
Health-related quality of life in patients with advanced colorectal cancer: a predictive nomogram including BMI, sex and age
Introduction
Colorectal cancer epidemiology
Colorectal cancer represents a high burden on the society worldwide, ranking as third in terms of incidence and second in terms of mortality among all cancer types. The World Health Organizations has estimated approximately 1,800,000 new diagnoses of and 860,000 new deaths from colorectal cancer worldwide in 2018 (1). The incidence/mortality ratio gives the idea that nearly half of colorectal cancers are incurable and lead to premature death. The number of subjects with a past or active diagnosis of colorectal cancer is steadily increasing, especially among elderly with relevant comorbidities, and is estimated to double in 30 years’ time (2).
Quality of life in oncology and colorectal cancer
When the disease is deemed incurable, maintenance of health-related quality of life (HRQoL) and patient reported outcomes (PROs) are paramount (3). Nonetheless, reports on HRQoL measures have so far been suboptimal in the cancer literature. In a recent telephone inquiry involving nearly ten thousand subjects across seven different European countries, HRQoL was reported by 60–80% of the respondents to be even more important than extending the overall survival in the hypothetical case of a ‘serious illness like cancer limiting the time to live’ (4).
HRQoL is identified itself as the primary endpoint in some studies. Beside this, it has also been established, in many oncological settings (including lung, breast, colorectal, and prostate cancers), as a powerful predictive factor of other primary objectives such as overall survival (5-8). Moreover, HRQoL maintenance is a fundamental requirement for innovative therapies since toxicity-related HRQoL impairment may significantly limit their implementation, and therefore it is often set as secondary endpoint in clinical trials (9-11).
So far PROs have been mainly used for research purposes. However, more recently their importance has been recognized also for practical application in order to improve the quality of the care delivered by Healthcare Systems. Strategies to systematically record PROs and include them in routine clinical practice are now taken into consideration (12). In this regard, they can be used as intervention tools to modulate nutrition support, physical activity and physiotherapeutic strategies (13). A number of questionnaires have been developed to capture and measure HRQoL (14) with challenges still remaining about optimal patient adherence. Furthermore, lack of guidelines to help clinicians respond to issues of patient HRQoL and the cost-benefit ratio in terms of clinician workload and the overloading of health services are other matters of debate (15).
As far as HRQoL in metastatic colorectal cancer (mCRC) patients is concerned, a recent population-based reports have evidenced that this disease may be associated with a significant HRQoL burden including bowel, urinary, and sexual problems even 1–3 years after the diagnosis (16).
In centers where PRO recording is not routinely used in mCRC management, it would be at least desirable to facilitate the identification of frail mCRC patients for whom full HRQoL assessment is indispensable (17).
Obesity and quality of life in colorectal cancer patients
Obesity is a complex physiopathological state that involves significant changes in the systemic metabolic profile (18,19), circulating levels of insulin and other growth hormones (20), baseline innate immunity activation (21) and vasomotor response (22-24). Such changes pave the way for an increase in the risk of cardiovascular diseases, cancer and diabetes (25-27). Nonetheless, a possible survival advantage has been hypothesized for certain oncological settings in presence of metastatic disease, raising the possibility that an ‘obesity paradox’ and a protective role of high BMI would exist for some cancer patients (28,29). However, the effect of obesity on HRQoL of mCRC patients is under-reported
Aim of the study
The aim of the present study was to investigate the effect of BMI and other clinical variables on HRQoL in metastatic colorectal cancer patients treated with standard chemotherapy and generate a possible HRQoL-specific predictive score. Analyzed variables (anthropometric, sociodemographic, lifestyle, and clinical variables) are universally collected and readily available in electronic charts. The subgroup of mCRC patients identified as at risk according to the predictive score should be offered full HRQoL assessment and subsequent tailored supportive strategies in the routine clinical practice of Oncology Units.
We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/apm-20-2194).
Methods
Study design
This is a prospective monocentric observational pilot study (TV-ONCO study, NCT03873064, local Ethics Committee Approval ID: R.S. 102/17) where consecutive adult patients referred to the Medical Oncology Unit of Tor Vergata University Hospital (Rome, Italy) with a histologically confirmed diagnosis of colorectal cancer and measurable metastatic disease were recruited. Patients who were to start a first-line chemotherapy treatment were requested, following informed written consent, to complete the EORTC-QLQ-C30 questionnaire (30), and basic information regarding sex, age, body mass index (BMI), height, level of education, marital status, alcohol and caffeine consumption and smoking habit were recorded.
