
Prognostic value of pretreatment prognostic nutritional index and lactated dehydrogenase in locally advanced nasopharyngeal carcinoma patients
Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor in southern Asia, with high incidence rates of 20–30 cases per 100,000 (1). Due to its complex anatomical location and high sensitivity of irradiation, concurrent chemoradiotherapy (CCRT) was the main treatment for locally advanced NPC (2) in the past. With the development of intensity modulated radiotherapy (IMRT) and its extensive application in NPC, local control rate has been improved, but the distant metastasis rate remains high (3). Increased clinical evidence supports induction chemotherapy (IC) could control and eliminate subclinical micro metastasis (4), therefore IC followed by CCRT is recommended to further reduce distant metastasis risk. At present, TNM staging is regarded as a crucial tool for clinical prognosis and outcome of NPC. However, even if patients with the same TNM stage receiving similar regimens, there are still significant differences in clinical response and prognosis, possibly because TNM staging system classifies diseases according to anatomy and does not reflect biological heterogeneity (5). Therefore, identification of biomarkers associated with prognosis may complement the inadequacy of the TNM staging system in prognosis prediction in NPC. Recently, a growing number of markers have been identified as relevant factors affecting the prognosis of NPC patients, including Epstein-Barr virus DNA loads, systemic immune-inflammation index (SII) (6), epidermal growth factor receptor overexpression and microRNA signature. However, these markers are not routinely applied in clinical practice because of high acquisition cost and great variability in experimental time. Therefore, it is urgent to screen out some inexpensive and clinically accessible markers for predicting the prognosis of NPC patients.
In recent years, an increasing number of studies have focused on the effects of nutrition and immune status on the prognosis of cancer patients. The prognostic nutritional index (PNI), which was based on serum albumin concentration and total lymphocyte count in peripheral blood, first introduced to assess the immunological and nutritional status of patients undergoing gastrointestinal surgery (7). Recently, the prognostic value of PNI in a variety of malignant tumors has been confirmed, including gastric cancer, colorectal cancer, hepatocellular cancer and pancreatic cancer (8). However, there are limited trials related to pretreatment PNI in NPC. In previous studies, most patients with metastatic NPC received different treatment regimens, thus, it was difficult to correctly analyze the impact of these indexes, and whether PNI is related to the prognosis in locally advanced NPC patients might be questioned. Therefore, to eliminate this therapeutic heterogeneity, the prognostic value of PNI in newly diagnosed locally advanced NPC patients at stage III-IVA receiving IC followed by CCRT was further studied.
As we know, transforming normal cells into malignant cells often leads to abnormal serum enzyme synthesis, even before tumor morphology changes (9). Therefore, enzyme studies have recently received widespread attention. Lactate dehydrogenase (LDH) is the most important enzyme that converts pyruvate to lactate at the end of glycolysis. Some studies demonstrated that poor survival in NPC patients with elevated serum LDH levels, however, most patients in these studies were from highly endemic areas (10), such as Guangdong, Fujian, etc. This may create a high risk of selection bias and affect the true conclusions. In addition, previous findings have been inconsistent regarding the prognostic impact of LDH in NPC (11). Therefore, new studies from low to middle incidence areas are necessary to improve the reliability of serum LDH levels as a prognostic factor for NPC, especially in locally advanced patients.
Therefore, a retrospective study was conducted to investigate the prognostic value of pretreatment PNI, LDH and their combination in locally advanced NPC patients receiving IC followed by CCRT, which could be regarded as a complement to the conventional TNM staging system, to improve survival prediction and guide appropriate treatment plans for NPC patients. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2033).
