
Risk factors for mortality of coronavirus disease 2019 (COVID-19) patients during the early outbreak of COVID-19: a systematic review and meta-analysis
Introduction
According to the World Health Organization, a novel pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, previously known as 2019-nCoV) is designated as coronavirus disease 2019 (COVID-19) (1). SARS-CoV-2 belongs to the coronavirus family together with SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), but has more rapid transmission than SARS-CoV and MERS-CoV (2-4), which leads to a dramatic increase in the number of confirmed cases during a short period, thereby posing a serious threat for health systems worldwide. Till August 4, 2020, a cumulative total of 18,142,718 confirmed COVID-19 cases and 691,013 deaths have been reported in 216 countries (5).
Fever and cough are main clinical manifestations of COVID-19 patients (6). Most of COVID-19 patients have a favorable outcome, but a minority of them may develop severe pneumonia, dyspnea and hypoxemia, and progress into respiratory or multi-organ failure and even death (7). Based on 55,924 laboratory confirmed cases in China, the overall national mortality rate is 3.8%, but the fatality rate of patients over 80 years old is up to 22% (8). Besides, male, pre-existing comorbidities, elevated inflammatory markers, and complications [i.e., acute respiratory distress syndrome (ARDS), acute cardiac injury, acute kidney injury and sepsis] were associated with an increased risk of death (9-15).
In the early stages of COVID-19 outbreak, because effective vaccines and antiviral drugs for SARS-CoV-2 are lacking, the management of critically ill patients is often challenging. Thus, it is very essential to identify the risk factors associated with poor outcome of COVID-19 patients and perform early interventions for high-risk patients. The present study aimed to systematically review the evidence on the in-hospital mortality of COVID-19 patients and elucidate the risk factors of mortality in COVID-19 patients.
Methods
This meta-analysis was conducted based on Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines and results were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (available at http://dx.doi.org/10.21037/apm-20-2557).
Registration
This study was registered at PROSPERO (registration number: CRD42020169921).
Search strategy
All relevant studies regarding mortality of COVID-19 patients were retrieved via the PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP, and Wanfang databases. The search terms were (“2019-nCoV” OR “SARS-CoV-2” OR “COVID-19” OR “new coronary pneumonia” OR “corona virus” OR “novel coronavirus” OR “nCoV” OR “severe acute respiratory syndrome coronavirus 2”) AND (“death” OR “died” OR “die” OR “mortality” OR “survival” OR “survivor” OR “fatal” OR “outcome” OR “decease” OR “deadly” OR “lethal” OR “fatality”). The last search was performed on May 26, 2020.
Study selection
There was neither publication language nor publication status restriction. All eligible studies should report the mortality and/or risk factors for death in COVID-19 patients. Exclusion criteria were as follows: (I) duplicates; (II) case reports, reviews or meta-analyses, guidelines, consensus, experimental or animal studies, comments, notes, and correspondences; (III) irrelevant papers; (IV) data regarding the mortality and/or risk factors cannot be extracted; and (V) duplicate study population. As for duplicate studies, we selected only one original study with more comprehensive clinical and laboratory data. Case-control studies were excluded from the proportion meta-analyses regarding mortality of COVID-19 patients due to their potential patient selection bias.
Data extraction
The following data were extracted from the included studies: the first author, publication year, region, source of cases, enrollment period, follow-up periods, number of COVID-19 patients, number of COVID-19 patients with severe disease, number of non-survivors and survivors, age, gender, and other potential risk factors for death.
Study quality
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of included studies. It includes study selection (four items), comparability (two items), and exposure/outcome (three items). The highest NOS score was 9, and studies with a NOS score of >6 were considered as high quality.
