
Research progress on the molecular mechanisms of hepatic metastasis in lung cancer: a narrative review
Introduction
Background
In recent years, lung cancer has been the most invasive disease in China, and about one-third of lung cancer patients have liver metastasis and a poor prognosis. The occurrence and development of various histological types of lung cancer is a multistep, multipath dynamic progression process, involving multiple tumor signal transduction pathways (1). Some studies have shown that certain genes, signaling pathways, chemicals, proteins, RNAs, and cells have the ability to induce cell mutation and proliferation. Many molecules related to lung cancer, such as microRNA (miRNA)-126, transforming growth factor-β (TGF-β), miRNA-338, CSC, and vascular endothelial growth factor (VEGF) enter the blood system and participate in the remote growth process (2,3). The process of liver metastasis in lung cancer includes the transportation, stagnation, and growth of lung cancer cells to the liver (4). In recent years, the use of targeted therapy and precision medicine in treating lung cancer and liver metastasis has increasingly focused on intervening in these 3 steps. Research into the mechanism of metastasis is critical to improving the efficiency of early diagnosis and exploring new treatment models for lung cancer liver metastasis, and thus has considerable clinical significance. In addition, research at the microscopic molecular level may discover the key targets and pathways of lung cancer cells in the process of information transmission and facilitate the quick identification of high-risk signs in the clinical setting for early intervention in lung cancer liver metastasis.
We present the following article in accordance with the Narrative Review reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1675).
Objectives
The aim of the present study was to investigate the issues related to the prognosis of patients with lung cancer metastasis. We reviewed literature published in the past 20 years concerning the molecular mechanism of liver metastasis of lung cancer, including genes, proteins, RNAs, pathways, and chemicals. This review provides relatively complete summary of the tumor prognosis research follow-up data, and offers new directions and focal points for the study of markers in the early diagnosis of lung cancer, etiological treatment, and improvement of existing treatment regimens.
At present, the prognosis of patients with metastatic lung cancer mainly depends on the time of diagnosis and treatment. Most patients with obvious clinical symptoms are diagnosed at an advanced stage, so the survival rate is poor, especially for patients with metastasis. The present study discusses the relevant evidence concerning the markers for the early diagnosis of lung cancer patients that may allow for early diagnosis and early treatment. However, the current clinical treatment of patients with lung cancer metastasis is mostly symptomatic and etiological, and can only relieve the related symptoms. This review begins with a discussion on the mechanism of lung cancer metastasis, focuses on the deep molecular mechanisms, and delineates research ideas and directions for the etiology and treatment of patients with lung cancer metastasis.
The new immune checkpoint inhibitors has been found to improve the survival time for patients with tumors, including Yervoy, Keytruda, Atezolizumab. But some studies have shown that the curative effect of the treatment of lung cancer patients with liver metastasis is weak. To further explore the mechanism behind this, we have reviewed the relevant evidence regarding how genes and proteins impact the curative effect, with the aim of improving treatment options.
Methods
In the present study, our primary focus was the invasion and metastasis of lung cancer. Information was collected from China National Knowledge Infrastructure (CNKI), Wanfang Med Online, China Biology Medicine disc, and EBSCOhost databases using the following keywords: “lung cancer”, “liver metastasis”, “genes”, “proteins”, and “signaling pathways”. According to their relevance to the mechanism of tumorigenesis, the role of 6 molecular levels in the liver metastasis of lung cancer was analyzed. To ensure the review had a certain degree of continuity, currency, and relevance, research from 1999 to 2020 was analyzed, the analyzed literature had an international perspective and included both non-Chinese (27 inclusions in English and 1 in Japanese) and Chinese publications (73 inclusions). In all, there were 86 journal papers and 15 master and doctoral theses, which included the recent work of researchers, scholars, and doctors in recent years, which had high academic credibility derived from sound clinical experience.
We present the following article in accordance with the Narrative Review Checklist.
Discussion
Research progress in hepatic metastasis in lung cancer
Since the 20th century, a number studies on the mechanisms of lung cancer metastasis have been published, and many different molecules have been identified as the central agent in the metastasis of lung cancer (3). More recently, significant progress into the role of genes, proteins, miRNAs, pathways, and chemicals in the process of tumor metastasis has been made, while the focus of studies of hepatic metastasis in lung cancer has shifted to examining the molecular level.
Liver metastasis in lung cancer
The most common methods of invasion and metastasis in lung cancer are tumor cell shedding in the extracellular matrix (ECM), invasion of adjacent tissues and basement membrane, infiltration into blood vessels or lymphatic vessels, transportation through blood and lymph modes, exosmosis at distant sites, and the formation of metastatic foci. These steps depend on tumor stem cells, the proliferation of tumor cells, apoptosis, escape, angiogenesis, circulatory exudation, and the proliferation of distant metastasis (5). The main contents and correlation factors are shown in Table 1. The primary mechanisms for the startup phase, which include the activation of tumor infiltration, bone marrow, the formation of tumor blood vessels, and the loss of cell polarity, are related to the RHoC, LOXVEGF, CSF1, ID1TWIST1, METFGFRMMP 9, and NEDD9, etc. In the transfer phase, the main mechanisms for vascular remodeling, immune evasion, and the seepage of tumor cells are related to genes, including EREG, Cox-2, MMP1, CCL5, and ANGPTL4. At the stage of final colonization and toxic effect, the main mechanisms for corresponding organ-specific function are related to CXCR4, RANKL, CTGF, interleukin 11, and endothelin 1.
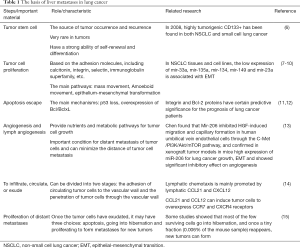
Full table
Tumor stem cells
In recent years, studies have shown that, although tumor stem cells are scarce in tumors, they have a strong ability to self-renew and differentiate. Most researchers believe that tumor stem cells are the source of tumor occurrence and recurrence. Non-small cell lung cancer (NSCLC) metastasis is driven by liver cancer stem cells (LCSCs) and is closely related to the Wnt/β-catenin-FoxM1-twist pathway. A recent study also found that the Notch pathway is involved, and miRNA may regulate CSCs by influencing these pathways (16). Therefore, the proliferation and invasion of lung cancer cells can be controlled by regulating the number of tumor hepatocytes. miRNA-99a is underexpressed in NSCLC tissues, which inhibits epithelial-mesenchymal transition (EMT) and stem cell characterization to reduce the number of tumor stem cells by targeting E2F2 and EMR2. Hsa-miRNA-124a inhibits the stem cell-like characteristics of NSCLC cell lines and enhances sensitivity to gefitinib by targeting the inhibition of ubiquitin-specific protease 14 (USP14). Therefore, hsa-miRNA-124a and USP14 can be used as tumor biomarkers to diagnose NSCLC (17).
