
microRNA-181a promotes the proliferation of hypertrophic scar fibroblasts and inhibits their apoptosis via targeting phosphatase and tensin homolog
Introduction
The skin is the human body’s largest organ. The healing process after injury to the skin is long. The ideal scar is flat and almost invisible; however, excessive fibrous tissue repair during the wound healing process can lead to hypertrophic scarring (HS) (1). Although HS is not a malignant disease, it can seriously affect an individual’s daily life.
Studies have shown that microRNAs (miRNAs or miRs) may be involved in the formation and development of HS. miRNAs are a type of non-coding single-stranded small RNA with an approximate length of 21–25 nucleotides. By mediating post-transcriptional regulation, they exert various biological functions, including proliferation, apoptosis, and invasion (2). For instance, Zhang et al. reported that the downregulation of miR-137 expression can induce the proliferation and metastasis of skin fibroblasts, and promote HS formation and development (3). Furthermore, a study by Wu et al. showed that miR-155 was downregulated in both HS tissues and HS-derived fibroblasts, and therefore suggested it as a potential therapeutic target for HS (4). Through a miRNA microarray analysis, Rang et al. observed the significantly up-regulation of miR-181a in HS tissues and cells, and that miR-181a promoted the proliferation of fibroblasts and inhibited their apoptosis (5).
The phosphatidylinositol kinase 3/protein kinase B (PI3K/AKT) signaling pathway has been shown to be closely related to HS formation (6), and multiple reports have demonstrated that its activation is mediated by phosphatase and tensin homolog (PTEN) (7). Through the mining of the ENCORI gene bank, PTEN was found to be the downstream target gene of miR-181a. Nonetheless, few studies to date have investigated the role of miR-181a in HS. Therefore, in the hope of unveiling a new therapeutic strategy for HS, the present study aimed to verify the targeting relationship between miR-181a and PTEN, and to explore the effects of miR-181a and PTEN on the proliferation and apoptosis of HS fibroblasts at the cellular level. We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/apm-21-604).
Methods
Experimental materials and main reagents
HS tissues and the normal skin tissues from 20 patients who underwent scar resection in our hospital between March 2016 and December 2019 were collected. Among them were 7 males and 13 females between the ages of 18 and 62 years old. All patients were diagnosed with HS, confirmed via clinical and pathological diagnosis. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Each patient signed an informed consent form, and approval for the study was granted by the ethics committee of Xinxiang Central Hospital.
The human embryo skin fibroblast cell line ESF-1 and the human HS fibroblast cell line HSFb were purchased from the American Type Culture Collection. Fetal bovine serum (FBS) and Roswell Park Memorial Institute (RPMI) 1640 Medium were obtained from Gibco (USA, California), and penicillin-streptomycin solution (1%) was purchased from Merck KGaA (Germany, Darmstadt). TRIzol reagent and a Lipofectamine 2000 transfection kit were obtained from Invitrogen (USA, California). A miScript reverse transcription kit was purchased from Qiagen GmbH (Germany, Hilden City). A SYBR premix Ex TaqTM II kit and a Cell Counting Kit (CCK)-8 kit were purchased from Tongren Institute of Chemistry (Japan, Kumamoto Prefecture). A BCA kit and an electrochemiluminescence (ECL) kit were acquired from Beijing Pulilai Gene Technology Co., Ltd. An Annexin V-FITC/PI apoptosis detection kit was purchased from BD Biosciences (USA, New Jersey). Primary antibodies for type I collagen (Col-1), type III collagen (Col-3), B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X protein (Bax), caspase-3, cleaved caspase-3 (c-caspase-3), caspase-9, and cleaved caspase-9 (c-caspase-9), together with horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G (IgG) secondary antibody, were purchased from Cell Signaling Technology (USA, Essex).