Statistical analysis
All meta-analyses were performed using STATA version 12.0 (Stata Corp., College Station, Texas, USA) and Review Manager software version 5.4 (Cochrane collaboration, the Nordic Cochrane Centre, Copenhagen, Denmark). The meta-analyses were conducted by using a random-effect model. We pooled the in-hospital mortality in COVID-19 patients, and then calculated the pooled proportion with 95% confidence interval (CI). We collected the risk factors for death in COVID-19 patients, and then calculated the odds ratios (ORs) or mean difference (MD) with 95% CIs. The heterogeneity among studies was evaluated by Cochrane Q test and the I2 statistics, and I2 >50% and/or P<0.1 were considered to have statistically significant heterogeneity. Publication bias was assessed with Egger test. P<0.1 was considered as a statistically significant publication bias. Subgroup analyses, meta-regression analyses, and sensitivity analyses would be performed to explore the sources of heterogeneity among studies. Subgroup analyses were conducted according to the sample size (>100 versus ≤100), source of cases (single-center versus multiple-center), NOS (>6 versus ≤6), region (Asia versus Europe versus North America), study design (retrospective versus prospective), longest follow-up duration (>30 days versus ≤30 days), and proportion of patients with severe disease (>50% versus ≤50%). Meta-regression analyses were also grouped in terms of the variables mentioned above. Scattered plots were drawn to show the trend in overall in-hospital mortality according to the proportion of severe COVID-19 patients included. The correlation between them was evaluated using Spearman correlation analysis in the IBM SPSS 22.0 (IBM Corp, Armonk, NY, USA). Coefficients were calculated. A two-sided P<0.05 indicates a statistical significance.
Results
Study selection
A total of 7,003 studies were identified via the 6 databases, and 6 studies were identified via a manual search. Finally, 80 studies with 25,385 COVID-19 patients were included (Figure 1). All included studies are listed in the Appendix.
Study characteristics
Characteristics of the included studies were listed in Table 1. Forty-three studies were published as full texts, 32 was published in press (i.e., available online ahead of print), and 5 studies were preprinted. The sample size ranged from 8 to 2,964. Sixty-one studies were performed in Asia, 11 in Europe, and 8 in North America; 72 of them were retrospective and 8 were prospective; 56 and 24 studies were single-center and multi-center studies, respectively.
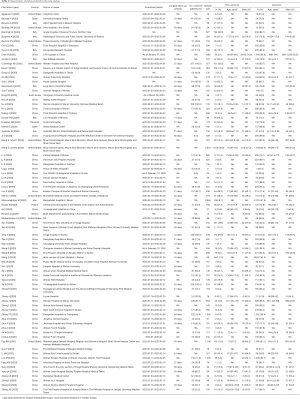
Full table
Study quality
The NOS score ranged between 3 and 8. Twenty-two studies were considered to be of high quality and 3 were of low quality (Table S1).
Mortality
The results of the meta-analyses regarding in-hospital mortality of COVID-19 patients are summarized in Table 2.
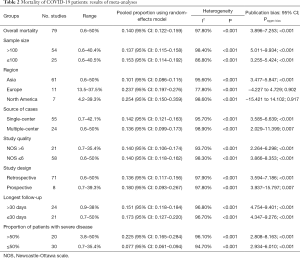
Full table
Overall analyses
Eighty studies reported the in-hospital mortality of COVID-19 patients, and the pooled in-hospital mortality of COVID-19 patients was 14% (95% CI: 12.2–15.9%). The heterogeneity was statistically significant (I2 =97.8%; P<0.001). Fifty studies reported the number of severe COVID-19 patients, and the pooled incidence of severe COVID-19 patients was 49.6% (95% CI: 43.6–55.6%). The heterogeneity was statistically significant (I2 =99.9%; P<0.001).
Subgroup analyses
The pooled in-hospital mortality of COVID-19 patients was 10.1%, 23.7%, and 25.4% in Asia, Europe, and North America, respectively. The pooled in-hospital mortality of COVID-19 patients was 13.7% and 15.2% in studies with a sample size of >100 and ≤100, respectively. The pooled in-hospital mortality of COVID-19 patients was 14.2% and 13.6% in single-center and multiple-center studies, respectively. The pooled in-hospital mortality of COVID-19 patients was 13.6% and 18% in retrospective and prospective studies, respectively. The pooled in-hospital mortality of COVID-19 patients was the same between the studies with NOS >6 and NOS ≤6 (both 14%). The pooled in-hospital mortality of COVID-19 patients was 15.1% and 17.3% in studies with the longest follow-up duration of >30 days and ≤30 days, respectively. The pooled in-hospital mortality of COVID-19 patients was 22.5% and 7.7% in studies with the proportion of patients with severe disease of >50% and ≤50%, respectively. The heterogeneity was statistically significant in all subgroup analyses.
Meta-regression analyses
The results of meta-regression analyses are shown in Table S2. Meta-regression analyses indicated that region (Asia versus Europe versus North America) (P=0.0001), proportion of patients with severe disease (>50% versus ≤50%) (P<0.001), rather than sample size (>100 versus ≤100) (P=0.456), source of cases (single-center versus multiple-center) (P=0.756), NOS (>6 versus ≤6) (P=0.956), study design (retrospective versus prospective) (P=0.403), and longest follow-up duration (>30 versus ≤30 days) (P=0.624), might be related to the heterogeneity.