Methods
Patients
Between January 2013 and December 2017, 213 patients diagnosed with locally advanced NPC patients receiving IC followed by CCRT at Union Hospital Cancer Center were retrospectively reviewed. The enrolled patients based on inclusion criteria: (I) age ≥16 years but ≤70 years; (II) histologically confirmed NPC; (III) Karnofsky performance score (KPS) ≥70; (IV) complete medical records, including nasopharyngeal speculum, contrast-enhanced MRI of the nasopharynx and neck, chest CT, abdominal ultrasonography and whole-body bone scan for staging, finally re-staged III–IVA NPC based on the 8th edition of the AJCC staging system; (V) completion of prescribed IC followed by CCRT; (VI) complete record of total lymphocyte count, serum albumin and LDH level within 1 week before therapy. Patients who met any of the following criteria were excluded: (I) a history of anticancer therapy; (II) evidence of concomitant tumors at diagnosis; (III) insufficient heart, lung, liver, and renal function; (IV) severe anemia, acute infection or autoimmune diseases.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Cancer center of Union Hospital of Tongji Medical College of Huazhong University of Science and Technology (No. 2019S1054) and individual consent for this retrospective analysis was waived.
Treatment
For locally advanced NPC patients, IC followed by CCRT is prescribed based on our institution guidelines. The total prescribed IMRT dose was 70 Gy/33 F to the gross tumor volume of the nasopharynx (GTVnx), 68 Gy/33 F to the gross tumor volume of the positive neck lymph nodes (GTVnd), 66 Gy/33 F to the high-risk sites of microscopic extension defined as clinical target volume 1 (CTV1), and 60 Gy/33 F to the clinical target volume 2 (CTV2). PTVs were delineated by adding 5 mm and 3 mm to the GTV and CTV, respectively. The fractionated dose was 1.8 to 2.2 Gy at 1 fraction per day on 5 days per week. The regimens of IC were as follows: 1) TPF regimen: docetaxel (75 mg/m2/day, day 1), cisplatin (75 mg/m2/day, day 1), and 5-fluorouracil (750 mg/m2/day, day 1-5); and 2) TP regimen: docetaxel (75 mg/m2/day, day 1) and cisplatin (75 mg/m2/day, day 1). IC were prescribed every 3 weeks for three cycles. Moreover, concurrent chemotherapy consisted of cisplatin sensitization with a total dose of 200 mg/m2.
Data collection and clinical endpoints
Clinical characteristics data were retrieved from the patients’ electronic medical records, including gender, age, the history of smoking and drinking, WHO pathological type, EBV DNA levels, Tumor classification, Nodal classification, AJCC stage and peripheral blood indexes within the 1 week before treatment. All peripheral blood was collected in EDTA anticoagulant test tubes and tested for serum albumin, total lymphocyte count and LDH within 1 week before therapy. The definition of the PNI is described as follows (12): PNI = serum albumin level (g/L) + 5× total lymphocyte count (109/L).
The following end points were: overall survival (OS), which was defined as the time between first treatment and death or last follow-up; Progression-free survival (PFS), defined as the time that had elapsed between initial treatment and the date of disease progression or death from any cause. Locoregional relapse-free survival (LRFS) was defined as the time from pathological diagnosis to local relapse. Distant metastasis-free survival (DMFS) was defined as the time from pathological diagnosis to the time of distant metastasis detection.
Follow-up
All patients were evaluated every 3 months for the first 2 years after completion of treatment, every 6 months between the third to fifth year, and then annually thereafter. A complete physical examination, including a nasopharyngeal speculum, contrast-enhanced MRI of the nasopharynx and neck, chest CT, abdominal ultrasonography, and a whole-body bone scan, was performed semiannually. The latest follow-up was conducted at the end of January 2020. All patients were followed up by regular records of each clinic recheck or phone calls.
