men and women.
Statistical analysis
Data were analyzed using SPSS v20 for Windows (IBM, Armonk, NY, USA). Descriptive statistics were computed for patient characteristics and are expressed as median [interquartile range (IQR)] or frequencies and relative frequencies. Differences in patient characteristics were analyzed with the Mann-Whitney U test, Pearson χ2 test, or Fisher’s exact test as appropriate, and Spearman’s correlation coefficient (rs) was used to evaluate correlations between variables. Logistic regression was performed for dichotomous outcomes of major postoperative complications, and any variable with a significant association in the univariate analysis was included in the multivariate analysis. Univariate and multivariate odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Missing data were excluded. All P values were 2-sided, and those <0.05 were considered statistically significant.
Results
Study population and general characteristics
During the study period, 163 cases comprising 97 males and 66 females met the inclusion criteria and were selected for analysis. The median age was 60 years (range, 22–86 years). The median BMI was 23.4 kg/m2 (range, 14.9–34.1 kg/m2), with 13 (8.0%) patients having a BMI <18.5 kg/m2, 103 (63.2%) a BMI of 18.5–24.9 kg/m2, 41 (25.2%) a BMI of 25–29.9 kg/m2, and 6 (3.7%) a BMI >30 kg/m2. Data for the preoperative spirometry test and complete blood counts were available for 159 (97.5%) and 162 (99.4%) cases, respectively, and data on albumin and CRP were not available for 1 (0.6%) case. The demographic and clinical characteristics of the study population are shown in Table 1.
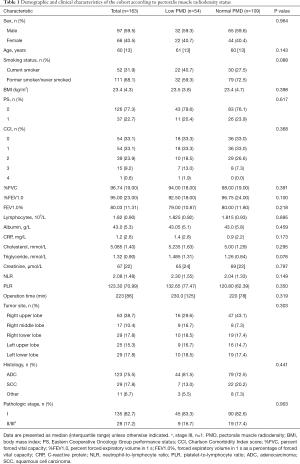
Full table
Pectoralis muscle mass and radiodensity
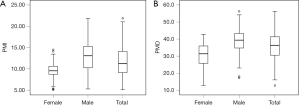
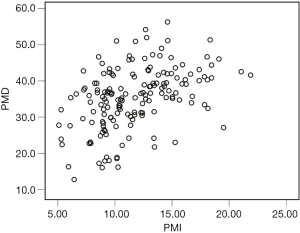
The median PMI and PMD were 11.23 cm2/m2 (IQR, 4.99 cm2/m2) and36.3 HU (IQR, 11.1 HU), respectively (Figure 2), and PMI was positively correlated with PMD (rs=0.459, P<0.001; Figure 3). The median PMI was significantly higher in male patients (13.08 cm2/m2; IQR, 5.05 cm2/m2) than in female patients (9.55 cm2/m2; IQR, 2.10 cm2/m2) (P<0.001; Figure 2A), and PMI <11.56 cm2/m2 in male patients and 8.98 cm2/m2 in female patients was defined as low PMI. The median PMD was significantly higher in male patients (39.3 HU; IQR, 8.6 HU) than in female patients (31.4 HU; IQR, 10.5 HU) (P<0.001; Figure 2B), and PMD <36.6 HU in male patients and 21.8 HU in female patients was defined as low PMD.
Pectoralis muscle radiodensity and demographic and clinical characteristics
The association between PMD and demographic and clinical characteristics is shown in Table 1. No significant differences were observed between patients with low PMD vs. normal PMD.
Postoperative complications
A total of 23 (14.1%) patients experienced major postoperative complications, 18 (11.0%) of whom were grade III (data on albumin and CRP were missing in 1case), 3 (1.8%) were grade IV and were admitted to the intensive care unit (ICU), and 2 (1.2%) were grade V and died within 30 days of the operation because of postoperative complications. Low PMI was significantly associated with more major postoperative complications (P=0.036) and grade III complications (P=0.032), whereas low PMD was associated with more major postoperative complications (P=0.036) and grade IV complications (P=0.035) (Table 2).