Sensitivity analyses
The sensitivity analysis showed that none of these included studies could significantly influence the results of the meta-analysis.
Risk factors
A total of 31 studies reported the detailed data regarding the association of demographic and clinical characteristics, laboratory data, imaging features, and complications with mortality of COVID-19 patients, of which 25 performed both univariate and multivariate analyses (Table S3).
The detailed results of meta-analyses are presented in Table 3 and forest plots in the Supplementary Materials.
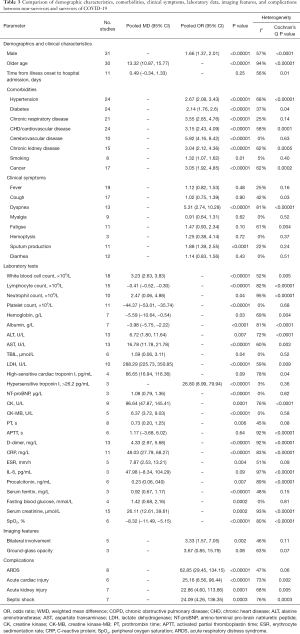
Full table
Demographics
Meta-analyses indicated that male (OR =1.66, 95% CI: 1.37–2.01; P<0.00001) and older age (MD =13.32, 95% CI: 10.87–15.77; P<0.00001) were significant risk factors for death of COVID-19 patients.
Comorbidities
Meta-analyses indicated that hypertension (OR =2.67, 95% CI: 2.08–3.43; P<0.00001), diabetes (OR =2.14, 95% CI: 1.76–2.6; P<0.00001), chronic respiratory disease (OR =3.55, 95% CI: 2.65–4.76; P<0.00001), chronic heart disease/cardiovascular disease (OR =3.15, 95% CI: 2.43–4.09; P<0.00001), cerebrovascular disease (OR =5.92, 95% CI: 4.16–8.42; P<0.00001), chronic kidney disease (OR =3.04, 95% CI: 2.12–4.36; P<0.00001), cancer (OR =3.05, 95% CI: 1.92–4.85; P<0.00001), and smoking (OR =1.32, 95% CI: 1.07–1.62; P=0.01) were significant risk factors for death of COVID-19 patients.
Clinical symptoms
Meta-analysis indicated that dyspnea (OR =5.31, 95% CI: 2.74–10.28; P<0.00001) and expectoration (OR =1.88, 95% CI: 1.39–2.55; P<0.0001) at admission were significant risk factor for death of COVID-19 patients, but fever (OR =1.12, 95% CI: 0.82–1.53; P=0.48), cough (OR =1.02, 95% CI: 0.75–1.39; P=0.90), myalgia (OR =0.91, 95% CI: 0.64–1.31; P=0.62), fatigue (OR =1.47, 95% CI: 0.93–2.34; P=0.10), hemoptysis (OR =1.25, 95% CI: 0.38–4.14; P=0.72), and diarrhea (OR =1.14, 95% CI: 0.83–1.56; P=0.43) at admission were not significantly associated with mortality.
Laboratory tests
Meta-analysis indicated that increased levels of white blood cells (WBC) (MD =3.23, 95% CI: 2.63–3.83; P<0.00001), alanine aminotransferase (ALT) (MD =6.72, 95% CI: 1.80–11.64; P=0.007), aspartate aminotransferase (AST) (MD =16.78, 95% CI: 11.78–21.78; P<0.00001), total bilirubin (TBIL) (MD =1.59, 95% CI: 0.06–3.11; P=0.04), lactate dehydrogenase (LDH) (MD =288.29, 95% CI: 225.73–350.85; P<0.00001), high-sensitive cardiac troponin I (hs-cTnI) (MD =66.65, 95% CI: 16.94–116.36; P=0.009), D-dimer (MD =4.33, 95% CI: 2.97–5.68; P<0.00001), C-reactive protein (MD =48.03, 95% CI: 27.79–68.27; P<0.00001), and serum creatinine (MD =26.11, 95% CI: 12.61–39.61; P=0.0002) at admission were significant risk factors for death of COVID-19 patients; and decreased levels of albumin (MD =−3.98, 95% CI: −5.75 to −2.22; P<0.0001), lymphocytes (MD =−0.41, 95% CI: −0.52 to −0.30; P<0.00001), hemoglobin (MD =−5.59, 95% CI: −10.64 to −0.54; P=0.03), platelet (MD =−44.37, 95% CI: −53.01 to −37.74; P<0.00001), and peripheral oxygen saturation (SpO2) (MD =−8.32, 95% CI: −11.49 to −5.15; P<0.00001) at admission were significant risk factors for death of COVID-19 patients. Additionally, hs-cTnI >26.2 pg/mL as a categorical variable (OR =26.80, 95% CI: 8.99–79.94; P<0.00001) at admission was significantly associated with an increased risk of COVID-19 mortality.
