
Relationship between the changes in thromboxane B2, 6-keto-prostaglandin Fla, and blood glucose levels and progressive ischemic stroke
Introduction
The occurrence of ischemic stroke could cause widespread neuronal death due to insufficient blood perfusion, lack of oxygen and glucose for brain tissue metabolism, insufficient energy production, and local accumulation of some toxic metabolites, such as excitotoxic products, acid metabolites, oxidative stress products, and inflammatory mediators (1). In recent years, the incidence of ischemic stroke has gradually increased, affected by factors such as poor lifestyle and eating habits. The disability and fatality rate of ischemic stroke is high, which seriously threatens the life of patients, and greatly increases the economic burden on their family and society (2). Progressive ischemic stroke has a relatively high incidence in ischemic cerebrovascular disease, which has gradually attracted the attention of many scholars and become a hot and challenging field in stroke research (3).
There are many studies on the relationship between the change of blood glucose (BG) level and progressive ischemic stroke (4,5). However, the correlation in thromboxane B2 (TXB2), 6-keto-prostaglandin Fla (6-k-PGFla) levels, and progressive ischemic stroke in China and abroad has not been clearly reported yet. In this study, we focused on the relationship between TXB2, 6-k-PGFla, and BG levels and progressive ischemic stroke, and observed the predictive value of the 3 indicators in the occurrence of progressive ischemic stroke, in order to provide a theoretical basis for the improvement of clinical stroke diagnosis rate. We present the following article in accordance with the STARD reporting checklist (available at http://dx.doi.org/10.21037/apm-21-774).
Methods
General information
Clinical data of patients with progressive ischemic stroke admitted to our hospital from December 2016 to December 2018 were selected for collection and sorting. The inclusion criteria were as follows: (I) patients who were diagnosed as progressive ischemic stroke by computed tomography (CT) and medical resonance imaging (MRI) examinations (6) for the first time; (II) patients aged under 80 years old; (III) patients with no other malignant tumors; (IV) patients who had not used platelet inhibitors, anticoagulants, and drugs affecting the levels of TXB2, 6-k-PGFla, and BG within 1 month before the study; (V) patients with balanced diet, reasonable supplement high carbohydrate, high fat, high protein; (VI) patients do reasonable exercise regularly. The exclusion criteria were as follows: (I) patients with incomplete clinical data; (II) patients with mental disorders, language disorders, or Alzheimer’s disease; (III) patients with inherited or acquired hemorrhage constitution; (IV) patients with diabetes mellitus.
After being selected according to the inclusion and exclusion criteria, a total of 106 patients who met the criteria were finally included in this study as the observation group. In addition, 110 patients who received physical examination in our hospital during the same period were recruited as the control group. In the observation group, there were 63 males and 43 females; aged 61–77 years old, with an average age of 58.32±2.49 years; weighed 45–66 kg, with an average weight of 52.75±2.28 kg. In the control group, there were 62 males and 48 females; aged 62–79 years, with an average age of 58.45±2.54 years; weighed 44–63 kg, with an average weight of 52.68±2.25 kg. There was no statistically significant difference in general information between the two groups of participants (P>0.05). This study was approved by the Second People’s Hospital of People of Deyang, Deyang (DEYL-2021-01). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent was taken from all the patients.
Detection methods
The participants with progressive ischemic stroke had blood taken on an empty stomach the next morning after admission, and the blood of control group participants was taken on an empty stomach during physical examination. The fasting venous blood sample (5 mL per person) was collected in a heparinized blood collection tube, the samples were stored at 0 °C, and sent for analysis in a mixture of ice and water. Before detection, the samples were immediately centrifuged at 3,000 r/min for 10 minutes, the supernatant was collected for anticoagulation treatment, and stored in a refrigerator at −4 °C until testing. The BG level was measured by the automatic biochemical analyzer (Olympus, Tokyo, Japan). The TXB2 level was measured by enzyme-linked immunoassay double antibody sandwich method with a detection kit (Thermo Fishier, MIT, USA). The 6-k-PGFla level was measured by enzyme-linked immune competitive suppression method with a kit provided by Jingkang Biological Engineering Company (Shanghai, China). All the tests were strictly performed according to the manufacturer instructions.
