
Retrospective analysis of factors associated with serum levels of fibroblast growth factor-21 in patients with diabetes
Introduction
The key roles of fibroblast growth factor-21 (FGF-21) in multiple pathophysiological processes of the human body have been increasingly evidenced. Preliminary basic research by our research group proved that FGF-21 can inhibit vascular calcification via diverse mechanisms (1-4). At the same time, studies have found elevated levels of serum FGF-21 to be associated with an increased risk of coronary heart disease (5-8); although, Chou et al. reported that serum FGF-21 levels are unable to predict cardiovascular events in the general population without a history of cardiovascular disease (8). Chou et al. also found that serum FGF-21 levels bore no relationship with carotid artery intima-media thickness, ankle-brachial index, or coronary artery calcification (8). However, other studies are inconsistent with this. For instance, the levels of serum FGF-21 in patients with hypertension, myocardial infarction, and heart failure have been reported by some researchers to be significantly increased. On the contrary, myocardial ischemia has also been associated with a decrease in circulating FGF-21 levels (8-10). Thus, it can be seen that a large number of studies have produced complex results, which often show inter-study inconsistency and contradictions. We believe that one of the reasons for the variability in study results to date is likely to be related to different conditions, physiques, living habits, and medications of patients. At the same time, on the basis of previous research, we know that there are many risk factors for myocardial infarction.
A variety of diseases and factors, including genetic and environmental factors, may cause myocardial infarction (11-13). These factors can dilute the predictive value of a certain risk factor, and only those that are most closely related to coronary atherosclerotic plaque, such as blood pressure and blood lipids, are able to predict the risk of myocardial infarction (14-16). Even in the best designed clinical studies, patients are sometimes observed to have multiple risk factors but no obvious coronary stenosis, which shows risk factors to be only a statistical concept. At present, the era of precision medicine is ongoing, and genetic testing can accurately evaluate and predict the occurrence of diseases and treatment effects. Individualization is one feature of precision medicine, which relates to different patients having different evaluation strategies and indicators (17,18). Therefore, in this study, we aimed to screen out specific predictors for specific patients. It is necessary to gradually narrow the scope of research to a certain type of patient.
Years of research have shown conditions such as hypertension, coronary heart disease, liver disease, and kidney disease to have a certain degree of association with FGF-21 levels; however, FGF-21 is not helpful for the clinical diagnosis, treatment, evaluation, and prediction of these conditions, so it cannot be applied in clinical practice. However, FGF-21 has been evidenced to be more closely related to diabetes. Thus, research to further investigate FGF-21 in diabetic patients is called for. Moreover, as diabetes is etiologically and mechanistically complicated, and can be divided into many subtypes, subdivision of diabetic patients could prove to be especially worthwhile in illuminating the extent of the relationship of FGF-21 with different subtypes (18). As part of such research, it is important to consider the factors associated with circulating FGF-21 levels in patients with diabetes. Therefore, to preliminarily test this conjecture and to examine the influence of related factors on serum FGF-21 levels in patients with diabetes, we further analyzed the relationship of serum FGF-21 levels with medication status, exercise status, and body mass index (BMI) in patients with diabetes. We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/apm-21-525).
Methods
Study population
Patients with diabetes who were treated in Beijing Aerospace General Hospital from August 2013 to February 2018 were included. The inclusion criteria for patients were: (I) type 2 diabetes; (II) aged ≥18 years; (III) had received medication for hyperglycemic treatment; (IV) complete clinical data; (V) provided signed informed consent; and (VI) frozen blood samples were kept on admission. The exclusion criteria were: (I) connective tissue disease; (II) malignant tumor; (III) heart failure; (IV) patient had decreased renal function or was undergoing dialysis; (V) active liver disease; (VI) pregnancy; (VII) use of diuretics; and (VIII) drug therapy for diabetes had not been initiated. This study was approved by the Ethics Committee of Beijing Aerospace General Hospital (No. 2003-02). All procedures involving human participants in this study were performed in accordance with the Declaration of Helsinki (as revised in 2013). The requirement for individual consent for this retrospective analysis was waived.