Tumor cell proliferation
The diffusion of tumor cells is based on the adhesion molecules between tumor cells and the cell matrix. For example, the mass movement of tumor cells is mainly regulated by the flat foot protein. Amoeboid movement regulates the rupture and dissolution of the ECM through the RhoA/ROCK signaling pathway, causing deformation and movement of tumor cells.
EMT refers to the process by which tumor cells secrete enzymes to degrade the ECM and form platy pods with fixed specific ECM for metastasis, and then move forward under the contraction of actin (18). Recent studies have found a series of EMT-related miRNAs in NSCLC. The upregulated expression of miRNA-128-3p in NSCLC tissues promotes the occurrence of EMT by targeting 2 key transcription factors, SNAIL and ZEB1, indicating that transcription factors play an important role by inducing EMT (5).
Apoptotic escape
The migration and apoptotic escape of tumors are important causes of hepatic metastasis in lung cancer. The process of apoptosis is complex and can be activated under a variety of conditions and signals. The main mechanisms of tumor resistance to apoptosis are p53 loss and the overexpression of Bcl/BclxL (11,12).
Angiogenesis and lymphangiogenesis
In lung cancer, angiogenesis is mainly related to VEGF, nerve growth factor, HIF-1α, and other molecules. The major factors involved in lymphangiogenesis are VEGFC and VEGFD, both of which are induced by inflammatory cytokines and play a role in lymphangiogenesis by binding to VEGFR-2, VEGFR-3, and neuro ciliary proteins on lymphatic capillaries (19). miRNA plays an important regulatory role in tumor angiogenesis: miRNA in tumor cells can influence endothelial cell activity through non-cellular autonomic mechanisms, while miRNA in endothelial cells can regulate the autonomic behavior of cells (20).
Infiltration, circulation, and exudation
The exudation of tumor cells into blood vessels is one of the critical steps of tumor metastasis and can be roughly divided into 2 stages. The first stage is via adhesion and the second stage is through the blood vessel wall. Physical factors and adhesion molecules play an important role in the first stage. Cytokines, critical regulatory molecules of vascular permeability, and cell movement, figure prominently in the second stage (14). Tumor entry into the vascular lumen results from chemokines acting on blood vessels, for instance when tumor-related macrophages aggregate and secrete EGF in blood vessels and at the edges of the tumor, which attracts tumor cells into the blood vessels and promotes vascular chemotaxis. Vascular extravasation is also a form of escape from apoptosis of tumor cells, and its mechanisms re similar to those of the infiltration into blood vessels.
Proliferation of distant metastasis
After circulation and exudation, most cells die. Most of the retained cells lack sufficient blood oxygen, nutrients, and growth factors to form a dormant state, and dormant cells may maintain a balance between proliferation and apoptosis for a long period (15). This may be the cause of tumor recurrence.
Gene and lung cancer metastasis
Many genes are involved in the regulation of hepatic metastasis in lung cancer, and determine whether or not malignant lung tumors metastasize.
As seen in Table 2, some genes play dominant roles in lung cancer metastasis. The role played by each gene is summarized and outlined below. The genes involved in lung cancer metastasis have been divided into those that inhibit tumor metastasis and those that cause tumor metastasis.
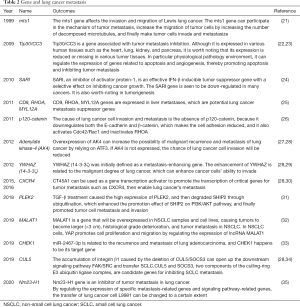
Full table
In 1999, Xu et al. found that the multiple tumor suppressor 1 (mts1) gene promotes the breakdown of microtubules, thereby increasing tumor cell mobility (21). In 2011, Zhang et al. found that the p120 catenin gene reduces the adhesion of cells, which is believed to contribute to the metastasis of lung cancer cells (23). Yang’s research in 2016 showed that CT45A1 could activate the migration and invasion ability of CXCR4, which is a crucial gene for lung cancer tumor metastasis (28,30). In 2012, Pozuelo-Rubio found that YWHAZ can enhance the invasive ability of cancer cells (29). In 2018, Deng et al. found that a series of changes caused by the high expression of PLEK2 could promote the metastasis of lung cancer tumor cells (31). In 2019, Dai found that CHEK1 was related to the recurrence and metastasis of lung adenocarcinoma (33). In the same year, Zhou et al. showed that the regulation of long non-coding RNA (lncRNA) MALAT1 expression could also promote lung cancer cell metastasis (32).
The mechanism by which genes inhibit tumor metastasis mainly involves the expression of genes related to angiogenesis in a specific physiological and pathological environment, and the inducement of apoptosis (30). In 2009, Tong et al. found that the Tip30/CC3 gene was responsible for tumor metastasis suppression in various human tissues (22,23). Studies by Dash and others in 2010 showed that the downregulation of the SARI gene weakened IFN-β–induced tumor suppression (24). In 2009, the CD9, RHOA, and MYL12A genes, and in 2020, the Nm23-H1 gene, were found to be expressed in liver metastasis, and may be potential lung cancer suppressor genes in hepatic metastasis (25,32,35). It can cause the invasion and change of transfer. In 2019, CUL5 and SOCS3 were identified as candidate genes for inhibiting small cell lung cancer (SCLC) transfer (28,34).
Different genes will have varying effects on lung cancer metastasis depending on their under expression or overexpression. In 2012, Jan et al. found that AK4 promotes the metastasis of malignant tumors to other organs in an ATF3-dependent manner, and the expression of AK4 is dual (27).
Proliferation, migration, and signaling pathways of lung cancer cells with miRNA
miRNAs are a class of non-coding small molecular RNAs with a length of about 22 nt, which regulate the activity of target genes through gene silencing at the post-transcriptional level. An increasing number of studies suggest that miRNAs are involved in tumor metastasis. Therefore, research on the relationship between miRNA and tumor metastasis has become a focal point for exploring the regulatory mechanisms of liver metastasis in lung cancer. The representative studies on miRNAs in recent years, including on miRNA-216, miRNA-182, miRNA-338, miRNA-451, miRNA-218, and miRNA-448, are summarized in Table 3.