Other data on socioeconomic status and working conditions were not systematically collected. They were often missing in medical records and were not included in the present analysis. First-line chemotherapy was started within 1 week of questionnaire completion. A prospectively maintained database linked to the central medical records system was used for the necessary clinical information.
The study was approved by the Local Ethics and Scientific Committees (local Ethics Committee Approval ID: R.S. 102/17) and performed in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all individual participants included in the study.
Statistical considerations
EORTC-QLQ-C30 data were managed and analysed according to the instruction manual (31). Global Health Score (GHS) was the primary objective. It was derived from the last two questions of the EORTC-QLQ-C30 and the final score for each participant was re-scaled from 0% to 100%, with higher scores indicating better HRQoL. For the purpose of the analysis, GHS was dichotomized according to its median value in the study population (high GHS, coded as 0, vs. low GHS, coded as 1).
No candidate predictor pre-selection was performed since the subject matter was thought to be relatively underexplored and no a priori exclusion of variables was decided.
Standard univariable and multivariable logistic regressions were performed to identify significant predictors of low GHS. Variables with a significant effect at the univariate analysis (P value <0.05) were selected for the multivariate analysis. C-statistics, Nagelkerke’s R2 and the Brier score were used to provide performance measures of the multivariable predictive model.
Before running the univariate logistic regression analysis (LRA), continuous variables (age, height and BMI) were grouped into deciles and qualitatively assessed for their association to the outcome by visually inspecting smoothed frequency curves of low vs. high GHS (‘cdplot’ function of R software). When the association appeared to be not linear, the continuous variable was dichotomized with the dichotomizing value chosen based on the curve shape (see supplementary material, Figure S1).
The resulting multivariable model was internally validated with 100 bootstrap resampling. Apparent calibration of the model was assessed by analyzing the relationship between predicted risk and actual incidence of low GHS for increasing risk in the study population.
By using the variables found to be significant in the multivariate analysis, a nomogram was built to estimate the predicted risk of suffering from low GHS.
Risk cut-offs identified thanks to the nomogram were analysed by means of receiver operating characteristic (ROC) curve analysis to determine sensitivity and specificity for low GHS prediction.
The clinical usefulness of the model was assessed by means of decision curve analysis to determine the net benefit of using the model for low GHS patient identification (32).
All analyses were performed with R software version 3.5.1. All tests were considered statistically significant for two tail P values <0.05.
Results
Between January 2015 and November 2018, 181 patients with mCRC were invited to participate in the study, of whom 173 returned the EORTC QLQ-C30 having completed questions 29 and 30, i.e., the Global Health Score (GHS), with a compliance rate of 96%. Patients’ characteristics are presented in Table 1.
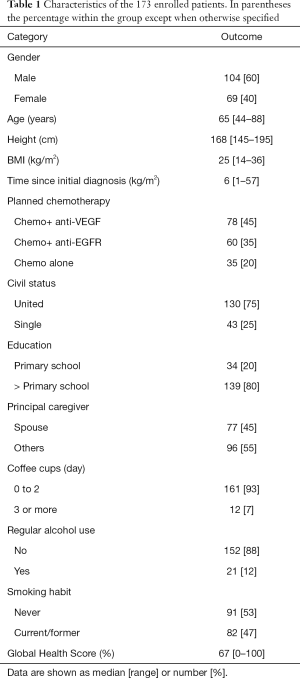
Full table
For the purpose of the analysis, GHS was dichotomized-based on the median value of 67%, in high GHS (≥67%, coded as 0) and low GHS (<67%, coded as 1).
For the univariate LRA for GHS prediction the following variables were included: age, education level (primary school coded as 1, higher educational stages coded as 0), height, BMI, marital status (single coded as 1, married or living with a partner coded as 0), principal caregiver (spouse coded as 0, others coded as 1), consumption of coffee cups a day (<3 coded as 1, 3 or more coded as 0), alcohol use (yes coded as 1, no coded as 0), smoking habit (current/former coded as 1, never coded as 0).
Before running the univariate LRA, continuous variables (i.e., age, height and BMI) were grouped into deciles and qualitatively assessed for their association to the outcome by visually inspecting smoothed frequency curves of low vs. high GHS by using the ‘cdplot’ function of R software (Figure S1).