Cell culture and transfection
ESF1 cells and HSFb were seeded in a 6-well plate at a density of 5×104 cells/well and cultured in RPMI 1640 Medium containing 10% FBS and 1% penicillin-streptomyces. The cells were then placed at 37 °C with 5% CO2 in a constant-temperature incubator. After 24 hours of incubation, HSFb were transfected with miR-negative control (miR-NC), miR-181a mimics, miR-181a inhibitor, pcDNA3.1-PTEN (pc-PTEN), or small interfence-PTEN (si-PTEN) plasmids (sequence: 5'-GACGGGAAGACAAGUUCAUTT-3') according to the instructions accompanying the Lipofectamine 2000 transfection kit. The transfected cells were then incubated at 37 °C in a constant-temperature incubator containing 5% CO2 for a further 48 hours before subsequent experiments.
Determination of miR-181a and PTEN expression
Total RNA was extracted from normal skin (normal) tissue, HS tissue, ESF-1 cells, and HSFb using TRIzol reagent. The miScript reverse transcription (RT) kit was used for synthesis of the corresponding cDNA. The following condition was used for RT: 16 °C for 30 minutes, 42 °C for 30 minutes, and 85 °C for 5 minutes, then maintenance at 4 °C. The SYBR Premix Ex TaqTM II kit was then used for the measurement of miR-181a and PTEN expression. The thermal cycling conditions for polymerase chain reaction (PCR) were as follows: 95 °C for 5 minutes, 95 °C for 15 seconds, and 60 °C for 30 seconds, for a total of 40 cycles. The 2−∆∆Ct method was used to calculate the relative expression levels of miR-181a and PTEN with U6 and GAPDH used as an internal reference, respectively. The primer sequences used in this study are listed in Table 1.

Full table
Detection of the levels of PTEN protein and other proteins by western blot
The lysate was used to extract total protein from normal skin tissue, HS tissue, ESF-1 cells, and HSFb. The protein concentration was then measured using the BCA kit. After that, the 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate the proteins (30 µg/lane), which were then transferred to a polyvinylidene difluoride (PVDF) membranes. Next, the membrane was sealed with 5% skimmed milk powder for 1.5 hours at room temperature. Then, diluted antibodys of PTEN (1:1,000), Col-1 (1:1,000), Col-3 (1:1,000), Bcl-2 (1:1,000), Bax (1:1,000), caspase-3 (1:1,000), c-caspase-3 (1:1,000), caspase-9 (1:1,000), and c-caspase-9 (1:1,000) was added, before incubation overnight at 4 °C. After 3 washes with phosphate-buffered saline (PBS) buffer, goat anti-rabbit IgG secondary antibody (1:2,000) was added to the membrane, which was then incubated at room temperature for 1 hour. After incubation, the ECL kit was used for blot development. The gray value of each band was analyzed with Image J software, and the relative expression level of each protein was calculated.
Verification of the relationship between miR-181a and PTEN
Wild-type PTEN (WT-PTEN) and mutant PTEN (MUT-PTEN) were cloned into the pmiR-RB-ReportTM dual-luciferase reporter plasmid vector. HSFb in the logarithmic growth phase were seeded in a 24-well plate at a density of 1×104 cells/well and incubated overnight at 37 °C with 5% CO2 in a constant-temperature incubator. Then, HSFb were transfected with miR-NC and WT-PTEN, miR-NC and MUT-PTEN, miR-181a and WT-PTEN, or miR-181a and MUT-PTEN according to the instructions accompanying the Lipofectamine 2000 transfection kit. After 48 hours of transfection, the relative luciferase activity was measured in each well using Renilla luciferase activity as a reference.
Measurement of HSFb proliferation by CCK-8 experiment
HSFb were cultured in 96-well plates at a density of 5×103 cells/well. Either miR-NC, miR-181a mimics, miR-181a inhibitor, pc-PTEN, or si-PTEN was used to transfect the cells, and then cultured at 37 °C with 5% CO2 in a constant temperature incubator for a further 48 hours after transfection. Next, 10 µL of CCK-8 solution and 90 µL of RPMI 1640 Medium were added to each well. After incubation for 4 hours, a microplate reader was used to measure the absorbance value of each well at 450 nm.