Full table
Metastasis occurrence is a critical factor in the prognosis of lung cancer, and is a complex, multifactor, multistep, and multigene regulatory process. Studies have found that miRNA-126 is differentially expressed in lung cancer tissue versus paracarcinoma tissue (36). Guo et al. reported that transfected miRNA-126 inhibits the proliferation, apoptosis, invasion, and migration of NSCLC by regulating the expression of EGFR, AKT, and mTOR (36). Currently, there are few studies on miRNA-126 in lung cancer. Miko, Edit has proposed that miRNA-126targets SLC7A5 to delay the G1 phase of H69 cells, and therefore inhibits the proliferation of lung cancer cells (37). Crawford et al. reported that miRNA-126 inhibits the invasion and metastasis of NSCLC cell lines by regulating the expression of the CRK protein (38). Liu suggested that miRNA-126 inhibits the proliferation of lung cancer cells by acting on VERE (39). In Tang’s study, miRNA-126 was transfected into A549 lung cancer cells, resulting in its proliferation ability being significantly inhibited. In addition, the upregulated miRNA-126 expression of transfected miRNA-126 A549 cells was experimentally recorded, and the activity, proliferation capacity, invasion, and metastasis of lung cancer A549 cells, along with the EGFR/AKT/mTOR signaling pathway of the cells, were significantly inhibited after miRNA-126 expression was upregulated (40).
Studies have found that the expression of miRNA-182 is significantly related to tumor formation time, TNM stage, lymph node metastasis, and EGFR gene mutation, which suggests that miRNA-182 may be a carcinogen in NSCLC that participates in the regulation of disseminated metastasis of NSCLC cells and targets to drug resistance–related genes to regulate the occurrence and development of NSCLC (40). However, the specific molecular mechanism of miRNA-182 regulation in lung cancer is still unclear. Recent studies have found that miRNA-182 is significantly upregulated in a variety of solid tumors. Lei found that miRNA-182 is highly expressed in breast cancer cells and is negatively correlated with the prognosis of breast carcinoma (41), indicating that miRNA-182 is not a unique indicator of lung cancer.
Studies have shown that the expression of miRNA-338 in lung cancer cells and lung cancer tissues is significantly inhibited. This suggests that the recovery of the expression level of miRNA-338 can significantly inhibit the metastasis of lung cancer cells. This study of Wei confirmed the anti-proliferation effect of miRNA-338 (42). Chen et al.used bioinformatics analysis to identify ITGB3 as a potential target gene of miRNA-338, which was further confirmed by western blot and double luciferase activity assays. By analyzing the data from The Cancer Genome Atlas database, they also found that ITGB3 was negatively correlated with miRNA-338 in 441 lung cancer tissues, which confirmed that ITGB3, a gene associated with tumor metastasis, was a new miRNA-338 target gene (42).
Wang et al. reported that miRNA-451 is the most downregulated miRNA in NSCLC tissues, and acts as a tumor inhibitor in NSCLC by targeting ras-related protein 14. Tian et al. found that miRNA-451 could promote the PTEN protein level in NSCLC cells, leading to increased radiotherapy sensitivity of NSCLC cells (43). Liu et al. confirmed that MIF was a target of miRNA-451 and reduced the proliferation activity, colony formation ability, and migration and invasion of nasopharyngeal carcinoma cells (44). Studies have shown that the expression of miRNA-23a and miRNA-451 is downregulated in NSCLC cell lines by transient transfection. One research group used quantitative reverse transcription polymerase chain reaction (qRT-PCR) to detect the expression levels of SPRY2 and MIF in miRNA-23a cells. The results showed that SPRY2 and MIF might be potential targets of miRNA-23a and miRNA-451 in NSCLC cells. Multi-factor analysis results showed that in miRNA-451, a predictor of poor prognosis in NSCLC, has the multi-function ability of microRNA and is involved in tumor cell proliferation and growth, metastasis, and biological behaviors (45,46).
Studies have suggested that miRNA-218 can influence the proliferation, invasion, and metastasis of tumor cells in lung adenocarcinoma tissues, affecting prognosis, survival and recurrence, and metastasis (47). Luan et al. studied the expression of miRNA-218-1-3-p in NSCLC, and found that miRNA-218-1-3-p was under expressed in NSCLC and negatively correlated with lymph node metastasis (48).
The overexpression of miRNA-448 inhibits the proliferation of NSCLC cells. miRNA448 is under expressed in NSCLC tissues and cells, and inhibits adhesion between A549 cells and HUVEC cells, and the EMT protein levels, invasion levels, and cell migration levels of NSCLC cells (49).
Proteins related to the hepatic metastasis of lung cancer
Representative studies on proteins in recent years are summarized in Table 4.

Full table
Alpha-fetoprotein (AFP) and alkaline phosphatase (ALP)
Under normal circumstances, AFP is only synthesized in the fetal liver. Adults resume the function of producing this protein after hepatocellular carcinoma develops, after which the AFP levels in serum will sharply increase (57). After liver metastasis of NSCLC, the liver tissue cells destroyed by the compression of proliferation can create a large amount of AFPs, resulting in an increase in serum AFP (50). ALP has 6 isoenzymes, 4 of which are produced in placenta and cancer cells (51). Liver metastasis in patients with NSCLC can be reflected by AFP and AIP levels. Hepatitis B virus infection may be a risk factor for liver metastasis in patients with NSCLC. If patients with NSCLC have high levels of AFP and ALP, they should be wary of liver metastasis.
Cytokeratin 19 fragment antigen 21-1 (cyfra21-1) and neuron-specific enolase (NSE)
Studies have reported that serum tumor markers cytokeratin 19 fragment antigen 21-1 (cyfra21-1) and NSE are closely related to the risk of hepatic metastasis of lung cancer (58). According to a previously published study, patients with hepatic metastasis of lung cancer have higher levels NSE in serum than patients with metastases elsewhere (59). Other researchers have reported that NSE levels and their positive expression rates in patients with hepatic metastasis of lung cancer were significantly higher than those in patients with other metastatic sites (52). However, it is not clear if the hepatic metastasis of lung cancer is caused by elevated NSE or if the hepatic metastasis of lung cancer increases NSE expression. Thus, the pathogenesis of hepatic metastasis in lung cancer remains unclear. However, if the serum NSE level is significantly increased, it may indicate metastasis in lung cancer patients (53). Some studies have suggested that when cyfra21-1 and NSE levels increase, greater attention should be paid to the risk of distant metastases, especially liver metastasis, and timely and effective measures should be taken. Cyfra21-1 is a tumor marker that is important for the diagnosis of lung cancer (54). Studies have shown that cyfra21-1 has good specificity in the differential diagnosis of benign and malignant lung diseases (55) and is highly sensitive to the spread of lung cancer, especially hepatic metastasis.