Imaging features
Meta-analysis indicated that bilateral involvement was a significant risk factor for death of COVID-19 patients (OR =3.33, 95% CI: 1.57–7.05; P=0.002), but ground-glass opacity was not significantly associated with mortality (OR =3.67, 95% CI: 0.85–15.79; P=0.08).
Complications
Meta-analysis indicated that ARDS (OR =62.85, 95% CI: 29.45–134.15; P<0.00001), acute cardiac injury (OR =25.16, 95% CI: 6.56–96.44; P<0.00001), acute kidney injury (OR =22.86, 95% CI: 4.60–113.66; P=0.0001), and septic shock (OR =24.09, 95% CI: 4.26–136.35; P=0.0003) were significant risk factors for death of COVID-19 patients.
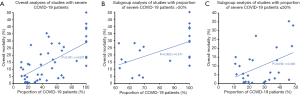
Scattered plots demonstrated an increased trend of overall mortality with the proportion of severe COVID-19 patients included (P<0.001, r=0.631) (Figure 2A
Discussion
The present meta-analysis suggested that the pooled in-hospital mortality of COVID-19 patients was 14%. By comparison, previous meta-analyses reported that the mortality of COVID-19 patients was relatively lower [i.e., 3.2% in the Hu’ meta-analysis (16) or 7.7% in our previous meta-analysis (6)]. This discrepancy can be explained by the difference in the severity of COVID-19 patients included among them. Indeed, the proportion of severe COVID-19 patients was higher in the present meta-analysis than the previous meta-analysis [49.6% versus 18% (16)], which might overestimate the overall mortality.
Severe disease status was an independent risk factor for death in COVID-19 patients (17,18). Our subgroup analyses also found that the in-hospital mortality of COVID-19 patients in studies with the proportion of patients with severe disease of >50% was higher than those ≤50% (22.5% versus 7.7%). Severe patients had more prominent laboratory abnormalities [i.e., leukopenia, lymphopenia, elevated levels of C-reactive protein and interleukin 6 (IL-6)] as compared to non-severe patients. Increased levels of C-reactive protein and inflammatory cytokines, such as IL-6, may induce “cytokines storm”, thereby aggravating systemic inflammatory response syndrome in patients with severe disease, which may be a driving factor of acute lung injury and ARDS and even death (19-21). The mortality in COVID-19 patients with ARDS was up to 39% (22). We also confirmed that ARDS was associated with a 62.85-fold increase in the risk of death in COVID-19 patients.
The mortality of COVID-19 patients greatly varies among regions. It seems to be the lowest in China (3.1%) and the highest in the United Kingdom (20.8%) and New York State (20.99%) (23). Our subgroup analysis also demonstrated that the in-hospital mortality of COVID-19 patients in Europe and North America were higher than Asia (23.7% and 25.4% versus 10.1%). This might be explained by the aging of patients in Europe and North America. It has been reported that 37.6% of COVID-19 patients are beyond 70 years old in Italy, but only 11.9% in China (24). As shown by our meta-analysis and others (23), age is a significant risk factor of death in COVID-19 patients. In other words, a higher proportion of elderly patients is often in parallel with an increased mortality. This phenomenon could be attributed to the relationship of aging with immune response impairment and chronic inflammation (25) and a high prevalence of comorbidities, such as hypertension, diabetes, and cardiovascular disease, in elderly patients. Obesity is common in Western countries with an increasing prevalence of obesity, and associated with poor prognosis of COVID-19 patients (26,27). Angiotensin-converting enzyme 2 (ACE2) is the receptor of SARS-CoV-2 infection target cells, and ACE2 expression level in adipocytes is higher than that in lung tissue. Obese people have more adipose tissue and therefore higher ACE2 levels. Among the obese population, the renin-angiotensin-aldosterone system is overactive, increasing the production of angiotensin II (26). Elevated angiotensin II levels in COVID-19 patients are related to the severity of lung injury (28), which will increase the risk of death. Additionally, it has been confirmed that obesity increases the risk of cardiovascular disease and its mortality (29). Besides, the difference in public prevention and control strategies of COVID-19 among countries is another major explanation for this variation (30).