Apoptosis of HSFb measured by flow cytometry
HSFb in the logarithmic growth phase were transfected with either miR-NC, miR-181a mimics, miR-181a inhibitor, pc-PTEN, or si-PTEN, and then cultured at 37 °C with 5% CO2 in a constant-temperature incubator. After 48 hours of incubation, the cells were washed 3 times with pre-chilled PBS buffer and then stained in the dark by using the Annexin V-FITC/PI Apoptosis Detection Kit. After 15 minutes of staining, cell apoptosis was then detected by flow cytometry.
Statistical analysis
SPSS 20.0 software developed by IBM (Armonk, New York, USA) was used for the experimental data analysis, and the GraphPad Prism 8.2.1 software was used to draw statistical graph. All tests were repeated 3 times, and the measurement data values were expressed as mean ± standard deviation (). Data between the 2 groups that conformed to a normal distribution were analyzed using t-test. Data between multiple groups were analyzed by one-way analysis of variance (ANOVA) and randomized block design ANOVA. P<0.05 was used for statistically significance.
Results
Expression of miR-181a in HS tissues and cells
Twenty HS tissues (HS group) and corresponding normal skin tissues (normal group) were used in this study. The expression of miR-181a in the HS group was significantly higher than the normal group (P<0.05). Furthermore, compared with ESF-1 cells, HSFb exhibited a significantly elevated expression of miR-181a (P<0.05), as shown in Figure 1.

PTEN expression in HS tissues and cells
As shown in Figure 2A,B, the RT-qPCR and WB results revealed a low expression of PTEN messenger RNA (mRNA) in HS tissues and HSFb (P<0.05). ENCORI prediction showed PTEN to be the downstream target gene of miR-181a; the targeted binding sequence is shown in Figure 2C. After transfection with miR-181a mimics and miR-181a inhibitor, the expression levels of miR-181a in HSFb were significantly increased and decreased, respectively (P<0.05), as shown in Figure 2D. The western blot showed that in HSFb, the expression of PTEN protein was significantly reduced following transfection with miR-181a mimics, while the expression of PTEN protein was significantly increased after transfection with miR-181a inhibitor. These results indicated that miR-181a negatively regulates PTEN protein expression (Figure 2E). The dual-luciferase reporter gene experiment showed that the luciferase activity of WT-PTEN was significantly inhibited (P<0.05) after miR-181a transfection, although we failed to observe a similar significant effect on the luciferase activity of MUT-PTEN (Figure 2F).
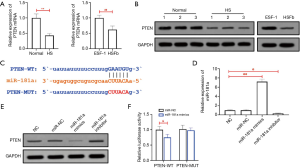
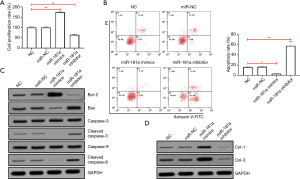
miR-181a regulates HSFb proliferation and apoptosis in vitro
The proliferation of HSFb was determined by the CCK-8 experiment. As shown in Figure 3A, HSFb proliferation was significantly enhanced by miR-181a mimic transfection (P<0.05), whereas the transfection of miR-181a inhibitor had the opposite effect (P<0.05). The apoptosis of HSFb was detected by flow cytometry, which showed that HSFb apoptosis was significantly inhibited following transfection with miR-181a mimics (P<0.05), while miR-181a inhibitor transfection significantly enhanced the early-stage apoptosis of HSFb (P<0.05) and also significantly elevated the total apoptotic rate (P<0.05) (Figure 3B). The levels of apoptosis-related proteins and collagens in HSFb were determined by western blot. The results showed that Bcl-2 Col-1, and Col-3 protein were significantly increased after transfection with miR-181a mimics (P<0.05), while the levels of Bax, c-caspase- 3, and c-caspase-9 protein were significantly inhibited (P<0.05). As expected, following transfection of HSFb with miR-181a inhibitor, the expression of Bcl-2 protein was significantly reduced (P<0.05), as was the protein expression of Col-1 and Col-3 (P<0.05), whereas the expression levels of Bax, c-caspase-3, and c-caspase-9 were significantly upregulated (P<0.05) (Figure 3C,D).