Several measures can guide the clinical identification of liver metastasis risk in patients with NSCLC. These include comparing NSCLC patients with and without liver metastasis in regards to their history of hepatitis B virus infection. Other research may involve examining tissue polypeptide-specific antigen and cyfra21-1 of NSCLC markers in vivo, and identifying the differences in NSE levels, AFP, and ALP levels This will provide a reference for future clinical work.
Serum cyfra21-1 and NSE levels of patients with hepatic metastasis of lung cancer are higher than those without hepatic metastasis of lung cancer, which has certain auxiliary value for the clinical diagnosis of lung cancer metastasis. And the detection of serum cyfra21-1 and NSE is a simple process.
Lactate dehydrogenase (LDH)
In recent years, the incidence of lung cancer has risen. Most clinical diagnoses are made at advanced stages, but by this time, treatment is unlikely to be effective (60). At present, tumor markers, including lung cancer cell gene types, proteins, related serum proteins and peptides, have been used as the basis for the diagnosis of lung cancer (61). Tumor markers are products of the aberrant expression of oncogenes or normal genes, and are critical for disease diagnosis, treatment, and prognostic assessment (62). Studies have confirmed that the level of oxidoreductase LDH (redox LDH), which is closely related to glycolysis, is higher in patients with malignant tumors. The increase of LDH is related to the TNM phase of the cancer, such as lung cancer, gastric cancer, esophageal cancer, and distant lymph node metastasis (63). Researchers in China and worldwide use LDH levels as a diagnostic criterion for lymphoma, and some currently use LDH levels for the diagnosis of lung cancer (64).
Lung cancer is a common clinical malignant tumor of the respiratory system, accounting for 12.6% of new cancers and 17.8% of cancer mortality (65). The tumor itself is a malignant disease of wasting. It is reported that even under anaerobic conditions, tumor cells can still undergo a vigorous anaerobic fermentation reaction and consume a large amount of glucose to acquire the necessary energy for proliferation (66). LDH is an important oxidoreductase in anaerobic fermentation. It catalyzes the biotransformation of pyruvate and lactic acid (67). According to different molecular structures, LDH can be divided into 5 isozymes of LDH1–5. Generally, LDH content in cells is higher than the LDH content in serum, and the LDH content in liver, skeletal muscle, cardiac muscle, red blood cells, and kidneys is high. When a tumor develops, the abnormal increase in LDH in serum is mainly due to the damage to the tumor cells themselves, causing the LDH in the cells to overflow into the blood. In addition, after normal cells are invaded or infiltrated by the tumor, the cells are destroyed to release LDH into human blood. It has been therefore speculated that due to the advantages of anaerobic lysis of tumor cells, elevated LDH can be detected in the serum of various tumor tissues. When serum LDH is abnormally elevated, acute myocardial infarction, pulmonary embolism, chronic viral hepatitis, cirrhosis, and liver and hematological malignancies should be excluded, and hepatic metastasis in lung cancer should be considered. In cases where the imaging characteristics have not changed significantly, the presence of distant metastasis of lung cancer can be ascertained by dynamically observing the serum changes of LDH; this method solves the bottleneck in diagnosing the distant metastasis of lung cancer, and thus has considerable clinical value (56).
Signaling pathways and lung cancer metastasis
In gene expression, signaling pathways in molecules act as links to tumor gene expression receptor and are directly involved in tumor metastasis. Inhibiting tumor invasion and promoting tumor metastasis are 2 different roles of pathways in lung cancer metastasis.
There are several signaling pathways that promote tumor metastasis. As seen in Table 5, from 2006 to 2019, considerable advances have been made in the study of the Pl3-AKT-mTOR pathway. After being activated in tumors, it participates in the regulation of cell proliferation, differentiation, and migration. Its mechanism is to interrupt cell adhesion by interrupting the PI3-AKT pathway and inducing tumor neovascularization (68-71).
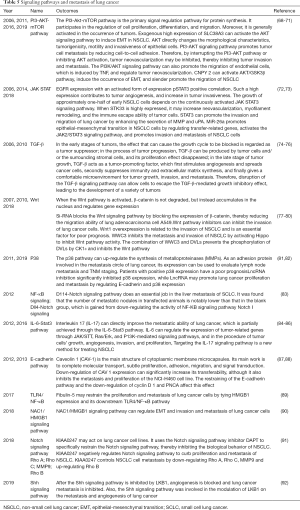
Full table
From 2006 to 2008, Wu et al. studied the JAK-STAT pathway and noted similar conclusions (72). Other previously published studies have found that the increased expression of pSTAT3 increases the activation of EGFR, while the high expression of STK33 induces the neovascularization of tumor cells, enhances the immune escape ability of tumor cells, facilitates the formation of tumor blood vessels, and promotes tumor aggressiveness (73).
Studies by Wu et al. in 2006 and 2010 found that TGF-β provides a favorable microenvironment for metastasis by spreading cancer cells and stimulating cell reproduction (74-76). In 2012 and 2016, Li and Wang also reported that interleukin 6 can realize the construction of corresponding microenvironments through the JAK/STT, Ras/Erk, and P13K-mediated signal pathways (84-86).
In 2011 and 2019, Zhang and Wei et al. suggested that p38 expression upregulates metalloproteinase (MMP) synthesis and promotes lung cancer proliferation and metastasis. lncRNAs can regulate E-cadherin and p38 expression to promote lung cancer proliferation and transfer (81,82).
In 2013, Song et al.’s study found that the downregulation of CAV1 expression restrained the metastasis of the NCI-H460 cell line, thereby curbing the E-cadherin pathway and downregulating cyclin D1 and proliferating cell nuclear antigen (PCNA), but significantly increased E-cadherin’ sability to transfer in vitro (87).
There are several pathways that play a role in suppressing tumor metastasis. Studies from 2007 to 2018 showed that the combination of WWC3 and dishevelled (DVL) proteins could prevent CK1ε phosphorylation, and, if the Wnt signaling pathway was blocked, could inhibit the invasion of lung cancer cells (77-80). A 2012 study by Kuramoto et al. showed that the D114-Notch signaling pathway inhibits liver metastasis in SCLC through a mechanism which involves downregulating the activity of Notch I in the nuclear factor-ĸβ signaling pathway (83). In a 2018 study by Xu, Notch signaling pathway inhibitor DAPT was used to negatively regulate the Notch signaling pathway and downregulate RhoA, RhoC, and MMP9, and upregulate RhoB, thereby inhibiting the metastasis and invasion of NSCLC (91). In addition, in 2019, Zheng et al. also found that after the Sonic hedgehog (Shh) signaling pathway was transplanted by LKB1, Shh’s role in regulating lung cancer metastasis and angiogenesis was also suppressed (92).