Full table
Risk factors for major postoperative complications
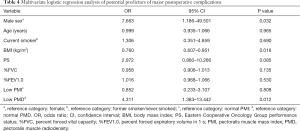
Univariate logistic regression analysis indicated that male sex (OR =8.842, 95% CI: 1.997–39.156; P=0.004), older age (OR =1.058, 95% CI: 1.004–1.115;P=0.035), smoking (OR =5.220, 95% CI: 2.046–13.318; P=0.001), lower BMI (1 kg/m2 increase, OR =0.816, 95% CI: 0.702–0.950; P=0.009), PS (OR =3.219, 95% CI: 1.276–8.120; P=0.013), lower %FVC (1% increase; OR =0.958, 95% CI: 0.929–0.987; P=0.005), lower %FEV1.0 (1% increase; OR =0.970, 95% CI: 0.946–0.995; P=0.017), low PMI (OR =2.545, 95% CI: 1.041–6.226; P=0.041), and low PMD (OR =2.545, 95% CI: 1.041–6.226; P=0.041) before surgery were significantly associated with an increased risk of major postoperative complications (Table 3). Multivariate analysis confirmed that male sex (OR =7.663, 95% CI: 1.186–49.501; P=0.032), lower BMI (1 kg/m2 increase, OR =0.760, 95% CI: 0.607–0.951; P=0.016), and low PMD (OR =4.311, 95% CI: 1.383–13.442; P=0.012) before surgery were independent risk factors for major postoperative complications (Table 4).
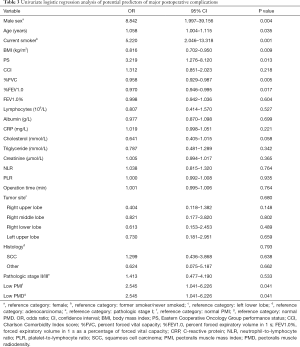
Full table
Full table
Discussion
This is the first study to investigate the relationship between skeletal muscle radiodensity and postoperative complication risk in patients with NSCLC who have undergone thoracoscopic lobectomy. In our disease- and treatment-specific cohort, the multivariate analysis revealed that low pectoralis muscle radiodensity measured on the first axial CT image above the aortic arch and expressed as PMD was a significant independent risk factor for major complications after first-line VATS lobectomy, whereas low pectoralis muscle mass expressed as PMI was not.
Previous studies have reported conflicting findings on the association between preoperative muscle mass and complications after surgery in NSCLC patients (8,9,11-17), and there may be several reasons for this discrepancy. First, a variety of methods have been used to measure the quantity of muscle across studies. For example, it was suggested that the psoas muscle area, which has been used in some studies (9,12,14,15), is not representative of total skeletal muscle area (18,19). Additionally, although body muscle composition is highly influenced by sex, Miller et al. did not perform a sex-specific analysis in their study (11). Second, the definition of postoperative complications was not consistent across studies. These differences likely resulted in significant heterogeneity in the findings, including the association between skeletal muscle mass and postoperative complications. Finally, most of the studies did not perform covariate-adjusted analysis (9,12-14,17).
We focused on the pectoralis muscle as data are readily obtained from CT scans which are performed as part of standard preoperative assessments and do not require specific research protocols. Until recently, CT-based measurements of pectoralis muscle mass were seldom used in studies investigating the relationship between muscle quantity and posttreatment outcomes in patients with NSCLC. One study reported that a smaller pectoralis muscle area was associated with worse overall survival (8); in another cohort of 283 of patients with small cell lung cancer and NSCLC and 16 with lung metastasis who underwent lobectomy (of which 278 were performed by VATS), the height-adjusted cross-sectional area of the pectoralis muscle was not associated with overall complication rates, rate of pneumonia, readmission, or length of stay in the ICU (11). In the present study, low PMI was a risk factor for major postoperative complications in the univariate analysis but not in the multivariate analysis.
Muscle quality is a relatively new term that encompasses muscle architecture and composition. Although there is no standard method for its clinical assessment, the value of muscle quality as determined from CT images for predicting postoperative outcomes has received increasing attention in surgical oncology. Lower muscle density was found to be associated with a higher risk of postoperative complications in patients with gastric, colorectal, periampullary, and pancreatic cancers (10,20-31), and low skeletal muscle radiodensity was linked to shorter survival in advanced NSCLC patients receiving first-line palliative chemotherapy (32). Our study is a first step in determining whether preoperative skeletal muscle radiodensity can be used to stratify patients with NSCLC undergoing first-line VATS lobectomy according to postoperative complication risk.