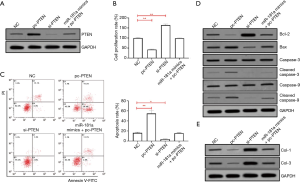
PTEN regulates HSFb proliferation and apoptosis in vitro as a downstream target gene of miR-181a
After transfection with pc-PTEN and si-PTEN plasmids, the expression of PTEN protein in HSFb was significantly affected (P<0.05) (Figure 4A). The results of CCK-8 experiments showed that HSFb proliferation was significantly inhibited following transfection with pc-PTEN (P<0.05), whereas si-PTEN transfection had the opposite effect (P<0.05). Also, as shown in Figure 4B, the simultaneous transfection of miR-181a mimics and pc-PTEN had no obvious effect on the proliferation of HSFb. The results of flow cytometry showed that the apoptotic rate of HSFb was significantly increased after pc-PTEN transfection (P<0.05), and the increase was most significant in the early apoptotic rate (P<0.05); in contrast, the transfection of si-PTEN inhibited the apoptosis of HSFb (P<0.05). However, as shown in Figure 4C, the simultaneous transfection of miR-181a mimics and pc-PTEN had no significant effect on the apoptosis rate of HSFb (P<0.05). The western blot results showed that transfection with pc-PTEN inhibited the expression of Bcl-2 protein (P<0.05), as well as the expression of Col-1 and Col-3 protein (P<0.05), in HSFb, but upregulated the protein expression of Bax, c-caspase-3, and c-caspase-9 (P<0.05). Moreover, after si-PTEN transfection, the protein expression levels of Bcl-2, Col-1, and Col-3 were significantly increased in HSFb (P<0.05), whereas those of Bax, c-caspase-3, and c-caspase-9 were inhibited (P<0.05). Finally, simultaneous transfection of miR-181a mimics and pc-PTEN had no obvious effects on the protein expression levels of Bcl-2, c-caspase-3, c-caspase-9, Col-1, or Col-3 in HSFb (Figure 4D,E).
Discussion
HS is 1 of the most common skin conditions, occurring in 30% of patients who suffer burns and scalds (8). Although HS can be treated with surgery, it may recur after surgical treatment. Multiple studies have shown that fibroblasts play a role in the processes of tissue repair and wound healing. In particular, the proliferation and apoptosis of HSFb are critical in the formation of HS (9). Therefore, elucidating the molecular mechanisms of HSFb proliferation and apoptosis may aid in the development of new treatment methods for HS.
In recent years, numerous studies have shown that miRNAs play an important role in the pathogenesis of HS (5,10-12). Although miR-181a has been confirmed to be involved in the occurrence and development of colon cancer (13), lung cancer (14), breast cancer (15), and thyroid cancer (16), few studies have focused on its expression and biological functions in HS. Zhang et al. suggested that HS results from HSFb proliferation and inhibition of HSFb apoptosis (17). In the current study, the expression of miR-181a in HS tissues was found to be significantly higher than that in normal skin tissues, and the expression of miR-181a in HSFb was also significantly higher than that in ESF-1 cells. Therefore, we speculate that miR-181a may be a regulator involved in the proliferation and apoptosis of HSFb. To clarify the specific role of miR-181a in HSFb proliferation and apoptosis, we achieved the upregulation or inhibition of miR-181a expression in HSFb via transfection. The results showed that inhibiting the expression of miR-181a not only significantly suppressed the proliferation of HSFb but also induced early apoptosis.