Pre-existing comorbidities correlated with an increased risk of mortality in COVID-19 patients, probably because patients with hypertension and diabetes have higher circulating ACE2 levels (31,32). A wider distribution of ACE2 in cardiac epithelial cells as well as respiratory, kidney, and liver is associated with organ failure in patients with SARS (33-35). Therefore, it is postulated that patients with cardiovascular disease are more prone to use angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin-receptor blockers (ARBs), thereby elevating the ACE2 expression and then increasing the risk of SARS-CoV-2 infection and disease progression (36).
Acute cardiac injury was also associated with poor outcomes in COVID-19 patients. Similarly, previous studies suggested that COVID-19 patients with abnormal troponin I, which is a marker of acute myocardial injury, had worse prognosis (37,38). Underlying mechanisms for explaining this phenomenon are as follows. First, the release of proinflammatory cytokines, endothelial dysfunction, and increased oxidative stress can lead to a hypercoagulable state, which is prone to coronary arterial thrombosis and triggers acute coronary syndrome (25). Second, SARS-CoV-2 binds to ACE2 receptor, which is widely expressed in cardiomyocytes, thereby attacking cardiac epithelial cells and inducing cardiac injury (39-41).
Lung pathology of critically ill patients showed occlusion and micro-thrombosis in pulmonary vessels (42). Additionally, severe COVID-19 patients, especially those with sepsis, are often at a hypercoagulable state (20,43). Our study confirmed that D-dimer level, a convenient biomarker of thrombotic events (44), was associated with the mortality of COVID-19 patients.
Of note, non-survival group had a significantly higher proportion of male than survival group. This may be attributed to the difference in the levels and types of sex hormones between males and females. Estrogen can modulate the responses of adaptive and innate immunity, which can reduce the susceptibility of females to viral infections (45). On the contrary, males are more susceptible to SARS-CoV-2 infection (46).
Major limitations of the present work should be that all studies included in this meta-analysis were observational with different patient characteristics and follow-up periods, a majority of studies were retrospective, and some of them were of low quality, which might produce the potential selection bias and recall bias. Additionally, the heterogeneity in most of meta-analyses was significant. Although we performed subgroup, meta-regression, and sensitivity analyses, the source of heterogeneity was not clearly identified.
In conclusion, based on the systematic review and meta-analysis, the in-hospital mortality of COVID-19 patients was up to 14%. Older age, male, comorbidities (i.e., hypertension, diabetes, cardiovascular diseases, and respiratory diseases), clinical presentations with dyspnea and expectoration, and laboratory abnormalities (i.e., WBC, AST, ALT, serum creatinine, C-reactive protein, LDH, hs-cTnI, and D-dimer), should be important predictors for mortality of COVID-19 patients. Moreover, patients who develop ARDS, acute cardiac injury, acute kidney injury, and septic shock are at higher risk of death.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MOOSE and PRISMA reporting checklists. Available at http://dx.doi.org/10.21037/apm-20-2557
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-2557
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2557). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- WHO. Situation Report - 22 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=fb6d49b1_2. Accessed on August 4, 2020.
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020;382:1199-207. [Crossref] [PubMed]
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270-3. [Crossref] [PubMed]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref] [PubMed]
- WHO. Coronavirus disease 2019 (COVID-19) Situation Report. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed on August 4, 2020.
- Wu YY, Li HY, Xu XB, et al. Clinical features and outcome of treatment for novel coronavirus pneumonia: a meta-analysis. Zhonghua Gan Zang Bing Za Zhi 2020;28:240-6. [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). WHO. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19---final-report-1100hr-28feb2020-11mar-update.pdf?sfvrsn=1a13fda0_2&download=true. Accessed on August 4, 2020.