Statistical analysis
Categorical variables were expressed as number and percentage, with differences between groups determined using the χ2 test. PNI and LDH in enrolled patients were found to be skewed by normality test. Then quartiles of PNI and LDH were calculated, with the median level as the cutoff value. Correlations between variables were assessed using the Pearson correlation coefficient. Survival curves were analyzed between different PNI and LDH subgroups, using the Kaplan-Meier method and compared using the log-rank test. PNI-LDH groups were defined according to the combination of different levels of PNI and LDH, and the area under ROC curve (AUC) was compared to evaluate the diagnostic performance of PNI, LDH and PNI-LDH. Univariate and multivariate analyses were calculated by the Cox proportional hazards regression model. The significant risk factors identified by univariate analysis were then entered into the multivariate analysis. Statistical analysis was conducted with SPSS 25.0 and GraphPad Prism 8.0. A two-tailed P value less than 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 213 re-staged III-IVA NPC patients were eligible for analysis, including 155 (72.8%) males and 58 (27.2%) females, with ages ranging from 24 to 69 years (median 45 years). Patients with a history of smoking and drinking were 101 (47.4%) and 87 (40.8%), respectively. According to the World Health Organization (WHO) pathological type, 25 (11.7%) patients had type I (keratinizing squamous cell carcinoma), 35 (16.4%) had type II (nonkeratinizing differentiated), and 153 (71.8%) had type III (nonkeratinizing undifferentiated). Based on EBV DNA status, 104 (48.8%) cases were positive. Of the 213 patients, 115 (54.0%) patients were re-staged in stage III and 98 (46.0%) patients were in stage IVA according to the 8th edition of the AJCC staging system. And there were 138 patients receiving TPF regimen, while 75 patients received the TP regimen.
Cutoff values of parameters
Quartiles of PNI and LDH were calculated, and we defined the median level as the cutoff value, then patients were divided into low and high PNI, LDH subgroups. The baseline characteristics of the patients in different groups were shown in Table 1. According to clinical and demographic characteristics, we compared age, sex, smoking and drinking history, EBV DNA status, WHO pathological type, TNM stage and IC regimen respectively. However, only EBV DNA status and N stage (P<0.05) showed statistically significant difference between groups in LDH.
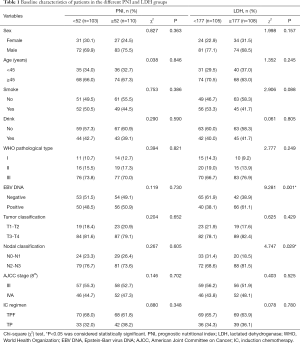
Full table
In total, the median follow-up time was 46 months, ranging from 26 months to 83 months. At the end of the time, 54 (25.4%) patients suffered from tumor progression, and 20 (9.4%) patients died. The 5-year OS, PFS, LRFS and DMFS rates were 86.7%, 73.4%, 83.6% and 84.1%, respectively.
Prognostic value of PNI and LDH
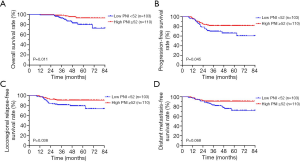
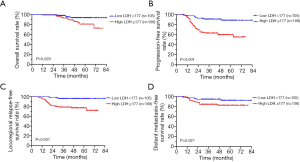
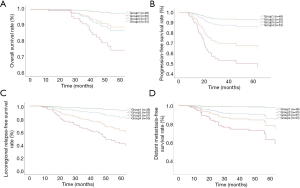
The correlation between clinicopathological factors and survival outcomes was shown in Figures 1,2. According to the median value, 213 patients were divided into low PNI group (n=103) (48.4%) and high PNI group (n=110) (51.6%). The survival difference between the two PNI subgroups revealed that compared with the low PNI group, a higher PNI group demonstrated inferior OS (P=0.011, Figure 1A), PFS (P=0.045, Figure 1B), LRFS (P=0.038, Figure 1C) and DMFS (P=0.068, Figure 1D). At the same time, 105 and 108 patients were divided to low (LDH <177) and high LDH (LDH ≥177) groups respectively. Fortunately, an evident survival difference between the two groups was proved in our analysis. As shown in Figure 2, a higher LDH level had a poorer OS (P=0.029, Figure 2A), PFS (P<0.001, Figure 2B), LRFS (P<0.001, Figure 2C) and DMFS (P=0.021, Figure 2D).
Univariate and multivariate Cox regression analysis
To further identify the risk factors linked to survival outcomes, PNI, LDH and other clinicopathologic factors were evaluated by univariate analysis and the Cox regression model. In the univariate analysis, age, sex, EBV DNA status, WHO pathological type, TNM staging, IC regimen, different PNI and LDH level were enrolled. As shown in Table 2, EBV DNA status, TNM staging, pretreatment PNI and LDH were corroborated as potential factors for survival outcomes (P<0.05).