PTEN is a dual phosphatase located on chromosome 10q23.3 that can inhibit tumor development. It can exert an anti-apoptotic effect by regulating the activity of the PI3K/AKT signaling pathway, as well as promote cell proliferation (18). Studies have shown that the loss of PTEN expression is associated with the formation of HS (7,19,20); consistent with this, our study found that the expression of PTEN mRNA in HS tissues and HSFb was significantly downregulated. miRNAs usually exert their biological functions by influencing the translation of downstream target genes. This study found that miR-181a has partially complementary sequences with PTEN at its 3 prime untranslated region. Subsequently, we performed a dual-luciferase reporter gene experiment, which also confirmed the targeting relationship between miR-181a and PTEN. Furthermore, miR-181a can negatively regulate the expression of PTEN protein. In this study, it was confirmed that the upregulation of PTEN expression in HSFb can inhibit their proliferation and induce apoptosis.
The overexpression of Col-1 and Col-3 is also considered to be 1 of the causes of HS (21). It has been demonstrated that the expression levels of Col-1 and Col-3 in HS tissues and HSFb are significantly increased compared to those in normal skin tissues and cells (22). The results of the present research showed that while inhibiting miR-181a or upregulating PTEN in HSFb can significantly inhibit the expression of Col-1 and Col-3, simultaneous upregulation of miR-181a and PTEN has no obvious effect on the expression of Col-1 and Col-3 in HSFb. The above results indicate that miR-181a regulates Col-1 and Col-3 expression in HSFb by targeting PTEN; in other words, it acts as an anti-fibrotic factor in HS.
In summary, the results of this study showed that miR-181a promotes the expression of Col-1 and Col-3, and regulates the proliferation and apoptosis of HSFb by targeting PTEN, thus promoting the formation of HS. Therefore, miR-181a and PTEN could be potential therapeutic targets for HS. Although drugs that inhibit miR-181a have yet to be developed, drugs that upregulate PTEN, such as sorafenib, have been used clinically. Mutations or deletions of the PTEN gene are found in most patients with liver cancer (23), and sorafenib can upregulate the expression of PTEN through a variety of mechanisms to inhibit the occurrence of liver cancer (24). Given the regulatory effect of sorafenib on PTEN in liver cancer cells, we hypothesize that sorafenib may also upregulate the expression of PTEN in HSFb, thereby inhibiting the formation of HS. The above hypothesis will be the focus of our next research, in which we will evaluate the effects and safety of sorafenib in the treatment of HS by performing animal experiments, and explore new ways and methods for treating HS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/apm-21-604
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-604
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-604). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Xinxiang Central Hospital (No. ZXYY-KY-0398), and written informed consent was obtained from the patients and their families.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee H J, Jang YJ. Recent Understandings of Biology, Prophylaxis and Treatment Strategies for Hypertrophic Scars and Keloids. Int J Mol Sci 2018;19:711. [Crossref] [PubMed]
- Chen L, Heikkinen L, Wang C, et al. Trends in the development of miRNA bioinformatics tools. Brief Bioinform 2019;20:1836-52. [Crossref] [PubMed]
- Zhang Q, Guo B, Hui Q, et al. miR-137 Inhibits Proliferation and Metastasis of Hypertrophic Scar Fibroblasts via Targeting Pleiotrophin. Cell Physiol Biochem 2018;49:985-95. [Crossref] [PubMed]