By inspecting the frequency plots, a higher occurrence of low GHS was seen for the 6th to 10th decile of age (age >65 years) and for the 1st to 3rd decile of BMI (BMI ≤23). Therefore, the age variable was dichotomized <65 years (coded as 0) vs. >65 years (coded as 1), and the BMI variable was dichotomized ≤23 (coded as 1) vs. BMI >23 (coded as 0). Height was found to be linearly associated with GHS and therefore kept as a continuous variable.
At the univariate LRA, sex, age and BMI demonstrated a significant association (P<0.05) to GHS (Table 2) and were used for the multivariable LRA.
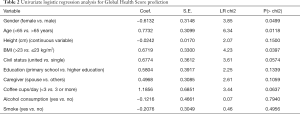
Full table
Multivariable LRA showed that the three variables retained a significant association with GHS (P values from 0.04 to 0.004, Table 3) and a full predictive model including age, BMI and sex was built, that produced a C-statistics of 67%, a Nagelkerke’s R2 index of 12% and a Brier score of 0.228, P=0.001. Coefficients of the single variables of the full model are reported in Table 3.
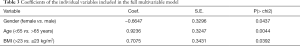
Full table
Apparent consistency and performance of the model was good, as demonstrated by a calibration analysis of predicted vs. actual probability of having low GHS (Figure S2).
Internal validation was carried out with a set of 100 bootstrap resample. Bootstrap resampling provided a corrected mean Nagelkerke’s R2 index of 11%, and C-statistics of 65%, slightly inferior to the original values.
The three variables were used to generate a nomogram with the following scoring system: BMI ≤23 =77 points, females =72 points, age >65 years =100 points (Figure 1).
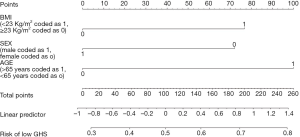
According to the nomogram, a total score ≥72 (presence of at least one of the three risk factors) was associated with a predicted risk >28% of having a low GHS. Female patients with a BMI ≤23 and older than 65 years (all three risk factors present) had a predicted risk of 79% of reporting low GHS.
A ROC curve analysis was performed to define the sensitivity and specificity of the 28% risk threshold.
The nomogram-derived 28% risk cut-off was associated with a sensitivity of 90% and a specificity of 34% (Figure 2).
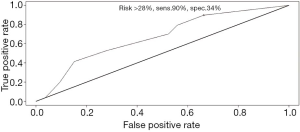
In the study population, patients with a predicted risk >28% had an actual prevalence of low GHS of 58% as compared to 23% of patients with a predicted risk of ≤28%, (odds ratio 3.54, P<0.0004). The actual prevalence of low GHS among patients with all three factors was 74%.
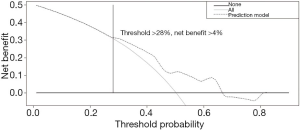
To determine the net benefit of using the generated nomogram to select patients in greater need of full HRQoL evaluation and appropriate supportive care strategies, a decision curve analysis was performed (Figure 3). By using the 28% risk threshold, there was an added net benefit of ≥4% of patients adequately identified as low GHS patients as compared to the ‘including all’ policy.
Discussion
In the present pilot study, we set up a quality of life nomogram using simple and readily available variables at baseline. The nomogram had the objective of increasing the awareness on mCRC patient features at risk of deteriorated quality of life. Even though HRQoL is widely recognized as an important outcome to take into consideration, it is not yet broadly and routinely measured in Oncology Units and clinical instruments predicting HRQoL have seldom been implemented in daily practice (33,34).
Assessment of HRQoL should represent a crucial aspect in the decision-making process as the most ‘patient-centric’ parameter, however it is often neglected in routine practice even if it does not require particular facilities or devices.
To the best of our knowledge, this is the first quality of life nomogram specifically built for patients with mCRC candidate for a first-line chemotherapy. Using nomograms may, at times, be laborious and time-consuming, however the availability of specific apps on mobile devices or tablets has significantly improved this process. Nevertheless, our nomogram is quite simple and easy to apply. It is based on dichotomized variables and the final output can be summarized by stating that the presence of at least one of three possible risk factors (female sex, age >65 years or BMI ≤23) puts the patient at risk of poor HRQoL. The AUC (67%) and the added net benefit at a risk cutoff of 28% (4%) should be regarded as sub-optimal, however the main intent of the nomogram is to rapidly screen patients who would need a second-level evaluation with minimization of the risk of missing true positive cases. The sensitivity of the model was indeed as high as 90%.