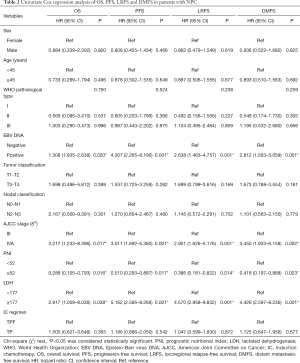
Full table
Variables that reached a significant difference in the univariate Cox regression analysis were further analyzed in multivariate Cox regression analysis in Table 3. With regard to PNI, a higher PNI was found to be an independent protecting factor in NPC patients for OS (HR: 0.317, P=0.027), PFS (HR: 0.560, P=0.040), LRFS (HR: 0.498, P=0.015) and DMFS (HR: 0.608, P=0.040). Similarly, a higher LDH was significantly associated with inferior OS (HR: 2.707, P=0.040), PFS (HR: 5.318, P=0.001), LRFS (HR: 5.491, P=0.001) and DMFS (HR: 4.926, P=0.001).
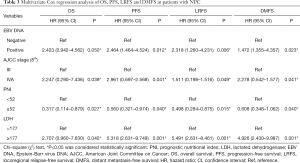
Full table
Combined prognostic value of the PNI and LDH
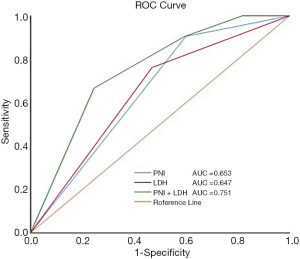
Pretreatment PNI and LDH were divided into four groups based on different levels: low PNI and low LDH patients defined as group 1, low PNI and high LDH patients defined as group 2 (high risk), high PNI and low LDH patients defined as group 3 (low risk), high PNI and high LDH patients defined as group 4. The number of patients in each group was 48 (22.5%), 55 (25.8%), 57 (26.8%) and 53 (24.9%), respectively. In order to adjust the covariates of different groups, we incorporate the meaningful variables in multivariate analysis into the COX analysis, and draw the survival curve on this basis. The survival curve we analyzed were shown in Figure 3. Our results revealed that group 2 had significantly poorer OS (P=0.012, Figure 3A), PFS (P=0.001, Figure 3B), LRFS (P=0.001, Figure 3C) and DMFS (P=0.001, Figure 3D) than others groups. Furthermore, the prognostic value of the PNI, LDH, and the combination of them was evaluated by comparing the AUC area. The AUC of the PNI, LDH, and their combination were 0.653 (P=0.021), 0.647 (P=0.028), and 0.751 (P=0.001), respectively (Figure 4), indicating that PNI-LDH is superior to either score alone.
Discussion
At present, the gold standard for predicting the prognosis in locally advanced NPC is TNM staging, however, patients with the same stage tend to have different prognosis after receiving similar treatment, as the TNM staging system does not take some potential prognostic factors into consideration. With the improvement of IMRT, the local control rate has been improved, the main cause of treatment failure has been identified as distant metastasis (3). Therefore, it is essential to effectively identify other promising prognostic factors to improve the ability of the TNM staging system to predict prognosis. In recent years, many studies have shown that some indexes can be used as prognostic markers of NPC treated with IMRT, including EBV DNA, miR-BARTs, promoter methylation of tumor related genes (13), systemic immune-inflammation index (SII) (14) and the albumin-globulin ratio (AGR) (15).
The PNI, which is determined by the serum albumin concentration and total lymphocyte count in the peripheral blood, first introduced by Onodera et al., (16) and can reflect the immune and nutritional status of patients. It is well known that serum Alb is a good indicator of inflammation and nutritional status in patients, which can help stabilize cell growth and DNA replication, buffer various biochemical changes, and maintain sex hormone homeostasis to prevent cancer. More and more studies have shown that serum Alb is closely related to the prognosis of gastric cancer and lung cancer, even in NPC (17). High levels of Alb help to maintain the stability of growth factors, inflammatory factors, and oxygen saturation in the internal environment, and prevent tumor progression. Low levels of Alb suggest impaired immune function, increased vascular permeability and changes in microenvironment, which are conducive to tumor progression (18). At the same time, lymphocytes are an important part of the host's adaptive immune response to cancer cells. In tumors, lymphocytes always function in many ways, including inhibiting the differentiation of dendritic cells. It is reported that infiltrating lymphocytes are effective anti-tumor cells. The low level of it may indicate a poor lymphocyte-mediated immune response to cancer cells, suggesting a poor prognosis (19). Hence, PNI, which is composed of serum protein and lymphocytes, is also considered to be a factor related to tumor prognosis. Recent studies have shown that PNI is closely related to the survival of many types of cancer. However, there are limited trials related to PNI in NPC patients.