- Wu X, Li J, Yang X, et al. miR-155 inhibits the formation of hypertrophic scar fibroblasts by targeting HIF-1α via PI3K/AKT pathway. J Mol Histol 2018;49:377-87. [Crossref] [PubMed]
- Rang Z, Wang ZY, Pang QY, et al. MiR-181a Targets PHLPP2 to Augment AKT Signaling and Regulate Proliferation and Apoptosis in Human Keloid Fibroblasts. Cell Physiol Biochem 2016;40:796-806. [Crossref] [PubMed]
- Zhi Y, Wang H, Huang B, et al. Panax Notoginseng Saponins suppresses TRPM7 via the PI3K/AKT pathway to inhibit hypertrophic scar formation in vitro. Burns 2020;4179:30550-7. [PubMed]
- He T, Zhang Y, Liu Y, et al. MicroRNA-494 targets PTEN and suppresses PI3K/AKT pathway to alleviate hypertrophic scar formation. J Mol Histol 2019;50:315-23. [Crossref] [PubMed]
- Tyack Z, Simons M, Spinks A, et al. A systematic review of the quality of burn scar rating scales for clinical and research use. Burns 2012;38:6-18. [Crossref] [PubMed]
- Wang X, Chu J, Wen CJ, et al. Functional characterization of TRAP1-like protein involved in modulating fibrotic processes mediated by TGF-β/Smad signaling in hypertrophic scar fibroblasts. Exp Cell Res 2015;332:202-11. [Crossref] [PubMed]
- Guo B, Hui Q, Xu Z, et al. miR-495 inhibits the growth of fibroblasts in hypertrophic scars. Aging (Albany NY) 2019;11:2898-910. [Crossref] [PubMed]
- Wang X, Zhang Y, Jiang BH, et al. Study on the role of Hsa-miR-31-5p in hypertrophic scar formation and the mechanism. Exp Cell Res 2017;361:201-9. [Crossref] [PubMed]
- Li L, Han W, Chen Y, et al. MiR-3613-3p inhibits hypertrophic scar formation by down-regulating arginine and glutamate-rich 1. Mol Cell Biochem 2021;476:1025-36. [Crossref] [PubMed]
- Wei Z, Cui L, Mei Z, et al. miR-181a mediates metabolic shift in colon cancer cells via the PTEN/AKT pathway. FEBS Lett 2014;588:1773-9. [Crossref] [PubMed]
- Ping W, Gao Y, Fan X, et al. MiR-181a contributes gefitinib resistance in non-small cell lung cancer cells by targeting GAS7. Biochem Biophys Res Commun 2018;495:2482-9. [Crossref] [PubMed]
- Gu M, Wang L, Yang C, et al. Micro-RNA-181a suppresses progestin-promoted breast cancer cell growth. Maturitas 2018;114:60-6. [Crossref] [PubMed]
- Le F, Luo P, Yang QO, et al. MiR-181a promotes growth of thyroid cancer cells by targeting tumor suppressor RB1. Eur Rev Med Pharmacol Sci 2017;21:5638-47. [PubMed]
- Zhang J, Liu Z, Cao W, et al. Amentoflavone inhibits angiogenesis of endothelial cells and stimulates apoptosis in hypertrophic scar fibroblasts. Burns 2014;40:922-9. [Crossref] [PubMed]
- Chen CY, Chen J, He L, et al. PTEN: Tumor Suppressor and Metabolic Regulator. Front Endocrinol (Lausanne) 2018;9:338. [Crossref] [PubMed]
- Zhu HY, Li C, Bai WD, et al. MicroRNA-21 regulates hTERT via PTEN in hypertrophic scar fibroblasts. PLoS One 2014;9:e97114 [Crossref] [PubMed]
- Guo L, Chen L, Bi S, et al. PTEN inhibits proliferation and functions of hypertrophic scar fibroblasts. Mol Cell Biochem 2012;361:161-8. [Crossref] [PubMed]
- Shi J, Xiao H, Li J, et al. Wild-type p53-modulated autophagy and autophagic fibroblast apoptosis inhibit hypertrophic scar formation. Lab Invest 2018;98:1423-37. [Crossref] [PubMed]
- Zhou R, Zhang Q, Zhang Y, et al. Aberrant miR-21 and miR-200b expression and its pro-fibrotic potential in hypertrophic scars. Exp Cell Res 2015;339:360-6. [Crossref] [PubMed]
- Zhou J, Li X. Association of PTEN expression with liver function and inflammatory changes in patients with liver cancer after chemotherapy. Oncol Lett 2018;16:6633-7. [Crossref] [PubMed]
- Ruan Z P, Xu R, Lv Y, et al. PTEN enhances the sensitivity of human hepatocellular carcinoma cells to sorafenib. Oncol Res 2012;20:113-21. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


