
Correlation analysis of myonectin levels with metabolic and hormonal disorders in patients with polycystic ovary syndrome
Introduction
Polycystic ovary syndrome (PCOS) is a common reproductive endocrine metabolic disease, which is characterized by high androgen, menstrual dysfunction, and polycystic ovarian changes. The etiology of PCOS is multifactorial and has not yet been clarified. Studies have shown that familial and genetic factors may contribute to the predisposition of PCOS (1,2). Insulin resistance plays an important role in the development of PCOS, but its mechanism remains to be further elucidated (3-5). Although there are different opinions on the diagnosis of PCOS, high androgen and sparse ovulation or no polycystic ovarian changes are regarded as the important diagnostic basis. PCOS is associated with multiple metabolic abnormalities, including insulin resistance, type 2 diabetes mellitus (T2DM), obesity, and dyslipidemia. Myonectin, a newly discovered actin, belongs to the C1q/TNF-related protein (CTRP) family, also known as CTRP15. Myonectin is secreted by skeletal muscle and participates in glucose and lipid metabolism. Studies have shown that recombinant myonectin can promote the uptake of fatty acids in mice, thereby reducing the level of serum-free fatty acids (6). When fed with a high-fat diet (HFD), mice with myonectin deficiency can store more fat, and the degree of liver steatosis is lower, accompanied by increased insulin resistance and triglyceride (TG) levels (7). In addition, after oral administration of liposomes, the lipid clearance of myonectin-deficient mice was impaired (7). Two preclinical studies that concentrated on the relationship between myonectin and metabolic diseases showed that no increase or decrease of circulating myonectin was detected in subjects with T2DM, and the level of myonectin in obese people was lower than that in lean people (8,9). The above results indicate that myocintin is associated with a variety of endocrine and metabolic diseases, but whether it plays a role in the pathogenesis of PCOS is unknown (9). Therefore, we studied the correlation between myoctin level and the clinical characteristics of PCOS to explore its possible role in the pathogenesis of PCOS, and to provide a theoretical basis for looking for a new treatment method.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-458).
Methods
Participants
One hundred PCOS patients who sought medical advice from September 2017 to March 2019 in our hospital were selected as the PCOS group and had a mean age of 27.83 years (±5.62). One hundred healthy women matched with the PCOS patients’ age and body mass index (BMI) were selected as the control group and had a mean age of 28.14 years (±4.57). The inclusion criteria were as follows: (I) a clinical diagnosis consistent with PCOS; (II) no treatment that would affect glucose metabolism, lipid metabolism, or sex hormones; (III) no recent surgery or acute infection; (IV) onset of menarche more than 3 years and less than 36 years before enrolment in the study; (V) no history of pregnancy or abortion within 3 months. Exclusion criteria were as follows: (I) PCOS combined with Cushing’s syndrome, congenital adrenal hyperplasia or other adrenal diseases, thyroid dysfunction or other diseases causing menstrual cycle disorders or androgen excess; (II) PCOS accompanied by abnormal liver and kidney function: (III) PCOS associated with ovarian cyst or tumor. All participants in the study signed informed consent. This study was approved by the Ethics Committee of Wenzhou Hospital of Traditional Chinese Medicine Affiliated to Zhejiang Chinese Medical University (No. WZY2016-027). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Detection methods
- Fasting enous blood samples were collected on the 2nd to 5th day of menstruation. Six measures of sex hormone and sex hormone-binding globulin (SHBG) were detected by chemiluminescence immunoassay, while myonectin levels were detected by enzyme-linked immunosorbent assay (ELISA).
- Venous blood samples were taken after fasting and 1 and 2 h after oral glucose administration. The fasting blood glucose (FBG) and oral glucose tolerance test (OGTT) 2 h blood glucose levels were detected by the glucose oxidase method.
- Fasting venous blood samples were collected, and the levels of TG, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were detected by an Olympus automatic biochemical analyzer (OLYMPUS OPTICAL Co., Ltd.).
Outcome measures
- The levels of sex hormones, SHBG, and myonectin were compared between the two groups.
- The levels of FBG, OGTT 2 h, and the homeostasis model assessment of insulin resistance (HOMA-IR) in the two groups were compared [HOMA-IR = fasting insulin (FINS, µU/mL) × FBG (mmol/L)/22.5].