Chemicals and hepatic metastasis in lung cancer
Representative studies on chemicals in recent years are summarized in Table 6.
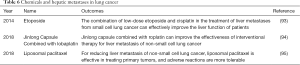
Full table
Etoposide
Researchers have analyzed and compared the clinical efficacy of etoposide combined with carboplatin and etoposide combined with cisplatin. The results of a phase III randomized controlled clinical study of the Greek Cancer Society showed that the two have roughly the same clinical efficacy. The combination of low-dose etoposide and cisplatin in liver metastasis of SCLC can effectively improve the liver function of patients (93). Irinotecan combined with lobaplatin chemotherapy is a potential high-efficiency rescue chemotherapy for patients with extensive stage SCLC relapse or metastasis after etoposide and platinum chemotherapy fails. It has a good therapeutic effect on liver metastasis and can significantly relieve liver function damage caused by liver metastasis with mild adverse reactions (96).
Jinlong capsule combined with lobaplatin
The Jinlong capsule is currently the only Chinese medicine approved for the adjuvant treatment of liver malignant tumors (97). It can inhibit cancer metastasis and recurrence, improve clinical symptoms, and induce tumor cell differentiation. To further understand the clinical effect of the drug on the liver metastasis of lung cancer, some researchers observed the effect of Jinlong capsule combined with lobaplatin on interventional therapy and immune function regulation in patients with non-small cell hepatic metastasis in lung cancer. Interventional treatment of NSCLC liver metastasis with Jinlong capsule combined with lobaplatin was found to improve the effectiveness of liver metastasis treatment for lung cancer. Combined with chemotherapy, lobaplatin has additive and synergistic effects, and may be a synergistic and attenuating agent for the interventional treatment of liver metastasis (94).
Liposomal paclitaxel
Paclitaxel is a broad-spectrum antitumor drug. It is a secondary metabolite extracted from Taxus plants. Its main mechanism is to promote the polymerization of microtubules and inhibit depolymerization, thereby hindering cell division and preventing the G2 and M phases of malignant tumor cells. Liposome is a bilayer structure with phospholipids constituting the skeletal membrane material. It has good histocompatibility and cell affinity. It can be quickly recognized and swallowed by the monocyte-macrophage system. It is mainly distributed in the reticular endothelium and develops in organs, such as the lungs, liver, and lymph nodes. The new formulation slowly releases paclitaxel, improves the stability of the drug, and reduces toxicity. Studies have shown that for patients with advanced NSCLC, gemcitabine and liposomal paclitaxel are equivalent in the treatment of primary foci, but for the relief of liver metastasis, the effect of liposomal paclitaxel is better, and adverse reactions are more tolerable. The incidence of gastrointestinal toxicity and thrombocytopenia in patients treated with liposomal paclitaxel was shown to be significantly lower than that in patients treated with gemcitabine chemotherapy (95); liposomal paclitaxel can thus better benefit patients and offer a better quality of life.
Crizotinib
Crizotinib can inhibit the growth of tumors by inhibiting the anaplastic lymphoma kinase (ALK) gene. It is the first drug to target ALK and has been used to treat patients with locally advanced or metastatic non-small cell lung cancer diagnosed as ALK-positive by FDA-approved testing methods Crizotinib is used as a dual blocker of ALK and c-MET genes or their variants, and it is more effective than chemotherapy in patients with ALK-positive lung cancer who have previously received treatment (98).
Erlotinib
Erlotinib therapy in patients with hepatic metastasis is complicated by elevated alanine transaminase levels (99). Hepatic metastasis in patients with lung adenocarcinoma predicts poor response to erlotinib as a second- or third-line therapy. Combination therapy, for example with Met-amplified tyrosine kinase inhibitor (MET-TKI), may be a good choice for patients with liver metastasis with poor prognosis.
Summary
The studies in this review used cell experiments, animal experiments, biological information analysis technology, and a series of methods to explore the molecular mechanisms of lung cancer liver metastasis, which include the genes, miRNA, proteins, pathways, chemicals, and microcosmic process processes involved in the spread of lung cancer to the liver. The above studies are not limited to a single level, but rather examine the process of metastasis through the gene–protein–receptor–pathway–biological function route, forming a relatively complete picture of the dynamic mechanism. For example, the above miRNA study is based on the differences of miRNA expression levels in tissues. First, miRNA expression is upregulated or downregulated to detect changes in the corresponding targeted protein level, and then the target protein receptor and its related pathways are inspected. Finally, the effects of the target protein receptor on the proliferation and invasion of lung cancer cells can be determined. This provides a holistic, point-to-surface approach for the future study of the mechanisms of lung cancer metastasis. The review also provides bioinformatics evidence for the study of the prognosis of liver metastasis of lung cancer.
However, most of the above studies were limited to the microscopic molecular dynamic changes and cell proliferation and invasion. They did not clarify a microscopic and macroscopic relationship, and did not map the molecular level material changes to the corresponding changes in clinical symptoms. This suggests that future studies should seek to examine the relationship between the molecular mechanism of lung cancer metastasis and the change of clinical symptoms to improve the prognosis of hepatic metastasis in lung cancer. Due to the fundamental nature of genes and miRNAs, future research directions are likely to focus heavily on this.
The mechanism of hepatic metastasis in lung cancer and its influencing factors are complex and diverse. Current treatment is still in its infancy. We believe future studies will more deeply explore the exact mechanisms of metastasis, which will in turn provide a method for early diagnosis, open avenues for novel treatment, and innovate breakthroughs in the prevention of hepatic metastasis in lung cancer.
Acknowledgments
English Language Editors: R. Scott and J. Gray from AME publishing company.
Funding: This study was supported in part by a grant of young talents in Shanghai, National Natural Science Foundation of China (81802255), Shanghai Pujiang Program (17PJD036) and a grant from Shanghai Municipal Commission of Health and Family Planning Program (20174Y0131), National key research & development project (2016YFC0902300), Major disease clinical skills enhancement program of three year action plan for promoting clinical skills and clinical innovation in municipal hospitals, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A), “Dream Tutor” Outstanding Young Talents Program (fkyq1901), key disciplines of Shanghai Pulmonary Hospital (2017ZZ02012), grant of Shanghai Science and Technology Commission (16JC1405900).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1675
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1675). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu H, Chen J. Progress in metastasis of lung cancer. Chinese Journal of Lung Cancer 2008:40-2.