Data collection
After enrollment, patient information including medical history, medication (glucose-lowering drugs, lipid-lowering drugs, angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists), lifestyle habits (smoking, alcohol consumption, and other unhealthy habits), as well as the results of physical and auxiliary examination, and laboratory tests. Considering that the medication statuses of most patients are complex and change regularly, it was impossible to determine the specific use time and dose of a drug. Therefore, we only recorded whether or not a drug was used and did not distinguish the specific drug dose. The main clinical factors included: duration of diabetes, the absence or presence of cardiovascular and cerebrovascular diseases, height, weight, blood glucose level, glycosylated hemoglobin level, blood pressure, blood lipids level, electrolytes, liver indicators (such as alanine aminotransferase, aspartate aminotransferase, total bilirubin, direct bilirubin protein, albumin, and globulin), renal function indicators (serum creatinine, urea nitrogen, and uric acid), cardiac ultrasound parameters (mainly including cardiac structure indicators, such as left ventricular end-diastolic diameter, and cardiac function indicators such as left ventricular ejection fraction). Blood for the FGF-21 test was acquired at enrollment and stored at 80 C after centrifugation. At the end of this study, FGF-21 was evaluated for each patient by enzyme-linked immune absorption using a kit bought from TaKaRa (Kyoto, Japan).
Statistical analysis
SPSS 17.0 statistical software (Illinois, Chicago, USA) was used for all statistical analyses. When the data conformed to a normal distribution, it was expressed as the mean ± standard deviation, and the t test was used for between-group comparison. When the data did not conform to a normal distribution, the rank-sum test was used. Qualitative data were expressed as the rate or composition ratio, and between-group comparison was performed with the χ2 test or Fisher’s exact test. Univariate and multivariate regression analyses were carried out to screen the factors related to serum FGF-21 level. P<0.05 indicated that a difference was statistically significant.
Results
Baseline characteristics of the enrolled patients
According to the inclusion and exclusion criteria, 2,118 patients with type 2 diabetes were included in the study. The patients were aged from 43 to 71 years, with the average age being (51.3±11.9) years old. Among them, there were 1,307 males (61.7%) and 811 females (38.3%), for whom the duration of diabetes ranged from 0.5 to 17 years, with an average duration of (4.2±2.7) years. Many patients in the group had hypertension, chronic obstructive pulmonary disease, chronic kidney disease, or history of stroke (and its sequelae), so most patients were taking multiple drugs concurrently, including antihypertensive drugs, lipid-lowering drugs, glucose-lowering drugs, antiplatelet drugs, anticoagulants, and drugs to protect kidney function. Therefore, in this study, it was impossible to analyze each specific drug in detail, and only the relationship between the category of drug and the circulating FGF-21 levels of patients could be analyzed. See Table 1 for details.
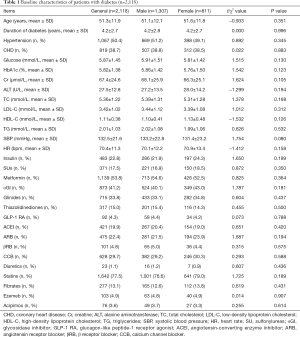
Full table
The relationship between drug use and serum FGF-21 levels in diabetic patients
The patients were divided into 3 groups according to the levels of serum FGF-21: low, medium, and high. The medication status of patients with low, medium, and high levels of serum FGF-21 is shown in Table 2. From the results of the overall analysis of drug types, it could be seen that in most cases, there was no clear relationship between the use of a drug and the levels of FGF-21. Furthermore, among patients with high levels of FGF-21, the proportion of obese patients was significantly higher than that in the groups with low or medium levels of FGF-21. After further grouping the patients according to BMI, we found that the levels of FGF-21 in obese patients were significantly higher than those of patients with a normal or low BMI. However, there was no statistical difference between the circulating levels of FGF-21 in patients with a low BMI and those with a normal BMI (Table 3). At the same time, we also observed that in the high-level group, the proportion of patients who did a large amount of exercise was significantly increased compared to the other 2 groups. Further grouped analysis based on the amount of exercise revealed that the greater the amount of exercise a patient did, the higher the level of serum FGF-21 (Table 4).
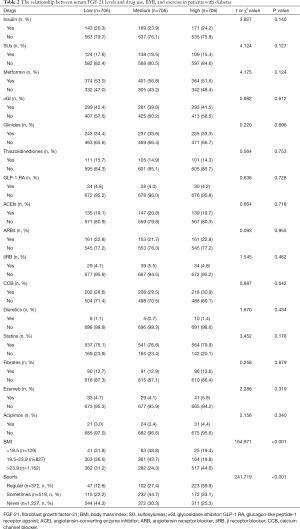
Full table
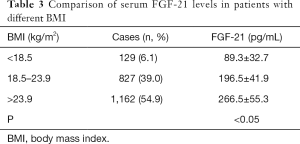
Full table
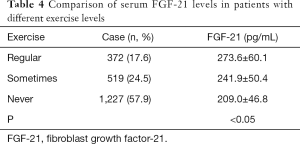
Full table
Discussion
The relationship between drugs and blood test results is a commonly arising and important clinical issue. Many blood parameters can serve as indicators of drug efficacy. For instance, the effective treatment of heart failure is evidenced by a continuous downward trend in plasma brain natriuretic peptide (18). Similarly, C-reactive protein gradually declines to normal levels with effective antibiotic treatment or inflammation control (19). Meanwhile, the success of some other drugs is indicated by an upward trend in certain indicators (20-22). Therefore, investigating the influence of various drugs on the expression levels of key molecules involved in pathophysiological processes can provide a basis for subsequent intervention strategies.