- The levels of blood lipid and BMI were compared between the two groups.
Statistical analysis
SPSS 21.0 software (SPSS, lnc., Chicago, IL, USA) was used to analyze the data, and results were expressed as mean ± standard deviation (SD). Data consistent with normal distribution were compared by independent sample t-tests. Pearson correlation analysis was used for the correlation analysis among all indices. P<0.05 was considered statistically significant.
Results
Comparison of general clinical information
There was no significant difference in age, BMI, systolic blood pressure (SBP), and diastolic blood pressure (DBP) between the two groups (P>0.05), which suggested that the two groups were comparable (Table 1).

Full table
Myosin and metabolic indicators
Compared with the control group, the levels of myostatin and HDL-C in the PCOS group were significantly decreased (P<0.05). FBG, HOMA-IR, OGTT 2h, and TG levels were significantly increased (P<0.05) (Table 2).
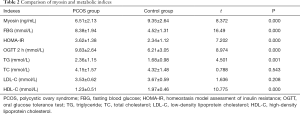
Full table
Sex hormone levels
Compared with the control group, the luteinizing hormone (LH) level of the PCOS group was significantly higher, and the SHBG level was significantly lower (P<0.05). There was no significant difference in follicle-stimulating hormone (FSH), estradiol (E2), testosterone, or progesterone between the two groups (P>0.05) (Table 3).

Full table
Pearson analysis of the correlation between myonectin and metabolic and hormonal indices of PCOS patients
The level of myonectin was negatively correlated with BMI, FBG, HOMA-IR, TG, and testosterone but was positively correlated with SHBG and HDL-C (Table 4).
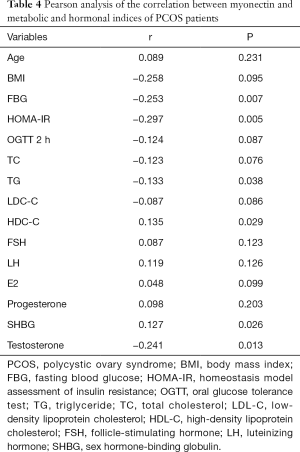
Full table
Multiple linear regression analysis of the correlation between myosin and the metabolism and hormones of PCOS patients
The level of myosin was negatively correlated with BMI, HOMA-IR, and TG but was positively correlated with SHBG and HDL-C (Table 5).
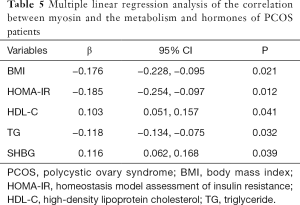
Full table
Discussion
PCOS is the most common endocrine disease in women of childbearing age in China. It is a group of clinical syndromes characterized by dysgenesis, endocrine abnormalities, metabolic disorders, and mental health problems (10). PCOS not only affects the fertility of patients but can also affect their pregnancy and long-term health. Therefore, it is crucial to clarify the pathogenesis and identify the early metabolic and hormonal disorders associated with PCOS to improve patients’ long-term quality of life. Cytokines and growth factors (muscle factors) secreted by skeletal muscle cells can participate in the metabolic regulation process through autocrine, paracrine, and endocrine effects (11,12). Myonectin is a new muscle factor discovered in recent years, which plays an important role in regulating glucose and lipid metabolism (6). However, there are few studies on the effect of myonectin in the metabolic and hormone disorders of PCOS. In this article, we compared the levels of myonectin between PCOS women and healthy women and the general clinical information, metabolism, and sex hormone-related indicators of the two groups of patients, and analyzed the correlation between myonectin, metabolism, and hormones.
Preclinical data analysis shows that myonectin has a potential application value in the treatment of metabolic diseases (7). Although there have been reports about the relationship between myonectin and metabolic disorders, the results in T2DM patients are not consistent. In patients with impaired glucose tolerance and T2DM, the level of myonectin is increased, which is positively correlated with body weight, TG, FBG, and insulin resistance. Moreover, subjects with a high level of myonectin are at increased risk of impaired glucose tolerance and diabetes (8). On the other hand, Li et al. pointed out that in subjects with T2DM, the level of myonectin decreased. Their research showed that the level of myonectin was negatively correlated with insulin resistance, BMI, visceral fat, TG, LDL-C, TC, and HbA1c but positively correlated with HDL-C (9). In another study, obese women experienced a significant increase in myonectin levels after eight weeks of aerobic exercise, accompanied by insulin resistance and significant weight loss (13). Our results suggest that the circulating myonectin levels in the PCOS group were lower than in the control group. In addition, the level of myonectin was negatively correlated with BMI, LH, FBG, HOMA-IR, and testosterone, and positively correlated with SHBG and an increased risk for PCOS.