The prognostic effect of PNI in metastatic NPC patients for the first time was studied by Yang et al. (20), which supporting that the pretreatment high PNI could predict lower risk of distant metastases with better DMFS. Wei et al. (21) and Topkan et al. (22) also revealed PNI is independent prognostic factors for OS in metastatic NPC patients. Followed by He et al. (23), a total of 377 newly diagnosed NPC patients treated with neoadjuvant chemotherapy plus CCRT at the Sichuan Cancer Hospital were reviewed, and revealed that the novel prognosis index PNI was related to OS (P<0.001)and PFS (P=0.008) for metastatic NPC patients. Furthermore, 539 patients with newly diagnosed non-metastatic NPC treated by CCRT were retrospectively analyzed by Miao et al. (24) and demonstrated that the 5-year LRFS, DMFS, DSS, and OS of PNI ≤52.0 group were significantly worse than the PNI >52.0 group. However, there was a study (25) showed that a statistically significant cut off value of PNI as 45.45 for OS in NPC patients, but no relation was found in terms of DMFS and LRFS. To sum up, in the existing studies for PNI on the prognosis of NPC patients, most of them were in metastatic stage, and demonstrated that a lower PNI was found as predictive factor in terms of worse OS and PFS, however, the role on LRFS and DMFS failed to reach an agreement. Therefore, in our study, 213 newly diagnosed locally advanced NPC patients receiving IC followed by CCRT were analyzed retrospectively, which could minimize the treatment regimen bias compared to previous studies. And the conclusion of our study demonstrated that compared with the low PNI group, a higher PNI was related to inferior OS (P=0.011) and PFS (P=0.045), LRFS (P=0.038) and a trend in DMFS (P=0.068), which is consistent with the previous results. Furthermore, we also affirmed the prognostic role of PNI with regard to LRFS and DMFS with non-metastatic NPC patients in our analysis. However, the values of PNI were inconsistent in studies, which may be due to the basic level of the included patients with different stages and the difference of sensitivity and reference value of reagent instrument. On the other hand, as a retrospective study with a relatively small sample size obtained at a single center, although PNI is an independent predictor of NPC prognosis, its sensitivity, and specificity are not necessarily very high, indicating that further prospective studies are required to determine the appropriate cutoff value.
Tumor proliferation has its unique metabolic characteristics, which involves the changes of many important molecular indexes in serum, including enzymes, proteins and hormones. Exploring these objective clinical indicators is extremely important for clinical practice. In order to produce energy, cancer cells give priority to the glycolysis pathway generated by LDH, a phenomenon historically known as the Warburg effect, which further promotes immunosuppression in tumor areas and leads to the proliferation of tumor cells (26). Several independent studies have shown that serum LDH levels predict poor prognosis in tumor related disease, including breast cancer, colorectal cancer, non-small cell lung cancer, endometrial cancer and gastric cancer (27). Studies have shown that high LDH level before treatment (LDH >245 U/L) is an independent risk factor for poor prognosis in NPC patients (28). Another study about NPC patients with liver metastasis after radical treatment demonstrated that a higher LDH was more likely to have liver metastasis (29). Similarly, as expected, a higher LDH level had a poorer OS (P=0.029), PFS (P<0.001), LRFS (P<0.001) and DMFS (P=0.021) in our study, which is consistent with the previous results. More importantly, we further confirmed the prognostic role of LDH in patients with locally advanced NPC in low to middle incidence areas. Hence, LDH could be recommended as an objective indicator of tumor prognosis in locally advanced NPC patients. In addition, we also explored other factors that may influence the survival outcomes in NPC patients. EBV DNA status was corroborated as potential factors affecting OS, PFS, LRFS and DMFS (P<0.05) in our univariate Cox regression analysis. And some studies found that patients with positive plasma EBV DNA levels may have a poorer prognosis because of increased cervical lymph node burden (30).