As mentioned earlier, FGF-21 may play an important role in the process of vascular calcification. Therefore, it is of great clinical significance to explore the effects of drugs frequently used in clinical practice on the expression of FGF-21. However, relatively few studies on the effects of drugs on FGF-21 expression have been conducted. Lee et al. subcutaneously injected recombinant insulin secretagogue into the abdomen of patients with type 2 diabetes and found that it reduced the patients’ levels of serum FGF-21 (23). Another study reported that rosiglitazone could reduce the serum levels of FGF-21 and improve insulin resistance in patients with diabetes (24). A study investigating the effects of metformin on the expression of serum FGF-21 noted that metformin elevated the serum levels of FGF-21 in rats fed a normal diet (25). Meanwhile, in another study, the non-sulfonylurea secretagogue mitiglinide was observed to reduce the serum FGF-21 levels of patients with type 2 diabetes; the authors proposed that mitiglinide may reduce plasma FGF-21 expression in diabetic patients through alleviating metabolic disorders and improving insulin resistance (26). In their study, Yang et al. found that liraglutide could increase the levels of FGF-21 in mice, thereby improving the resistance of the mice to “FGF-21” (27). Unlike Yang et al.’s mouse study, a clinical study involving patients with diabetes found that liraglutide use had no significant impact on serum FGF-21 levels (28). However, there is much inconsistency among the results of studies on this drug. For instance, Patel et al. found that the serum FGF-21 levels of newly diagnosed obese diabetic patients decreased after liraglutide was used (29), which was consistent with results reported by Lynch et al. (30). From all of these reports, it is evident that investigations of FGF-21 levels in diabetic patients have produced extremely inconsistent results to date. Furthermore, there is a lack relevant research on the relationship of circulating FGF-21 levels with drugs other than those described above. Thus, further investigations to address these inconsistencies and gaps in the knowledge would be worthwhile.
Through retrospective analysis, the current study demonstrated that the types of drugs commonly used to treat diabetes have no significant effect on the expression levels of circulating FGF-21 in diabetic patients. However, this result is inconsistent with those of some other studies. Possible explanations for our present result may be: (I) the drugs studied do not have a significant effect on the expression of circulating FGF-21 in humans. (II) The interactions between multiple drugs cancelled out the effects on FGF-21 expression in our patients. (III) Drugs may affect the expression levels of FGF-21 in tissues but not at the circulatory level. (IV) The large sample size, other factors, and the large number of drugs being taken concurrently by patients in our study concealed the effects of specific drugs on the expression of FGF-21. (V) The target population of the present study differs from those of other studies.
FGF-21 is an important regulator of human liver lipid metabolism. Type 2 diabetes is associated with obesity, lipid metabolism disorders, and other problems, which can result in increased levels of circulating FGF-21. The present study found that obese patients had higher circulating FGF-21 levels than non-obese patients, which is consistent with previous observations (31). However, among our diabetic patients, we also found that the greater the amount of exercise, the higher the circulating FGF-21 levels, which is consistent with the findings of related studies (32,33). However, there appears to be some contradiction regarding FGF-21 levels being high in both patients who were obese and patients who exercised regularly. Firstly, the elevation in the plasma levels of FGF-21 in obese patients suggests that high levels of FGF-21 may be an unfavorable factor in the human body. However, exercise, which is especially beneficial to patients with diabetes and obesity, also increased the level of FGF-21. Thus, it is unclear whether a high level of FGF-21 is a risk factor or a protective factor for diabetic patients. Secondly, if FGF-21 elevation is a protective factor, then the increase in its circulatory levels may be a feedback protection in obese patients. If this mechanism exists, then patients with diabetes and obesity should increase their levels of physical exercise, which is not consistent with current mainstream views. However, currently, this feedback mechanism is only a conjecture, and its verification through experimental research is a direction and focus of future study.