In the present study, PMI was positively correlated with PMD. The skeletal muscle mass index was recently shown to be related to skeletal muscle density in CT images at the level of the third lumbar vertebra in patients with colorectal cancer (20). However, other studies reported that skeletal muscle radiodensity, but not muscle mass, predicted complications after surgery for colorectal cancer (21-24) and periampullary cancer (30). We also found that low PMD, but not PMI, was an independent risk factor for major complications following VATS lobectomy. Low skeletal muscle radiodensity reflects a decrease in muscle quality due to increased intramuscular lipid deposition (33,34), and a higher muscle lipid content has been linked to loss of muscle strength (35). Increased muscle mass is not necessarily associated with enhanced function (36), and skeletal muscle strength was shown to be superior to skeletal muscle mass for predicting adverse outcomes (4). This could explain why skeletal muscle quality and not muscle mass was an independent risk factor for major postoperative complications in our analysis.
As a hallmark of cachexia, systemic inflammatory response is a well-established prognostic factor in cancer (37). Low skeletal muscle radiodensity was related to low albumin and elevated CRP and NLR in patients with colorectal cancer (38) and pancreatic-biliary cancer (39,40). We did not find any association between pectoralis muscle radiodensity and systemic inflammation, but this finding should be interpreted with caution, as we did not have any data pertaining to obstructive pneumonitis.
In a study of patients who underwent lobectomy for primary lung cancer, overall postoperative complication and mortality rates were significantly higher in underweight patients and lower in preobese and obese patients than in those with normal BMI (41). A similar relationship was recently reported in patients undergoing lobectomy for NSCLC (42). In the present study, in which 8.0% of patients had a BMI <18.5 kg/m2 and 3.7% had a BMI ≥30 kg/m2, lower BMI was identified as an independent risk factor for major complications following VATS lobectomy.
In a study of 238 patients who underwent lobectomy for NSCLC in which 14 (5.1%) patients underwent pneumonectomy, 3 (1.1%) underwent segmentectomy, 151 (55.5%) underwent minimally invasive surgery using the VATS approach, and 18 (6.6%) underwent neoadjuvant chemotherapy, male sex was associated with a higher rate of overall complications (16). Similarly, our study found male sex to be an independent risk factor for major complications after first-line VATS lobectomy for NSCLC.
In addition to male sex, lower BMI and low PMD before first-line VATS lobectomy were revealed to be independent risk factors for major postoperative complications in our study. BMI and sarcopenia are potentially modifiable risk factors; thus, to reduce postoperative complications, patients with NSCLC should receive preoperative assessment of BMI and diagnosis of sarcopenia before first-line thoracoscopic lobectomy and should then undergo preoperative exercise and nutritional support (43,44).
This study had some limitations. First, because of its retrospective nature, other direct measures of sarcopenia were not available. Second, the Clavien-Dindo classification is becoming one of the more commonly used methods to capture surgical complication data, but limits its measurement to the highest-grade complication and does not capture the interplay between multiple complications. It was recently reported that CT-derived body muscle composition measures may be linked to specific complications, but this was not examined in our study. Finally, because it was a single-center study, there was an inherent bias in the results attributable to factors such as surgical technique and postoperative management. Future larger, prospective study should conducted for verification.
In conclusion, inpatients undergoing first-line VATS lobectomy for NSCLC, low muscle quality but not low muscle mass predicted major postoperative complications. Thus, preoperative CT-derived skeletal muscle measures may be used to stratify patients with NSCLC into risk categories to facilitate clinical decision-making.