To our knowledge, this is the first study of the combined prognostic value of the PNI and LDH in locally advanced NPC. Interestingly, in line with our hypothesis, PNI-LDH was a powerful prognostic factor. We divided the patients into four groups according to the different levels of PNI and LDH. Patients with a lower PNI and higher LDH had significantly poorer OS (P=0.012), PFS (P=0.001), LRFS (P=0.001) and DMFS (P=0.001) than others groups. Therefore, as we have studied, the combination of the two parameters can indeed affect the prognosis of locally advanced NPC patients. Furthermore, through the comparison of binary logistic regression and ROC curve, we found that the AUC of the PNI-LDH was 0.751 (P=0.001), which was the largest in these three indexes and demonstrated that the combination of them was superior to either score alone.
However, our study also has some limitations. First, the number of patients is relatively small, which may potentially bias our findings. Secondly, we only studied the level of indicators before treatment, dynamic levels of the indicators will be more meaningful. Therefore, further multicenter, large-sample, prospective randomized controlled trials are needed to confirm the effect of combination of PNI and LDH on the prognosis of patients with locally advanced NPC.
Conclusions
Pretreatment PNI and LDH are independent prognostic factors affecting survival outcomes in locally advanced NPC patients receiving definitive IC followed by CCRT. In terms of prognostic ability, their combination was superior to either score alone, which was first reported in our study. Therefore, the evaluation of pretreatment PNI and LDH, as a supplement to the traditional TNM staging system, may be an important parameter for choosing different treatment strategies. However, further researches are needed to confirm the importance of PNI and LDH in determining the different responses to treatment in cancer patients.
Acknowledgments
We thank our friend MUNGUR Ishanee Devi for checking our manuscript and correcting some grammar problems for us.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2033
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2033
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2033). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Cancer center of Union Hospital of Tongji Medical College of Huazhong University of Science and Technology (No. 2019S1054) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. Erratum in: CA Cancer J Clin 2020;70:313. [Crossref] [PubMed]
- Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015;16:645-55. [Crossref] [PubMed]
- Qiu WZ, Peng XS, Xia HQ, et al. A retrospective study comparing the outcomes and toxicities of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy for the treatment of children and adolescent nasopharyngeal carcinoma. J Cancer Res Clin Oncol 2017;143:1563-72. [Crossref] [PubMed]
- Liu T, Sun Q, Chen J, et al. Neoadjuvant Chemotherapy with Fluorouracil plus Nedaplatin or Cisplatin for Locally Advanced Nasopharyngeal Carcinoma: a Retrospective Study. J Cancer 2018;9:3676-82. [Crossref] [PubMed]
- Sun W, Zhang L, Luo M, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck 2016;38:E1332-40. [Crossref] [PubMed]
- Wang L, Wang C, Wang J, et al. A novel systemic immune-inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2017;143:2077-86. [Crossref] [PubMed]
- Lee SH, Chung MJ, Kim B, et al. The Significance of the Prognostic Nutritional Index for All Stages of Pancreatic Cancer. Nutr Cancer 2017;69:512-9. [Crossref] [PubMed]
- Ikeya T, Shibutani M, Maeda K, et al. Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J Cancer Res Clin Oncol 2015;141:307-13. [Crossref] [PubMed]
- Cascante M, Centelles J, Veech R, et al. Role of thiamin (vitamin B-1) and transketolase in tumor cell proliferation. Nutr Cancer 2000;36:150-4. [Crossref] [PubMed]