Observation indicators
The general information of participants in the two groups were collected, including gender, name, age, and history of combined underlying diseases. The expression levels of TXB2, 6-k-PGFla, and BG in the two groups were compared. The reference range of fasting BG level was as follows (7): <6.1 mmol/L was normal, and ≥6.1 mmol/L was regarded as an abnormal increase; The reference range of fasting TXB2 reference range (8): <45.6 pg/mL was normal, and ≥45.6 pg/mL was regarded as an abnormal increase; The reference range of fasting 6-k-PGFla (9): <35.8 pg/mL was normal, and ≥35.8 pg/mL was regarded as an abnormal increase. Participants in the observation group were followed up for 2 years to clarify their prognosis through telephone follow-up or outpatient review. The follow-up deadline was December 2020, or the follow-up time ended when the patient died. The prognostic mortality of patients with progressive ischemic stroke with different TXB2, 6-k-PGFla, and BG expressions was compared. Multivariate logistic regression was used to analyze the related risk factors that affected the prognosis and survival of patients with progressive ischemic stroke. The receiver operating characteristic (ROC) curve was drawn, and the area under the curve (AUC) was calculated to analyze the predictive value of TXB2, 6-k-PGFla, and BG levels on the poor prognosis of patients with progressive ischemic stroke.
Statistical methods
Statistical analysis was performed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). The count data were expressed as n (%), analyzed by t-test. Measurement data were described as (), and analyzed by χ2 test. Multiple logistic regression analysis was conducted to analyze the risk factors affecting the prognosis of patients with progressive ischemic stroke. The ROC curve was drawn to analyze the predictive value of TXB2, 6-k-PGFla, and BG levels on the prognosis of patients with progressive ischemic stroke. Results with P<0.05 were considered statistically significant.
Results
TXB2, 6-k-PGFla and BG expression in the two groups
The expression levels of TXB2, 6-k-PGFla, and BG in the observation group were significantly higher than those in the control group (P<0.05, Table 1).
The prognosis of patients with progressive ischemic stroke
Among participants with progressive ischemic stroke, the prognostic mortality of those with abnormally increased levels of TXB2, 6-k-PGFla, and BG was significantly higher than that of those with normal expression of TXB2, 6-k-PGFla, and BG (P<0.05, Table 2).

Full table
Research on related factors affecting the prognosis of patients with progressive ischemic stroke
Age and gender were not related risk factors that affected the prognosis of patients with progressive ischemic stroke (P>0.05). However, factors such as hypertension, diabetes, collateral circulatory disorders, hyperlipidemia, early hemorrhagic transformation, TXB2 (abnormal increase), 6-k-PGFla (abnormal increase), and BG (abnormal increase) were related risk factors affecting the prognosis of patients with progressive ischemic stroke (P<0.05, Table 3).

Full table
The predictive value of TXB2, 6-k-PGFla, BG, and the combined detection of the 3 indexes on the prognostic mortality of patients with progressive ischemic stroke
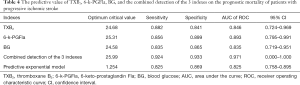
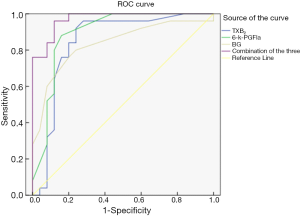
The ROC curve was used to analyze the predictive value of TXB2, 6-k-PGFla, BG, and the combined detection of the 3 on the prognostic mortality of patients with progressive ischemic stroke. Results showed that the AUC of TXB2, 6-k-PGFla, BG, and the 3 combined detections were 0.846, 0.893, 0.835, and 0.971, respectively, and the AUC of the 3 combined detections was the largest (Table 4 and Figure 1).
Full table
Discussion
Progressive ischemic stroke is a common cerebrovascular disease in clinical practice, with a high morbidity and extremely high rate of fatality and disability. Progressive ischemic stroke is a serious threat to the health and life of those affected, and is a prominent disabling and fatal disease (10). For patients with acute ischemic stroke, it is essential to optimize the treatment effect and reduce the rates of disability and fatality via timely diagnosis and the formulation of targeted treatment programs.