Acknowledgments
Funding: The present study was supported by Medical and Health Technology Projects of Guangzhou (20151A011086) and Science and Technology Foundation of Guangdong Province of China (2014A020212349).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-902
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-902
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-902). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The ethics committee of the Affiliated Cancer Hospital of Guangzhou Medical University approved this study (ethics approval number 2020-2). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: A meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 2013;16:244-9. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. Erratum in Age Ageing 2019;48:601. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. Introduction to the 2015 World Health Organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Kinsey CM, San José Estépar R, Van Der Velden J, et al. Lower pectoralis muscle area is associated with a worse overall survival in non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev 2017;26:38-43. [Crossref] [PubMed]
- Nakamura R, Inage Y, Tobita R, et al. Sarcopenia in resected NSCLC: Effect on postoperative outcomes. J Thorac Oncol 2018;13:895-903. [Crossref] [PubMed]
- Margadant CC, Bruns ER, Sloothaak DA, et al. Lower muscle density is associated with major postoperative complications in older patients after surgery for colorectal cancer. Eur J Surg Oncol 2016;42:1654-9. [Crossref] [PubMed]
- Miller JA, Harris K, Roche C, et al. Sarcopenia is a predictor of outcomes after lobectomy. J Thorac Dis 2018;10:432-40. [Crossref] [PubMed]
- Kawaguchi Y, Hanaoka J, Ohshio Y, et al. Sarcopenia predicts poor postoperative outcome in elderly patients with lung cancer. Gen Thorac Cardiovasc Surg 2019;67:949-54. [Crossref] [PubMed]
- Tsukioka T, Nishiyama N, Izumi N, et al. Sarcopenia is a novel poor prognostic factor in male patients with pathological Stage I non-small cell lung cancer. Jpn J Clin Oncol 2017;47:363-8. [Crossref] [PubMed]
- Hervochon R, Bobbio A, Guinet C, et al. Body mass index and total psoas area affect outcomes in patients undergoing pneumonectomy for cancer. Ann Thorac Surg 2017;103:287-95. [Crossref] [PubMed]
- Nakada T, Noda Y, Kato D, et al. Risk factors and cancer recurrence associated with postoperative complications after thoracoscopic lobectomy for clinical stage I non-small cell lung cancer. Thorac Cancer 2019;10:1945-52. [Crossref] [PubMed]
- Kim EY, Lee HY, Kim KW, et al. Preoperative computed tomography-determined sarcopenia and postoperative outcome after surgery for non-small cell lung cancer. Scand J Surg 2018;107:244-51. [Crossref] [PubMed]
- Suzuki Y, Okamoto T, Fujishita T, et al. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer 2016;101:92-7. [Crossref] [PubMed]
- Baracos VE. Psoas as a sentinel muscle for sarcopenia: A flawed premise. J Cachexia Sarcopenia Muscle 2017;8:527-8. [Crossref] [PubMed]
- Rutten IJG, Ubachs J, Kruitwagen RFPM, et al. Psoas muscle area is not representative of total skeletal muscle area in the assessment of sarcopenia in ovarian cancer. J Cachexia Sarcopenia Muscle 2017;8:630-8. [Crossref] [PubMed]
- Dolan RD, Almasaudi AS, Dieu LB, et al. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J Cachexia Sarcopenia Muscle 2019;10:111-22. [Crossref] [PubMed]
- Looijaard SMLM, Meskers CGM, Slee-Valentijn MS, et al. Computed tomography-based body composition is not consistently associated with outcome in older patients with colorectal cancer. Oncologist 2020;25:e492-e501. [Crossref] [PubMed]
- Berkel AEM, Klaase JM, De Graaff F, et al. Patient’s skeletal muscle radiation attenuation and sarcopenic obesity are associated with postoperative morbidity after neoadjuvant chemoradiation and resection for rectal cancer. Dig Surg 2019;36:376-83. [Crossref] [PubMed]
- Souwer ET, Moos SI, Van Rooden CJ, et al. Physical performance has a strong association with poor surgical outcome in older patients with colorectal cancer. Eur J Surg Oncol 2020;46:462-9. [Crossref] [PubMed]