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9. [Crossref] [PubMed]
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Wang X, Fang X, Cai Z, et al. Comorbid Chronic Diseases and Acute Organ Injuries Are Strongly Correlated with Disease Severity and Mortality among COVID-19 Patients: A Systemic Review and Meta-Analysis. Research (Wash D C) 2020;2020:2402961 [Crossref] [PubMed]
- Martins-Filho PR, Tavares CSS, Santos VS. Factors associated with mortality in patients with COVID-19. A quantitative evidence synthesis of clinical and laboratory data. Eur J Intern Med 2020;76:97-9. [Crossref] [PubMed]
- Parohan M, Yaghoubi S, Seraji A, et al. Risk factors for mortality in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male 2020;23:1416-24. [Crossref] [PubMed]
- Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol 2020;92:1875-83. [Crossref] [PubMed]
- Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med 2020;130:304-9. [PubMed]
- Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (COVID-19): A systematic review and meta-analysis. J Clin Virol 2020;127:104371 [Crossref] [PubMed]
- Sun DW, Zhang D, Tian RH, et al. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID-19 patients: A sentinel? Clin Chim Acta 2020;508:122-9. [Crossref] [PubMed]
- Luo M, Jiang B, Xu HJ, et al. Analysis of influencing factors of death in patients with COVID-19. Chinese Traditional and Herbal Drugs 2020;51:1450-4.
- Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033-4. [Crossref] [PubMed]
- Lin L, Lu L, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020;9:727-32. [Crossref] [PubMed]
- Ulhaq ZS, Soraya GV. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Mal Infect 2020;50:382-3. [Crossref] [PubMed]
- Hasan SS, Capstick T, Ahmed R, et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med 2020;14:1149-63. [Crossref] [PubMed]
- Bonanad C, Garcia-Blas S, Tarazona-Santabalbina F, et al. The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects. J Am Med Dir Assoc 2020;21:915-8. [Crossref] [PubMed]
- Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020;323:1775-6. [Crossref] [PubMed]
- Libby P. Inflammation in atherosclerosis. Nature 2002;420:868-74. [Crossref] [PubMed]
- Sanchis-Gomar F, Lavie CJ, Mehra MR, et al. Obesity and Outcomes in COVID-19: When an Epidemic and Pandemic Collide. Mayo Clin Proc 2020;95:1445-53. [Crossref] [PubMed]
- Sharma A, Garg A, Rout A, et al. Association of Obesity With More Critical Illness in COVID-19. Mayo Clin Proc 2020;95:2040-2. [Crossref] [PubMed]
- Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364-74. [Crossref] [PubMed]
- Pranata R, Huang I, Lim MA, et al. Impact of cerebrovascular and cardiovascular diseases on mortality and severity of COVID-19-systematic review, meta-analysis, and meta-regression. J Stroke Cerebrovasc Dis 2020;29:104949 [Crossref]
- Hradsky O, Komarek A. Demographic and public health characteristics explain large part of variability in COVID-19 mortality across countries. Eur J Public Health 2021;31:12-6. [Crossref] [PubMed]
- Patel SK, Velkoska E, Freeman M, et al. From gene to protein-experimental and clinical studies of ACE2 in blood pressure control and arterial hypertension. Front Physiol 2014;5:227. [Crossref] [PubMed]
- Soro-Paavonen A, Gordin D, Forsblom C, et al. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens 2012;30:375-83. [Crossref] [PubMed]
- Yang JK, Feng Y, Yuan MY, et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med 2006;23:623-8. [Crossref] [PubMed]
- Li R, Qiao S, Zhang G. Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. J Infect 2020;80:469-96. [Crossref] [PubMed]
- Yang JK, Lin SS, Ji XJ, et al. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 2010;47:193-9. [Crossref] [PubMed]
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8:e21 [Crossref] [PubMed]
- Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis 2020;63:390-1. [Crossref] [PubMed]
- Vrsalovic M, Vrsalovic Presecki A. Cardiac troponins predict mortality in patients with COVID-19: A meta-analysis of adjusted risk estimates. J Infect 2020;81:e99-100. [Crossref] [PubMed]
- Chen Y, Guo Y, Pan Y, et al. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun 2020;525:135-40. [Crossref] [PubMed]
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565-74. [Crossref] [PubMed]
- Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci 2004;25:291-4. [Crossref] [PubMed]
- Luo W, Yu H, Gou J, et al. Clinical Pathology of Critical Patient with Novel Coronavirus Pneumonia (COVID-19). Preprints 2020. Available online: https://wwwpreprintsorg/manuscript/2020020407/v4. Accessed 13 Aug 2020.
- Levi M, van der Poll T. Coagulation and sepsis. Thromb Res 2017;149:38-44. [Crossref] [PubMed]
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:2950-73. [Crossref] [PubMed]
- Jaillon S, Berthenet K, Garlanda C. Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol 2019;56:308-21. [Crossref] [PubMed]
- Vahidy FS, Pan AP, Ahnstedt H, et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: Cross-sectional analysis from a diverse US metropolitan area. PLoS One 2021;16:e0245556 [Crossref] [PubMed]