There are differences in the results of many studies on myonectin, which may be due to the heterogeneity of the study populations. PCOS subjects usually have lipid metabolism disorder, accompanied by lower HDL-C levels and higher TG levels. We found that circulating HDL-C levels were decreased in the PCOS patients, while TG levels were consistently higher than in the control group. Our study showed that the level of myonectin was negatively correlated with TG and positively correlated with HDL-C. Therefore, the decrease in myonectin levels may be an important cause of lipid metabolism disorder in PCOS patients.
Our research also has some limitations. In this study, we used the HOMA-IR index instead of the hyperinsulinemic-euglycemic clamp technique to evaluate the level of insulin resistance. Although this cross-sectional design study cannot confirm the causal relationship between molecules and diseases, it assists in determining the correlation between them. In conclusion, low levels of myonectin are associated with hormone and metabolic abnormalities in PCOS patients. Myonectin may increase the risk of PCOS by participating in the pathophysiological process of PCOS.
Future and prospect
Based on the current research progress of intestinal flora and glucose and lipid metabolism, it can be inferred that metabolic abnormality is the key factor in the occurrence of PCOS, and genetic polymorphism of specific population leads to its susceptibility to metabolic changes. This type of PCOS is known as metabolic associated polycystic ovary syndrome (MAPS). Combined with our study, the dysregulation of myocinetin expression may play an important role in the pathogenesis of MAPS. The proposal of MAPS is conducive to changing the current clinical practice and clinical research thinking with anti-androgen as the main strategy, emphasizing more on etiological treatment. Therefore, myocinetin may be a potential therapeutic target for MAPS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-458
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-458
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-458). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Wenzhou Hospital of Traditional Chinese Medicine Affiliated to Zhejiang Chinese Medical University (No.WZY2016-027). All participants in the study signed informed consent. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ehrmann DA, Liljenquist DR, Kasza K, et al. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91:48-53. [Crossref] [PubMed]
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774-800. [PubMed]
- Azziz R, Woods KS, Reyna R, et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 2004;89:2745-9. [Crossref] [PubMed]
- March WA, Moore VM, Willson KJ, et al. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 2010;25:544-51. [Crossref] [PubMed]
- Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 2005;352:1223-36. [Crossref] [PubMed]
- Seldin MM, Peterson JM, Byerly MS, et al. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 2012;287:11968-80. [Crossref] [PubMed]
- Little HC, Rodriguez S, Lei X, et al. Myonectin deletion promotes adipose fat storage and reduces liver steatosis. FASEB J 2019;33:8666-87. [Crossref] [PubMed]
- Li K, Liao X, Wang K, et al. Myonectin Predicts the Development of Type 2 Diabetes. J Clin Endocrinol Metab 2018;103:139-47. [Crossref] [PubMed]
- Li Z, Yang YL, Zhu YJ, et al. Circulating Serum Myonectin Levels in Obesity and Type 2 Diabetes Mellitus. Exp Clin Endocrinol Diabetes 2019; Epub ahead of print. [Crossref] [PubMed]
- Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol 2018;182:27-36. [Crossref] [PubMed]
- Pedersen BK. Muscles and their myokines. J Exp Biol 2011;214:337-46. [Crossref] [PubMed]
- Pedersen BK, Akerström TC, Nielsen AR, et al. Role of myokines in exercise and metabolism. J Appl Physiol (1985) 2007;103:1093-8. [Crossref] [PubMed]
- Pourranjbar M, Arabnejad N, Naderipour K, et al. Effects of Aerobic Exercises on Serum Levels of Myonectin and Insulin Resistance in Obese and Overweight Women. J Med Life 2018;11:381-6. [PubMed]
(English Language Editor: D. Fitzgerald)