- Boer BC, De Graaff F, Brusse-Keizer M, et al. Skeletal muscle mass and quality as risk factors for postoperative outcome after open colon resection for cancer. Int J Colorectal Dis 2016;31:1117-24. [Crossref] [PubMed]
- Herrod PJJ, Boyd-Carson H, Doleman B, et al. Quick and simple; psoas density measurement is an independent predictor of anastomotic leak and other complications after colorectal resection. Tech Coloproctol 2019;23:129-34. [Crossref] [PubMed]
- van Vugt JLA, Coebergh van den Braak RRJ, Lalmahomed ZS, et al. Impact of low skeletal muscle mass and density on short and long-term outcome after resection of stage I-III colorectal cancer. Eur J Surg Oncol 2018;44:1354-60. [Crossref] [PubMed]
- Zhuang CL, Shen X, Huang YY, et al. Myosteatosis predicts prognosis after radical gastrectomy for gastric cancer: A propensity score-matched analysis from a large-scale cohort. Surgery 2019;166:297-304. [Crossref] [PubMed]
- Lin J, Zhang W, Chen W, et al. Mass, density, and strength are necessary to diagnose sarcopenia in patients with gastric cancer. J Surg Res 2019;241:141-8. [Crossref] [PubMed]
- van der Kroft G, van Dijk DPJ, Rensen SS, et al. Low thoracic muscle radiation attenuation is associated with postoperative pneumonia following partial hepatectomy for colorectal metastasis. HPB (Oxford) 2020;22:1011-9. [Crossref] [PubMed]
- Van Rijssen LB, van Huijgevoort NC, Coelen RJ, et al. Skeletal muscle quality is associated with worse survival after pancreatoduodenectomy for periampullary, nonpancreatic cancer. Ann Surg Oncol 2017;24:272-80. [Crossref] [PubMed]
- Namm JP, Thakrar KH, Wang CH, et al. A semi-automated assessment of sarcopenia using psoas area and density predicts outcomes after pancreaticoduodenectomy for pancreatic malignancy. J Gastrointest Oncol 2017;8:936-44. [Crossref] [PubMed]
- Sjøblom B, Grønberg BH, Wentzel-Larsen T, et al. Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin Nutr 2016;35:1386-93. [Crossref] [PubMed]
- Goodpaster BH, Kelley DE, Thaete FL, et al. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985) 2000;89:104-10. [Crossref] [PubMed]
- Stephens NA, Skipworth RJ, Macdonald AJ, et al. Intramyocellular lipid droplets increase with progression of cachexia in cancer patients. J Cachexia Sarcopenia Muscle 2011;2:111-7. [Crossref] [PubMed]
- Marcus RL, Addison O, Kidde JP, et al. Skeletal muscle fat infiltration: Impact of age, inactivity, and exercise. J Nutr Health Aging 2010;14:362-6. [Crossref] [PubMed]
- Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): Results from two randomised, double-blind, phase 3 trials. Lancet Oncol 2016;17:519-31. [Crossref] [PubMed]
- Clarke SJ, Chua W, Moore M, et al. Use of inflammatory markers to guide cancer treatment. Clin Pharmacol Ther 2011;90:475-8. [Crossref] [PubMed]
- Okugawa Y, Toiyama Y, Yamamoto A, et al. Close relationship between immunological/inflammatory markers and myopenia and myosteatosis in patients with colorectal cancer: A propensity score matching analysis. JPEN J Parenter Enteral Nutr 2019;43:508-15. [Crossref] [PubMed]
- Rollins KE, Tewari N, Ackner A, et al. The impact of sarcopenia and myosteatosis on outcomes of unresectable pancreatic cancer or distal cholangiocarcinoma. Clin Nutr 2016;35:1103-9. [Crossref] [PubMed]
- van Dijk DP, Bakens MJ, Coolsen MM, et al. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J Cachexia Sarcopenia Muscle 2017;8:317-26. [Crossref] [PubMed]
- Thomas PA, Berbis J, Falcoz PE, et al. National perioperative outcomes of pulmonary lobectomy for cancer: The influence of nutritional status. Eur J Cardiothorac Surg 2014;45:652-9. [Crossref] [PubMed]
- Wang C, Guo M, Zhang N, et al. Association of body mass index and outcomes following lobectomy for non-small-cell lung cancer. World J Surg Oncol 2018;16:90. [Crossref] [PubMed]
- Friedman J, Lussiez A, Sullivan J, et al. Implications of sarcopenia in major surgery. Nutr Clin Pract 2015;30:175-9. [Crossref] [PubMed]
- Yamamoto K, Nagatsuma Y, Fukuda Y, et al. Effectiveness of a preoperative exercise and nutritional support program for elderly sarcopenic patients with gastric cancer. Gastric Cancer 2017;20:913-8. [Crossref] [PubMed]
(English Language Editor: B. Draper)