Previous studies have highlighted (11) that a high level of BG is an independent risk factor for prognostic mortality in patients with progressive stroke, and the complication of diabetes will double the risk of disease progression in stroke patients. In this study, the BG level of participants with progressive ischemic stroke was significantly higher than that of healthy people, and the prognostic mortality of patients with high BG was as high as 82.05%, further confirming the above view. An explanation of why high expression of BG affects the prognosis of progressive ischemic stroke patients might be that a long-term increase of BG level in their body will cause microvascular disease and circulatory disorders. Concurrently, it will also significantly increase the incidence of arteriosclerosis of the aorta and coronary arteries, leading to local arterial stenosis and occlusion, hypoperfusion of tissues and organs, increased blood viscosity, stasis of blood flow in the microcirculation, and finally progression of disease. The high expression of BG in stroke patients will increase the anaerobic glycolysis of ischemic stroke, aggravate the accumulation of lactic acid, and eventually lead to stroke metabolic disorders (12,13).
Kong et al. (14) reported that TXB2 is expressed at a low level in blood in a normal state, but when platelets are activated, a large amount of TXB2 is released into blood, which has the effect of promoting platelet aggregation and vasoconstriction. The metabolite 6-k-PGFla is synthesized during the production of arachidonic acid in the blood vessel wall triggered by vascular cyclooxygenase. It plays a role in lowering blood pressure by inhibiting platelet aggregation and relaxing blood vessels (15). Under normal physiological conditions, the levels of TXB2 and 6-k-PGFla are always in a relatively balanced state, which is important for maintaining the blood patency. In this study, TXB2 and 6-k-PGFla were both highly expressed in participants with progressive ischemic stroke, suggesting the formation of thrombus and tissue ischemia, or even bleeding tendency. A study has also pointed out (16) that in patients with progressive ischemic stroke, 6-k-PGFla is lowly expressed, which is biased with the results of this study. The bias may be due to the small ample size included in this study, and the varied time points of participant blood collection. After reviewing a large number of studies, we found that some researchers have reported that 6-k-PGFla is highly expressed in the early stage of thrombosis (17-19).
The results of this study showed that among the participants with progressive ischemic stroke, the prognostic mortality of those with abnormally increased expression of TXB2 and 6-k-PGFla was significantly higher than that of those with normal expression of TXB2 and 6-k-PGFla. Both TXB2 and 6-k-PGFla were both shown to be independent risk factors for the prognostic mortality in participants with progressive ischemic stroke in this study. As a factor released after platelet activation, TXB2 is shown to play an important role in the pathogenesis of cerebrovascular diseases (20). However, there are few studies on the relationship of TXB2 and progressive ischemic stroke. In this study, the ROC curve was drawn and the AUC was calculated. The results showed that the AUC of combined detection of the 3 indexes was 0.971, with a sensitivity of 0.924, and a specificity of 0.933, suggesting that the combined detection of the 3 indexes can be used as a sensitive indicator for predicting the prognostic mortality of patients with progressive ischemic stroke.
In summary, there are significant differences in the expression levels of TXB2, 6-k-PGFla, and BG between patients with progressive ischemic stroke and healthy people. Hypertension, diabetes, collateral circulatory disorders, hyperlipidemia, levels of TXB2 (abnormal increase), 6-k-PGFla (abnormal increase), and BG (abnormal increase) are related risk factors that affect the prognosis of patients with progressive ischemic stroke. In addition, the sensitivity and specificity of the combined detection of the 3 indexes are relatively high for the prognosis of patients with progressive ischemic stroke. Therefore, for clinicians, early diagnostic rate of progressive ischemic stroke might be improved by combining the detection of the 3 indexes to ensure good patients prognosis. However, due to the small sample size of this study, the bias was inevitable, in the future, we will expand the sample size to further study the expression levels of TXB2, 6-k-PGFla, and BG at different times after the onset of progressive ischemic stroke in order to obtain more valuable research results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-774