The clinical relevance and practical usefulness of the nomogram is linked to the fact that in many centers HRQoL assessment is not universally carried out during the initial patient consultation, whilst it is an issue that may arise later after treatment start. Information regarding sex, BMI and age are universally available in medical records, and, based on our nomogram, can be rapidly used to identify mCRC patients for whom full HRQoL assessment should be considered indispensable. The nomogram might possibly be incorporated into electronic medical records with automatic warning signal to prompt HRQoL questionnaire administration. The ‘on-treatment’ monitoring of HRQoL was beyond the scope of the present study, however the authors recognize that it is another area of clinical interest, which warrants specific study designs.
A recently reported population-based HRQoL assessment at 12 to 36 months of colorectal cancer diagnosis, revealed that as many as 65% of patients reported one or more HRQoL problems (16). In this report, which included more than 20000 patients (including both metastatic patients and patients with localized disease), advanced age (>85 years) and female sex were confirmed to be poor HRQoL predictors, with results comparable to those found in our cohort. BMI was not investigated.
In another study by Gray et al., nearly 500 colorectal cancer patients with either localized or advanced disease were asked to complete the EORTC-QLQ-C30. Female sex was found to be significantly associated with the global health status (P<0.001), but, taken on its own, had little correlation with the outcome in a multiple linear correlation model (correlation coefficient R2 =0.064). No integrated model was built in this study (35).
The impact of age and sex on HRQoL was also demonstrated in other cancer settings (36), while the impact of BMI on quality of life is under-reported. In mCRC patients low BMI is associated with sarcopenia and poorer clinical condition, and this is the probable explanation of the low HRQoL found for BMI <23 in our cohort (37-39).
Lis et al. looked at the association between nutritional status, BMI and HRQoL across different cancer types in a systematic review. Twenty-four studies were selected with the majority being on gastrointestinal cancer (8 studies). Poor nutritional status (which was associated with sarcopenia and low BMI) was confirmed to be a strong predictor of poor HRQoL in patients with cancer (40).
BMI, sex and age have also been used, together with other socio-economic, lifestyle and treatment-related factors, to predict quality of life in colorectal cancer survivors long after the diagnosis (~5–6 years after the initial diagnosis) (41).
In our study, patients were approached either in the outpatient clinic before the visit or at the moment of the hospital admission, and the close interaction with Health professionals in the period immediately after questionnaire administration yielded an impressively high response rate (96%), thus minimizing non-response bias (42). The nomogram we built included variables all independently associated with poor HRQoL (age >65 years, female sex and BMI <23 kg/m2). The presence of at least one of the three risk factors was associated with a predicted risk of poor HRQoL >28% and selection of patients according to this criterion was sufficient to provide a net benefit superior to an ‘all-inclusive’ policy when deciding who requires full HRQoL assessment.
A major drawback of our study is the absence of external validation, however the homogeneity of the patient group (all patients with mCRC who were chemotherapy naïve but fit for first-line chemotherapy) and the internal validation by bootstrapping partly made up for this limitation.
Detection of deteriorated HRQoL overall or in specific areas should always be accompanied with defined intervention plans. Research on effective supportive strategies (nutrition, pain control, physical activity, etc.) was not included in the present pilot study. However, authors acknowledge that explorative analyses should always look at possible therapeutic implications. Future development of the present research will include also supportive care algorithms, in particular during anticancer therapy.
Conclusions
In conclusion, we were able to build a model which predicts the risk of having a deteriorated baseline quality of life in patients with mCRC, using basic anthropometric and lifestyle parameters.
Our results indicate that special attention ought to be paid to elderly, females or low weight patients. Increasing awareness of patients at risk of poor HRQoL will help in the direction of systematic and accurate implementation of quality of life assessment in daily practice and prioritization of supportive care strategies. Validation of the present nomogram in independent cohorts and in oncological settings other than mCRC is also underway.
Acknowledgments
AN has carried out the present study within the Ph.D. program in Experimental and Systems Medicine, XXXII cycle, ‘Tor Vergata’ University of Rome.
C.M. has conducted the present study within the PhD Programme on Experimental System and Medicine (XXXV cycle) at the University of Rome Tor Vergata.