- Shen C, Zhou K, Che G. Research progress of microRNA and EGFR mutations in the mechanism of non-small cell lung cancer metastasis. Chinese Journal of Clinical Thoracic and Cardiovascular Surgery 2018;25:440-4.
- Qu A, He P, Niu H. Study on the mechanism of guanine nucleotide dissociation inhibitor 2 involved in lung cancer invasion and metastasis by regulating miRNAs . Shanxi Medical Journal 2016;45:1971-4.
- Zheng R, Ouyang M. Research progress of lung cancer metastasis mechanism. International Journal of Internal Medicine 2007:445-7.
- Liu X, Tong W, Wang S, et al. Molecular mechanism of lung cancer invasion and metastasis. Journal of the Chinese Academy of Medical Sciences 2016;38:108-12.
- Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 2008;15:504-14. [Crossref] [PubMed]
- Yang L, Yang J, Li J, et al. MircoRNA-33 a inhibits epithelial-to-mesenchymal transition and metastasis and could be a prognostic marker in non-small cell lung cancer. Sci Rep 2015;5:13677. [Crossref] [PubMed]
- Shi H, Ji Y, Zhang D, et al. MiR-135a inhibits migration and night and regulates EMT- related marker genes by targeting KLF8 in lung cancer cells. Biochem Biophys Res Commun 2015;465:125-30. [Crossref] [PubMed]
- Li J, Wang Y, Luo J, et al. miR-134 inhibits epithelial to mesenchymal transition by targeting FOXM1 in non-small cell lung cancer cells. FEBS Lett 2012;586:3761-5. [Crossref] [PubMed]
- Ke Y, Zhao W, Xiong J. MiR-149 inhibits non-small-cell lung cancer cells EMT by targeting FOXM1. Biochem Res Int 2013;2013:506731 [Crossref] [PubMed]
- Brisdelli F, Bennato F, Bozzi A, et al. ELF—MF attenuates quercetin—induced apoptosis in K562 cells through modulating the expression of Bcl-2 family proteins. Mol Cell Biochem 2014;397:33-43. [PubMed]
- Yang L, Zhou Y, Li Y, et al. Mutations of p53 and KRAS activate NF—KB to promote chemoresistance and tumorigenesis via dysregulation of cell cycle and suppression of apoptosis in lung cancer cells. Cancer Lett 2015;357:520-6. [Crossref] [PubMed]
- Chen Q, Jiao D, Wu Y, et al. MiR-206 inhibits HGF–induced epithelial and mesenchymal transition and angiogenesis in non-small cell lung cancer-Met via c/PI3k/Akt/mTOR pathway. Oncotarget 2016;7:18247-61. [Crossref] [PubMed]
- Wang L. The molecular mechanism of tumor cells exudating blood vessels, a key step in tumor metastasis. China National Kang Medical Science 2015:81-3.
- Naumov GN, MacDonald I, Weinmeister M, et al. Persistence of solitary mammary carcinoma cells in a secondary site a possible contributor to dormancy. Cancer Res 2002;62:2162-8. [PubMed]
- Venkatesh V, Nataraj R, Thangaraj G, et al. Targeting Notch signaling pathway of cancer stem cells. Stem Cell Investig 2018;5:5. [Crossref] [PubMed]
- Yu F, Liu J, Wu Z, et al. Tumor suppressive microRNA-124a inhibits stemness and enhances gefitinib sensitivity of non-small cell lung cancer cells by targeting ubiquitin-specific protease 14. Cancer Lett 2018;427:74-84. [Crossref] [PubMed]
- Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Mol Cancer Res 2010;8:629-42. [Crossref] [PubMed]
- Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 2006;7:359-71. [Crossref] [PubMed]
- Wang Y, Wang L, Chen C. New insights into the regulatory role of microRNA in tumor angiogenesis and clinical implications. Mol Cancer 2018;17:22. [Crossref] [PubMed]
- Xu J, Xie Q, Zhang Y, et al. Expression of tumor metastasis gene mts1 in lung cancer and its relationship with lymph node metastasis. Chinese Journal of Pathology 1999:51-2.
- Tong X, Li K, Luo Z, et al. Decreased TIP30 expression promotes tumor metastasis in lung cancer. Am J Pathol 2009;174:1931-9. [Crossref] [PubMed]
- Zhang M, Chen G. Research progress on lung cancer invasion and metastasis-related signaling pathways. J Clin Pulm Med 2011;16:1429-30.
- Dash R, Su ZZ, Lee SG, et al. Inhibition of AP-1 by SARI negatively regulates transformation progression mediated by CCN1. Oncogene 2010;29:4412-23. [Crossref] [PubMed]
- Li X. Differential expression of non-small cell lung cancer metastasis-related genes in tumor cells of primary, blood circulation and metastases. Third Military Medical University 2011.
- Zhang Y, Liu Y, Wang E. Deletion of p120-catenin gene in lung cancer cells promotes invasion and metastasis of lung cancer cells. Advances in Anatomical Sciences 2011;17:287-93.
- Jan YH, Tsai HY, Yang CJ, et al. Adenylate kinase-4 is a marker of poor clinical outcomes that promotes metastasis of lung cancer by downregulating the transcription factor ATF3. Cancer Res 2012;72:5119-29. [Crossref] [PubMed]
- Yang P. Proto-oncogene CT45A1 as a new gene transcription activator promotes lung cancer growth and metastasis. Suzhou University 2016.
- Pozuelo-Rubio M. 14-3-3 Proteins are Regulators of Autophagy. Cells 2012;1:754-73. [Crossref] [PubMed]
- Gao A. CT45A1 gene promotes the invasion and metastasis of lung cancer cells and its mechanism. Suzhou University 2015.
- Deng S. Study on the role and mechanism of PLEK2 in non-small cell lung cancer metastasis. Chengdu Medical College 2018.
- Zhou N, Guo J, Dai J, et al. Effects of YAP on Proliferation and Migration Ability of Non-small Cell Lung Cancer Cells. Journal of Jilin University Medicine Edition 2019;45:1320-6.
- Dai H. Genes related to liver metastasis potential of microRNAs related to recurrence and metastasis of stage I lung adenocarcinoma after surgery. Peking Union Medical College 2019.
- . Research reveals new mechanism of small cell lung cancer metastasis. Hainan Medical Journal 2019;30:546.