Based on the results of previous studies, it is difficult to draw a conclusion about whether circulating FGF-21 is increased or decreased in diabetic patients, or if it is a protective factor. Certain drugs which have been proven by multiple large-scale clinical trials to reduce patient risk and improve prognosis have also been shown to decrease circulating FGF-21 levels in patients, including liraglutide and metformin (34,35). However, the specific mechanisms of the overall benefit of these drugs may not be related to their effects on FGF-21. Meanwhile, other drugs have been shown to increase the levels of FGF-21 in in vivo studies; however, the results have been very inconsistent. The results of this study and other studies show that exercise can increase the levels of FGF-21 in diabetic patients, which contradicts the reduction in FGF-21 expression levels seen with the use of drugs. Therefore, follow-up research should be conducted to further analyze whether the effects of various factors directly or indirectly lead to changes in the expression levels of circulating FGF-21 in patients with diabetes. The value of retrospective research is to provide hint or clue for further studies. Solid evidence mainly come from RCTs or experimental studies. To overcome this problem, we should carry out prospective studies in the future.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-525
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-525
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-525). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Beijing Aerospace General Hospital (No. 2003-02). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Shi Y, Lu W, Hou Y, et al. Fibroblast growth factor 21 ameliorates vascular calcification by inhibiting osteogenic transition in vitamin D3 plus nicotine-treated rats. Biochem Biophys Res Commun 2018;495:2448-55. [Crossref] [PubMed]
- Shi Y, Wang S, Peng H, et al. Fibroblast Growth Factor 21 Attenuates Vascular Calcification by Alleviating Endoplasmic Reticulum Stress Mediated Apoptosis in Rats. Int J Biol Sci 2019;15:138-47. [Crossref] [PubMed]
- Cao F, Liu X, Cao X, et al. Fibroblast growth factor 21 plays an inhibitory role in vascular calcification in vitro through OPG/RANKL system. Biochem Biophys Res Commun 2017;491:578-86. [Crossref] [PubMed]
- Liu X, Cao F, Liu S, et al. BMP2/Smad signaling pathway is involved in the inhibition function of fibroblast growth factor 21 on vascular calcification. Biochem Biophys Res Commun 2018;503:930-7. [Crossref] [PubMed]
- Xiao Y, Liu L, Xu A, et al. Serum fibroblast growth factor 21 levels are related to subclinical atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol 2015;14:72. [Crossref] [PubMed]
- Wu L, Qian L, Zhang L, et al. Fibroblast Growth Factor 21 is Related to Atherosclerosis Independent of Nonalcoholic Fatty Liver Disease and Predicts Atherosclerotic Cardiovascular Events. J Am Heart Assoc 2020;9:e015226 [Crossref] [PubMed]
- Basurto L, Gregory MA, Hernández SB, et al. Monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-21 (FGF-21) as biomarkers of subclinical atherosclerosis in women. Exp Gerontol 2019;124:110624 [Crossref] [PubMed]
- Chou RH, Huang PH, Hsu CY, et al. Circulating Fibroblast Growth Factor 21 is Associated with Diastolic Dysfunction in Heart Failure Patients with Preserved Ejection Fraction. Sci Rep 2016;6:33953. [Crossref] [PubMed]
- Zhang W, Chu S, Ding W, et al. Serum Level of Fibroblast Growth Factor 21 Is Independently Associated with Acute Myocardial Infarction. PLoS One 2015;10:e0129791 [Crossref] [PubMed]
- Semba RD, Crasto C, Strait J, et al. Elevated serum fibroblast growth factor 21 is associated with hypertension in community-dwelling adults. J Hum Hypertens 2013;27:397-9. [Crossref] [PubMed]
- Palasubramaniam J, Wang X, Peter K. Myocardial Infarction-From Atherosclerosis to Thrombosis. Arterioscler Thromb Vasc Biol 2019;39:e176-85. [Crossref] [PubMed]
- Maglietta G, Ardissino M, Malagoli Tagliazucchi G, et al. Long-Term Outcomes After Early-Onset Myocardial Infarction. J Am Coll Cardiol 2019;74:2113-5. [Crossref] [PubMed]
- Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet 2017;389:197-210. [Crossref] [PubMed]
- Pedersen LR, Frestad D, Michelsen MM, et al. Risk Factors for Myocardial Infarction in Women and Men: A Review of the Current Literature. Curr Pharm Des 2016;22:3835-52. [Crossref] [PubMed]
- Dugani SB, Ayala Melendez AP, Reka R, et al. Risk factors associated with premature myocardial infarction: a systematic review protocol. BMJ Open 2019;9:e023647 [Crossref] [PubMed]
- Gulati R, Behfar A, Narula J, et al. Acute Myocardial Infarction in Young Individuals. Mayo Clin Proc 2020;95:136-56. [Crossref] [PubMed]
- Antman EM, Loscalzo J. Precision medicine in cardiology. Nat Rev Cardiol 2016;13:591-602. [Crossref] [PubMed]
- Petersmann A, Müller-Wieland D, Müller UA, et al. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp Clin Endocrinol Diabetes 2019;127:S1-7. [Crossref] [PubMed]
- Santaguida PL, Don-Wauchope AC, Oremus M, et al. BNP and NT-proBNP as prognostic markers in persons with acute decompensated heart failure: a systematic review. Heart Fail Rev 2014;19:453-70. [Crossref] [PubMed]
- Butler CC, Gillespie D, White P, et al. C-Reactive Protein Testing to Guide Antibiotic Prescribing for COPD Exacerbations. N Engl J Med 2019;381:111-20. [Crossref] [PubMed]
- Kumar S, Rai H, Kapoor A, et al. Pharmacological measures to increase HDL-C among high risk isolated low HDL cases: a randomized study amongst north Indians. Indian J Med Res 2013;138:873-81. [PubMed]
- Najafipour M, Zareizadeh M, Khokhi MA, et al. Comparative study of the effect of atorvastatin and fenofibrate on high-density lipoprotein cholesterol levels in patients with type 2 diabetes. J Adv Pharm Technol Res 2018;9:135-8. [Crossref] [PubMed]
- Lee J, Hong SW, Park SE, et al. Exendin-4 regulates lipid metabolism and fibroblast growth factor 21 in hepatic steatosis. Metabolism 2014;63:1041-8. [Crossref] [PubMed]
- Li K, Li L, Yang M, et al. Effects of rosiglitazone on fasting plasma fibroblast growth factor-21 levels in patients with type 2 diabetes mellitus. Eur J Endocrinol 2009;161:391-5. [Crossref] [PubMed]
- Fan H, Sun X, Zhang H, et al. Effect of Metformin on Fibroblast Growth Factor-21 Levels in Patients with Newly Diagnosed Type 2 Diabetes. Diabetes Technol Ther 2016;18:120-6. [Crossref] [PubMed]
- Wang B, Yang G, Yang M, et al. Mitiglinide treatment may decreases plasma fibroblast growth factor-21 levels in individuals with new-onset T2DM. Cytokine 2012;57:300-3. [Crossref] [PubMed]
- Yang M, Zhang L, Wang C, et al. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS One 2012;7:e48392 [Crossref] [PubMed]
- Liu JL, Gao ZH. Does GLP-1 suppress hepatocyte glucose production directly, via fibroblast growth factor 21? EBioMedicine 2019;41:5-6. [Crossref] [PubMed]
- Patel V, Joharapurkar A, Kshirsagar S, et al. Balanced Coagonist of GLP-1 and Glucagon Receptors Corrects Dyslipidemia by Improving FGF21 Sensitivity in Hamster Model. Drug Res (Stuttg) 2017;67:730-6. [Crossref] [PubMed]
- Lynch L, Hogan AE, Duquette D, et al. iNKT Cells Induce FGF21 for Thermogenesis and Are Required for Maximal Weight Loss in GLP1 Therapy. Cell Metab 2016;24:510-9. [Crossref] [PubMed]
- Nonogaki K, Kaji T, Yamazaki T, et al. Pharmacologic stimulation of central GLP-1 receptors has opposite effects on the alterations of plasma FGF21 levels induced by feeding and fasting. Neurosci Lett 2016;612:14-7. [Crossref] [PubMed]
- Staiger H, Keuper M, Berti L, et al. Fibroblast Growth Factor 21-Metabolic Role in Mice and Men. Endocr Rev 2017;38:468-88. [Crossref] [PubMed]
- Kim HJ, Song W. Resistance training increases fibroblast growth factor-21 and irisin levels in the skeletal muscle of Zucker diabetic fatty rats. J Exerc Nutrition Biochem 2017;21:50-4. [Crossref] [PubMed]
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016;375:311-22. [Crossref] [PubMed]
- Zilov AV, Abdelaziz SI, AlShammary A, et al. Mechanisms of action of metformin with special reference to cardiovascular protection. Diabetes Metab Res Rev 2019;35:e3173 [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