- Oei RW, Ye L, Kong F, et al. Pre-treatment Serum Lactate Dehydrogenase is Predictive of Survival in Patients with Nasopharyngeal Carcinoma Undergoing Intensity-Modulated Radiotherapy. J Cancer 2018;9:54-63. [Crossref] [PubMed]
- Zhang M, Wei S, Su L, et al. Prognostic significance of pretreated serum lactate dehydrogenase level in nasopharyngeal carcinoma among Chinese population: A meta-analysis. Medicine (Baltimore) 2016;95:e4494 [Crossref] [PubMed]
- Wang J, Liu Y, Mi X, et al. The prognostic value of prognostic nutritional index (PNI) and neutrophil to lymphocyte ratio (NLR) for advanced non-small cell lung cancer treated with platinum-based chemotherapeutics. Ann Palliat Med 2020;9:967-78. [Crossref] [PubMed]
- Su L, Zhang M, Zhang W, et al. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e6364 [Crossref] [PubMed]
- Jiang W, Chen Y, Huang J, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with nasopharyngeal carcinoma: a propensity score-matched analysis. Oncotarget 2017;8:66075-86. [Crossref] [PubMed]
- Zhou T, Yu ST, Chen WZ, et al. Pretreatment albumin globulin ratio has a superior prognostic value in laryngeal squamous cell carcinoma patients: a comparison study. J Cancer 2019;10:594-601. [Crossref] [PubMed]
- Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001-5. [PubMed]
- Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [Crossref] [PubMed]
- Liu LT, Chen QY, Tang LQ, et al. The Prognostic Value of Treatment-Related Lymphopenia in Nasopharyngeal Carcinoma Patients. Cancer Res Treat 2018;50:19-29. [Crossref] [PubMed]
- He JR, Shen GP, Ren ZF, et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck 2012;34:1769-76. [Crossref] [PubMed]
- Yang L, Xia L, Wang Y, et al. Low Prognostic Nutritional Index (PNI) Predicts Unfavorable Distant Metastasis-Free Survival in Nasopharyngeal Carcinoma: A Propensity Score-Matched Analysis. PLoS One 2016;11:e0158853 [Crossref] [PubMed]
- Wei GB, Lu YY, Liao RW, et al. Prognostic nutritional index predicts prognosis in patients with metastatic nasopharyngeal carcinoma. Onco Targets Ther 2016;9:5955-61. [Crossref] [PubMed]
- Topkan E, Mertsoylu H, Kucuk A, et al. Low Systemic Inflammation Response Index Predicts Good Prognosis in Locally Advanced Pancreatic Carcinoma Patients Treated with Concurrent Chemoradiotherapy. Gastroenterol Res Pract 2020;2020:5701949 [Crossref] [PubMed]
- He Q, Huang Y, Wan G, et al. A novel prognostic marker based on risk stratification with prognostic nutritional index and age for nasopharyngeal carcinoma patients who received neoadjuvant chemotherapy. Biomark Med 2019;13:1013-23. [Crossref] [PubMed]
- Miao J, Xiao W, Wang L, et al. The value of the Prognostic Nutritional Index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J Cancer Res Clin Oncol 2017;143:1263-73. [Crossref] [PubMed]
- Gundog M, Basaran H. Pretreatment low prognostic nutritional index and low albumin-globulin ratio are predictive for overall survival in nasopharyngeal cancer. Eur Arch Otorhinolaryngol 2019;276:3221-30. [Crossref] [PubMed]
- Angelin A, Gil-de-Gómez L, Dahiya S, et al. Foxp3 Reprograms T Cell Metabolism to Function in Low-Glucose, High-Lactate Environments. Cell Metab 2017;25:1282-93.e7. [Crossref] [PubMed]
- Li G, Gao J, Tao Y, et al. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. Chin J Cancer 2012;31:197-206. [Crossref] [PubMed]
- Wan X, Wei L, Li H, et al. High pretreatment serum lactate dehydrogenase level correlates with disease relapse and predicts an inferior outcome in locally advanced nasopharyngeal carcinoma. Eur J Cancer 2013;49:2356-64. [Crossref] [PubMed]
- Zhou G, Tang L, Mao Y, et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys 2012;82:e359-65. [Crossref] [PubMed]
- Chen M, Yin L, Wu J, et al. Impact of plasma Epstein-Barr virus-DNA and tumor volume on prognosis of locally advanced nasopharyngeal carcinoma. Biomed Res Int 2015;2015:617949 [Crossref] [PubMed]