We thank Dr. Christine Tracey for revising the English and contributing to the clarity of the information provided in the present article.
Funding: This work was partially supported by the European Social Fund, under the Italian Ministry of Economic Development (“HORIZON 2020” PON I&C 2014-2020 – F/050383/01-03/X32).
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/apm-20-2194
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2194
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2194). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Local Ethics and Scientific Committees (local Ethics Committee Approval ID: R.S. 102/17) and performed in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all individual participants included in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Bottomley A, Pe M, Sloan JSetting International Standards in Analyzing Patient-Reported Outcomes and Quality of Life Endpoints Data (SISAQOL) consortium, et al. Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards. Lancet Oncol 2016;17:e510-4. [Crossref] [PubMed]
- Higginson IJ, Gomes B, Calanzani N, et al. Project PRISMA. Priorities for treatment, care and information if faced with serious illness: a comparative population-based survey in seven European countries. Palliat Med 2014;28:101-10. [Crossref] [PubMed]
- Jacot W, Colinet B, Bertrand D, et al. OncoLR health network. Quality of life and comorbidity score as prognostic determinants in non-small-cell lung cancer patients. Ann Oncol 2008;19:1458-64. [Crossref] [PubMed]
- Efficace F, Biganzoli L, Piccart M, et al. EORTC-BCG-IDBBC-NDDG. Baseline health-related quality-of-life data as prognostic factors in a phase III multicentre study of women with metastatic breast cancer. Eur J Cancer 2004;40:1021-30. [Crossref] [PubMed]
- Mol L, Ottevanger PB, Koopman M, et al. The prognostic value of WHO performance status in relation to quality of life in advanced colorectal cancer patients. Eur J Cancer 2016;66:138-43. [Crossref] [PubMed]
- Gupta D, Braun DP, Staren ED. Prognostic value of changes in quality of life scores in prostate cancer. BMC Urol 2013;13:32. [Crossref] [PubMed]
- Schuurhuizen CSEW, Braamse AMJ, Konings IRHM, et al. Does severe toxicity affect global quality of life in patients with metastatic colorectal cancer during palliative systemic treatment? A systematic review. Ann Oncol 2017;28:478-86. [Crossref] [PubMed]
- Basch E, Deal AM, Kris MG, et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J Clin Oncol 2016;34:557-65. [Crossref] [PubMed]
- Dueck AC, Mendoza TR, Mitchell SANational Cancer Institute PRO-CTCAE Study Group, et al. Validity and Reliability of the US National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 2015;1:1051-9. [Crossref] [PubMed]
- LeBlanc TW, Abernethy AP. Patient-reported outcomes in cancer care – hearing the patient voice at greater volume. Nat Rev Clin Oncol 2017;14:763-772. [Crossref] [PubMed]
- Kaasa S, Loge JH, Aapro M, et al. Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol 2018;19:e588-653. [Crossref] [PubMed]
- Cull AM. Cancer-specific quality of life questionnaires: the state of the art in Europe. Eur J Cancer 1997;33:S3-7. [Crossref] [PubMed]
- Kotronoulas G, Kearney N, Maguire R, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol 2014;32:1480-501. [Crossref] [PubMed]
- Downing A, Morris EJ, Richards M, et al. Health-related quality of life after colorectal cancer in England: a patient-reported outcomes study of individuals 12 to 36 months after diagnosis. J Clin Oncol 2015;33:616-24. [Crossref] [PubMed]
- Rønning B, Wyller TB, Nesbakken A, et al. Quality of life in older and frail patients after surgery for colorectal cancer-A follow-up study. J Geriatr Oncol 2016;7:195-200. [Crossref] [PubMed]
- Piro MC, Tesauro M, Lena AM, et al. Free-amino acid metabolic profiling of visceral adipose tissue from obese subjects. Amino Acids 2020;52:1125-37. [Crossref] [PubMed]
- Candi E, Tesauro M, Cardillo C, et al. Metabolic profiling of visceral adipose tissue from obese subjects with or without metabolic syndrome. Biochem J 2018;475:1019-35. [Crossref] [PubMed]