- Ai C, Ma G, Deng Y, et al. Nm23-H1 inhibits lung cancer bone-specific metastasis by upregulating miR-660-5p targeted SMARCA5. Thorac Cancer 2020;11:640-50. [Crossref] [PubMed]
- Guo J, Wang Y, Sun W, et al. Effects of miRNA-126 on cell function in non-small cell lung cancer and related mechanisms. J Diff Dis 2019;18:191-6.
- Miko E, Margitai Z, Czimmerer Z, et al. MiR-126 inhibits proliferation of small cell lung cancer cells by targeting SLC7A5. FEBS Lett 2011;585:1191-6. [Crossref] [PubMed]
- Crawford M, Brawner E, Baitte K, et al. MicroRNA-126 inhibits invasion in non-small lung carcinoma cell lines. Biochem Biophys Res Commun 2008;373:607-12. [Crossref] [PubMed]
- Liu R, Gu J, Jiang P, et al. DNMT1-microRNA126 epigenetic circuit contributes to esophageal squamous cell carcinoma growth via ADAM9-EGFR-AKT signaling. Clin Cancer Res 2015;21:854-63. [Crossref] [PubMed]
- Tang X, Jiao D, Chen J, et al. effects of miRNA-126 on proliferation, migration, invasion and EGFR/AKT/mTOR signaling of lung cancer A549 cells. Chinese Journal of Pathophysiology 2016;32:458-63.
- Lei R, Tang J, Zhuang X, et al. Suppression of MIM by microRNA-182 activates RhoA and promotes breast cancer metastasis. Oncogene 2014;33:1287-96. [Crossref] [PubMed]
- Chen X, Wei L, Li J, et al. MiR-338 expression and its inhibition of lung cancer metastasis in lung adenocarcinoma. The Mechanism of China 2018;35:1277-9.
- Tian F, Han Y, Yan X, et al. Upregulation of microrna-451 increases the sensitivity of A549 cells to radiotherapy through enhancement of apoptosis. Thorac Cancer 2016;7:226-31. [Crossref] [PubMed]
- Liu N, Jiang N, Guo R, et al. MiR-451 inhibits cell growth and invasion by targeting MIF and is associated with survival in nasopharyngeal carcinoma. Mol Cancer 2013;12:123. [Crossref] [PubMed]
- Wang XC, Tian LL, Jiang XY, et al. The expression and function of miRNA-451 in non-small cell lung cancer. Cancer Lett 2011;311:203-9. [Crossref] [PubMed]
- Gu W, Ren X, Mou K, et al. Expression and clinical significance of miRNA-451 in non-small cell lung cancer. Journal of Clinical Pulmonary Science 2019;24:480-3.
- Zhang C, Ge S, Hu C, et al. miRNA-218, a new regulator of HMGB1, suppresses cell migration and invasion in non-small cell lung cancer. Acta Biochim Biophys Sin (Shanghai) 2013;45:1055-61. [Crossref] [PubMed]
- Luan I, Han B, Wang C, et al. Expression and clinical significance of microrna-218-1-3 p in non-small cell lung cancer. Journal of China Medical University 2014;43:181.
- Wang H, Sun D, Wang F, et al. The miRNA - 448 in non-small cell lung cancer cell proliferation, the influence of the transfer and epithelial mesenchymal. Mark Immune Analysis and Clinical 2019;26:1741-6.
- Wu F, Lou J. Application of circulating tumor cells in the diagnosis and treatment of lung cancer. Chinese Journal of Laboratory Medicine 2013;36:983-6.
- Peng G, Liu W, Xi L, et al. Expression of lung tissue-specific X protein in non-small cell lung cancer and its relationship with lung cancer micrometastasis. Guangdong Medical Journal 2015;7:1866-9.
- Jiang Z, Wang M, Xu J. Thymidine kinase 1 combined with CEA, CYFRA21-1 and NSE improved its diagnostic value for lung cancer. Life Sci 2018;194:1-6. [Crossref] [PubMed]
- Yu J, Wang J. The relationship between the changes of Cyfra21-1, NSE and LDH levels and liver metastasis of lung cancer. Progress in Modern General Surgery of China 2019;22:729-31.
- Jiang Z, Wang M, Xu J. Thymidine kinase 1 combined with CEA, CYFRA21-l and NSE improved its diagnostic value for lung cancer. Life Sci 2018;194:1-6. [Crossref] [PubMed]
- Zeng Y, Bao J, Zhao Y, et al. A sandwich-type electrochemical immunoassay for ultrasensitive detection of non-small cell lung cancer biomarker CYFRA21-1. Bioelectrochemistry 2018;120:183-9. [Crossref] [PubMed]
- Yuan Y, Wang J. The relationship between the changes of Cyfra21-1, NSE and LDH levels and liver metastasis from lung cancer. Progress in Modern General Surgery of China 2019;22:729-31.
- Kim H, Mendiratta S, Pecot C. Cell 2013;155:552-66. [Crossref] [PubMed]
- Li J, Liu C. Diagnosis of lung cancer combined with malignant pleural effusion in patients with PCT combined with LDH. Break value. International Journal of Respiration 2017;37:1137-40.
- Li M, Zheng M, Xu Z, et al. Diagnostic value of combined detection of serum NSE, SCC, CYFRA21-1 and CEA for lung cancer. Chinese Journal of Tropical Medicine 2016;16:584-7.
- Zang Y, Yu H, Li Y, et al. Investigation of symptom groups in patients with lung cancer. Chinese Journal of Nursing 2016;51:316-20.
- Ni J, Guo Z, Zhang L. Individual and combined detection of four lung cancer serum tumor markers in the Value of lung cancer diagnosis III. Chinese Journal of Internal Medicine 2016;55:25-30. [PubMed]
- Zhao J, Li X, Yu H, et al. Four lung cancer serum tumor markers in the diagnosis of lung cancer. Evaluation of joint application effects III. Genomics and Applied Biology 2018;37:90-8.
- Peng Y, Wang Y, Li J, et al. The role of serum NSE, PmGRP and LDH in the diagnosis and treatment of small cell lung cancer. Chinese Journal of Lung Cancer 2016;19:590-4. [PubMed]
- Deng J, Xu A. Serum growth differentiation factor 15 level in diagnosis and chemotherapy of lung cancer Research on the Value of Efficacy Evaluation III. Chinese General Practice 2017;20:1823-8.
- Zhang H. Discovery of key factors in the development of small cell lung cancer. Cancer Progress 2017;15:123-5.