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-774
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-774). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Second People’s Hospital of People of Deyang, Deyang (DEYL-2021-01). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- George P, Ramiro JI, Gomes JA, et al. Central Nervous System Fungal Infection-Related Stroke: A Descriptive Study of Mold and Yeast-Associated Ischemic Stroke. J Stroke Cerebrovasc Dis 2020;29:104759 [Crossref] [PubMed]
- Kefalopoulou ZM, Liossis SN, Sagona T, et al. An ischemic stroke as the presenting manifestation of rapidly progressive primary angiitis of central nervous system in a 17-year-old boy. J Neuroimmunol 2020;341:577190 [Crossref] [PubMed]
- Miró-Mur F, Urra X, Ruiz-Jaén F, et al. Antigen-Dependent T Cell Response to Neural Peptides After Human Ischemic Stroke. Front Cell Neurosci 2020;14:206. [Crossref] [PubMed]
- Baerts L, Brouns R, Kehoe K, et al. Acute Ischemic Stroke Severity, Progression, and Outcome Relate to Changes in Dipeptidyl Peptidase IV and Fibroblast Activation Protein Activity. Transl Stroke Res 2017;8:157-64. [Crossref] [PubMed]
- Yahn GB, Abato JE, Jadavji NM. Role of vitamin B12 deficiency in ischemic stroke risk and outcome. Neural Regen Res 2021;16:470-4. [Crossref] [PubMed]
- Dhakar MB, Sheikh Z, Kumari P, et al. Epileptiform Abnormalities in Acute Ischemic Stroke: Impact on Clinical Management and Outcomes. J Clin Neurophysiol 2020;16:78-82. [PubMed]
- Dillen Y, Kemps H, Gervois P, et al. Adult Neurogenesis in the Subventricular Zone and Its Regulation After Ischemic Stroke: Implications for Therapeutic Approaches. Transl Stroke Res 2020;11:60-79. [Crossref] [PubMed]
- Rashad S, Saqr KM, Fujimura M, et al. Author Correction: The hemodynamic complexities underlying transient ischemic attacks in early-stage Moyamoya disease: an exploratory CFD study. Sci Rep 2020;10:6217. [Crossref] [PubMed]
- Simeone P, Boccatonda A, Liani R, et al. Significance of urinary 11-dehydro-thromboxane B2 in age-related diseases: Focus on atherothrombosis. Ageing Res Rev 2018;48:51-78. [Crossref] [PubMed]
- Obilade OA, Akanmu AS, Broughton Pipkin F, et al. Prostacyclin, thromboxane and glomerular filtration rate are abnormal in sickle cell pregnancy. PLoS One 2017;12:e0184345 [Crossref] [PubMed]
- Lagier D, Tonon D, Garrigue P, et al. Thromboxane-prostaglandin receptor antagonist, terutroban, prevents neurovascular events after subarachnoid haemorrhage: a nanoSPECT study in rats. Crit Care 2019;23:42. [Crossref] [PubMed]
- Mohring A, Piayda K, Dannenberg L, et al. Thromboxane Formation Assay to Identify High On-Treatment Platelet Reactivity to Aspirin. Pharmacology 2017;100:127-30. [Crossref] [PubMed]
- Turner EC, Kavanagh DJ, Mulvaney EP, et al. Identification of an interaction between the TPα and TPβ isoforms of the human thromboxane A2 receptor with protein kinase C-related kinase (PRK) 1. Implications for prostate cancer. J Biol Chem 2018;293:12286. [Crossref] [PubMed]
- Kong HK, Gan CF, Xiong M, et al. Chronic Methylmercury Exposure Induces Production of Prostaglandins: Evidence From A Population Study and A Rat Dosing Experiment. Environ Sci Technol 2019;53:7782-91. [Crossref] [PubMed]
- Mishra PS, Vijayalakshmi K, Nalini A, et al. Etiogenic factors present in the cerebrospinal fluid from amyotrophic lateral sclerosis patients induce predominantly pro-inflammatory responses in microglia. J Neuroinflammation 2017;14:251. [Crossref] [PubMed]
- Hammad E, Kostandy M, El-Sabakhawi D. Effect of feeding sweet orange peels on bloodglucose and lipid profile in Diabetic and hypercholesterolemic. Bulletin of the National Nutrition Institute of the Arab Republic of Egypt 2018;51:70-90. [Crossref]
- Schwartzbaum J, Edlinger M, Zigmont V, et al. Associations between prediagnostic blood glucose levels, diabetes, and glioma. Sci Rep 2017;7:1436. [Crossref] [PubMed]
- Altschul DM, Starr JM, Deary IJ. Cognitive function in early and later life is associated with blood glucose in older individuals: analysis of the Lothian Birth Cohort of 1936. Diabetologia 2018;61:1946-55. [Crossref] [PubMed]
- Aihara M, Kubota N, Minami T, et al. Association between tear and blood glucose concentrations: Random intercept model adjusted with confounders in tear samples negative for occult blood. J Diabetes Investig 2021;12:266-76. [Crossref] [PubMed]
- Freckmann G, Link M, Pleus S, et al. Measurement Performance of Two Continuous Tissue Glucose Monitoring Systems Intended for Replacement of Blood Glucose Monitoring. Diabetes Technol Ther 2018;20:541-9. [Crossref] [PubMed]
(English Language Editor: J. Jones)