- Huang Z, Huang L, Waters MJ, et al. Insulin and Growth Hormone Balance: Implications for Obesity. Trends Endocrinol Metab 2020;31:642-54. [Crossref] [PubMed]
- Tesauro M, Schinzari F, Rovella V, et al. Tumor necrosis factor-alpha antagonism improves vasodilation during hyperinsulinemia in metabolic syndrome. Diabetes Care 2008;31:1439-41. [Crossref] [PubMed]
- Schinzari F, Tesauro M, Cardillo C. Increased endothelin-1-mediated vasoconstrictor tone in human obesity: effects of gut hormones. Physiol Res 2018;67:S69-81. [Crossref] [PubMed]
- Schinzari F, Tesauro M, Veneziani A, et al. Favorable Vascular Actions of Angiotensin-(1-7) in Human Obesity. Hypertension 2018;71:185-91. [Crossref] [PubMed]
- Schinzari F, Tesauro M, Cardillo C. Endothelial and Perivascular Adipose Tissue Abnormalities in Obesity-Related Vascular Dysfunction: Novel Targets for Treatment. J Cardiovasc Pharmacol 2017;69:360-8. [Crossref] [PubMed]
- Schinzari F, Iantorno M, Campia U, et al. Vasodilator responses and endothelin-dependent vasoconstriction in metabolically healthy obesity and the metabolic syndrome. Am J Physiol Endocrinol Metab 2015;309:E787-92. [Crossref] [PubMed]
- Campia U, Tesauro M, Di Daniele N, et al. The vascular endothelin system in obesity and type 2 diabetes: pathophysiology and therapeutic implications. Life Sci 2014;118:149-55. [Crossref] [PubMed]
- Formica V, Tesauro M, Cardillo C, et al. Insulinemia and the risk of breast cancer and its relapse. Diabetes Obes Metab 2012;14:1073-80. [Crossref] [PubMed]
- Sanchez A, Furberg H, Kuo F, et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol 2020;21:283-93. [Crossref] [PubMed]
- Pamoukdjian F, Aparicio T, Canoui-Poitrine F, et al. Obesity survival paradox in cancer patients: Results from the Physical Frailty in older adult cancer patients (PF-EC) study. Clin Nutr 2019;38:2806-12. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Fayers PM, Aaronson NK, Bjordal K, et al. The EORTC QLQ-C30 Scoring Manual. 3rd ed. Brussels, Belgium: European Organisation for Research and Treatment of Cancer; 2001.
- Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making 2006;26:565-74. [Crossref] [PubMed]
- Chipman JJ, Sanda MG, Dunn RLPROST-QA Consortium, et al. Measuring and predicting prostate cancer related quality of life changes using EPIC for clinical practice. J Urol 2014;191:638-45. [Crossref] [PubMed]
- Engelhardt EG, Révész D, Tamminga HJ, et al. Clinical Usefulness of Tools to Support Decision-making for Palliative Treatment of Metastatic Colorectal Cancer: A Systematic Review. Clin Colorectal Cancer 2018;17:e1-12. [Crossref] [PubMed]
- Gray NM, Hall SJ, Browne S, et al. Modifiable and fixed factors predicting quality of life in people with colorectal cancer. Br J Cancer 2011;104:1697-703. [Crossref] [PubMed]
- Hechtner M, Eichler M, Wehler B, et al. Quality of Life in NSCLC Survivors – A Multicenter Cross-Sectional Study. J Thorac Oncol 2019;14:420-35. [Crossref] [PubMed]
- Broughman JR, Williams GR, Deal AM, et al. Prevalence of sarcopenia in older patients with colorectal cancer. J Geriatr Oncol 2015;6:442-5. [Crossref] [PubMed]
- Choi MH, Oh SN, Lee IK, et al. Sarcopenia is negatively associated with long-term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle 2018;9:53-9. [Crossref] [PubMed]
- Sun G, Li Y, Peng Y, et al. Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis 2018;33:1419-27. [Crossref] [PubMed]
- Lis CG, Gupta D, Lammersfeld CA, et al. Role of nutritional status in predicting quality of life outcomes in cancer--a systematic review of the epidemiological literature. Nutr J 2012;11:27. [Crossref] [PubMed]
- Révész D, van Kuijk SMJ, Mols F, et al. Development and internal validation of prediction models for colorectal cancer survivors to estimate the 1-year risk of low health-related quality of life in multiple domains. BMC Med Inform Decis Mak 2020;20:54. [Crossref] [PubMed]
- Wakefield CE, Fardell JE, Doolan EL, et al. Participation in psychosocial oncology and quality-of-life research: a systematic review. Lancet Oncol 2017;18:e153-65. [Crossref] [PubMed]