- Peng Y, Wang Y, Li J, et al. Utility of NSE, Pro GRP and LDH in diagnosis and treatment in patients with small cell lung cancer. Chinese Journal of Lung Cancer 2016;19:590-4. [PubMed]
- Zhang X, Guo M, Fan J, et al. Prognostic significance of serum LDH in small cell lung cancer: A systematic review with meta-analysis. Cancer Biomark 2016;16:415. [Crossref] [PubMed]
- Tang J, He Q, Guo R, et al. Phosphorylated AKT overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer 2006;51:181-91. [Crossref] [PubMed]
- Zhu B, Zhou X. Research on PI3K / AKT pathway in lung cancer metastasis and drug resistance. Chinese Journal of Lung Cancer 2011;14:689-94. [PubMed]
- Fu D. SLC38A3 promotes metastasis of non-small cell lung cancer by inducing EMT by activating Akt signaling pathway. Dalian Medical University 2016.
- Dou Y. Preliminary study on the in vitro function and mechanism of CNPY2 gene invasion and metastasis of non-small cell lung cancer and cisplatin resistance. Lanzhou University 2019.
- Wu J. STK33 regulates PI3K / AKT and JAK / STAT in non-small cell lung cancer metastasis signaling pathway. Kunming Medical University, 2014.
- Song Q. The mechanism of miR-26a targeting ITGβ8 to regulate JAK2 / STAT3 signaling pathway to promote lung cancer cell metastasis. Tianjin Medical University 2018.
- Wu Q, Zhang M, Dong W, et al. Detection of serum transforming growth factor β1 in patients with lung cancer and its clinical significance. Journal of Clinical Pulmonary Medicine 2006.
- Minamiya Y, Miura M, Hinai Y, et al. Transforming growth factor-β1 29T>C genetic polymorphism is associated with lymph node metastasis in patients with adenocarcinoma of the lung. Tumour Biol 2010;31:437-41. [Crossref] [PubMed]
- Wilgus ML, Borczuk AC, Stoopler M, et al. Lysyl oxidase: a lung adenocarcinoma biomarker of invasion and survival. Cancer 2011;117:2186-91. [Crossref] [PubMed]
- Kim J, You L, Xu Z, et al. Wnt inhibitory factor inhibits lung cancer cell growth. J Thorac Cardiovasc Surg 2007;133:733-7. [Crossref] [PubMed]
- Teng Y, Wang X, Wang Y, et al. Effect of siRNA-mediated beta-catenin gene on Wnt signal pathway in lung adenocarcinomaA549 cell. Zhonghua Yi Xue Za Zhi 2010;90:988-92. [PubMed]
- Wang Q, Zhan P, Yu L, et al. Relationship between Wnt1 expression and prognosis in non-small cell lung cancer. Chinese Journal of Lung Cancer 2010.
- Han Q. WWC3 affects the activity of Wnt pathway and Hippo pathway through DVLs and LATS1 to inhibit lung cancer invasion and metastasis. China Medical University 2018.
- Zhang H, Zuo P. Research progress of lung cancer metastasis mechanism. Journal of Medical Research 2013;42:4-6.
- Wei L. LncRNA ATB promotes the proliferation and metastasis of lung cancer by activating the p38 signaling pathway. Chinese People's Liberation Army Medical University 2019.
- Kuramoto T, Goto H, Mitsuhashi A, et al. Dll4-Fc, an inhibitor of Dll4-notch signaling, suppresses liver metastasis of small cell lung cancer cells through the downregulation of the NF-κB activity. Mol Cancer Ther 2012;11:2578-87. [Crossref] [PubMed]
- Yeh H, Lai W, Chen H, et al. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene 2006;25:4300-9. [Crossref] [PubMed]
- Li Q, Han Y, Fei G, et al. IL-17 promoted metastasis of non-small-cell lung cancer cells. Immunol Lett 2012;148:144-50. [Crossref] [PubMed]
- Wang H. Study on the role and mechanism of TIM-4 in IL-6 promoting migration and invasion of NSCLC. Shandong University 2016.
- Song Y, Xue L, Du S, et al. Caveolin-1 knockdown is associated with the metastasis and proliferation of human lung cancer cell line NCI-H460. Biomed Pharmacother 2012;66:439-47. [Crossref] [PubMed]
- Zhang H, Zuo P. Research progress of lung cancer metastasis mechanism. Journal of Medical Research 2013;42:4-6.
- Zhang X, Wang H, Zhang M, et al. The HMGB1 / TLR4 pathway participates in the inhibitory effect of Fibulin-5 on lung cancer cell proliferation and metastasis. China Cancer Journal 2017;27:761-9.
- Liu Y. The role of NAC1 / HMGB1 signaling pathway in EMT and invasion and metastasis of lung cancer cells. Chongqing Medical University 2018.
- Xu Y. KIAA0247 inhibits proliferation, metastasis and invasion of non-small cell lung cancer through Notch pathway. China Medical University 2018.
- Zheng G, Song K, Zhao Y. Liver kinase B1 suppresses the metastasis and angiogenesis of lung cancer: involvement of the Shh signaling pathway. Neoplasma 2019;66:367-76. [Crossref] [PubMed]
- Chen L, Huang P, Zhang X, et al. Efficacy and safety evaluation of irinotecan combined with loboplatin in patients with liver metastasis from small cell lung cancer with etoposide chemotherapy failure. Hebei Medicine 2016;38:3098-100.
- Zhang X, Nan Z. Effects of Jinlong Capsule Combined with Loplatin on Non-small Cell Lung Cancer Patients with Liver Metastasis and Its Immune Function Regulation. China Drug and Clinical Medicine 2018;18:2170-1.
- Liu Y, Wang Z, Ren W, et al. Comparison of the effects of liposome paclitaxel and gemcitabine combined with cisplatin in the treatment of hepatic metastases in lung cancer. Journal of Hebei Medical University 2018;39:810-2.
- Liu C. Effect of small dose etoposide combined with cisplatin on liver function of patients with small cell lung cancer to improve liver function. Liver 2014;19:559-61.
- Liu Y, Gao J, Gu B, et al. Study on the effect of Jinlong capsule (JLC) on the induction and differentiation of tumor cells. Chinese Journal of Clinical Oncology 2004;31:380-3.
- He Y, Zhou C, Niu F. Comparison of the efficacy of crizotinib and chemotherapy in patients with advanced ALK-positive lung cancer. Evid Based Med 2014;14:45-7.
- He Y, Wang Y, Boyle T, et al. Hepatic Metastases is Associated with Poor Efficacy of Erlotinib as 2nd/3rd Line Therapy in Patients with Lung Adenocarcinoma. Med Sci Monit 2016;22:276-83. [Crossref] [PubMed]


