
Mechanism of hepatic stellate cell proliferation and apoptosis in rats by promoting circulation, removing stasis, and dredging collaterals based on the theory of liver collateral disease
Introduction
Hepatic fibrosis (HF) is one of the pathological processes underlying chronic liver disease that results from various causes and is also the process through which cirrhosis develops. HF is a complex process that develops dynamically. It is a multi-gene, multi-cell, and multi-factor co-regulation process that participates in the occurrence and development of liver fibrosis (1). Hepatic stellate cells (HSCs) are the key cells involved in HF, resulting in fiber deposition, liver tissue remodeling and disorder, and acceleration of HF (2). The pathogenic factors of liver fibrosis are activated by HSCs, and the activated HSCs follow the evolution of “HSCs-myofibroblast-extracellular matrix-hoof tissue and collagen fibers”. Therefore, HSCs are the core elements of liver fibrosis. Consequently, inhibiting HSC activation is an important therapeutic target for the treatment of liver fibrosis.
As the liver belongs to the yin in physique and yang in function, unobstructed liver collaterals can play a role in liver catharsis and regulate changes in qi and the blood. The liver acts through liver collaterals. HF can be induced by various means, such as qi transportation and distribution disorders, collateral qi stagnation, and qi-blood-body fluid movement disorders in the liver collaterals. Qi is a commander of the blood, and stagnation in qi leads to poor blood flow, blood stasis, and collateral obstruction, resulting in masses under the ribs and accumulation of turbid phlegm in the liver collaterals, finally leading to liver fibrosis and cirrhosis. Our previous study showed that Chinese medicine interventions can effectively control the progress of liver fibrosis in children with biliary atresia after Kasai surgery (3). Therefore, to further investigate the mechanisms of traditional Chinese medicine (TCM) in anti-liver fibrosis therapy, this study used Chinese medicine containing serum to intervene HSC-T6 according to the theory of liver collateral disease to explore its effect on the treatment of liver fibrosis. This provides the theoretical basis for the prevention and treatment of liver fibrosis by TCM. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1545).
Methods
Animals
A total of 27 Wistar male rats (weight: 220±20 g, grade: clean) were provided by Charles River Laboratories (Beijing, China). Experiments were performed under a project license (No. 1103241911038659) granted by ethics committee of Beijing Children’s Hospital, in compliance with the Guide for the Use of Laboratory Animals.
Cells
The HSC-T6 cell strain has an immortalized phenotype. The HSC-T6 cell strain and SV40-transfected HSC of rats were provided by Hunan Fenghui Biotechnology Co., Ltd. (Hunan, China).
Medicine
The preparation of the TCM, including the ingredients used, was in line with the Chinese Pharmacopoeia 2015. The ingredients included raw malt, herba artemisiae scopariae, longhairy antenoron herb, tetrapanax papyriferus, salviae miltiorrhizae, lycopus lucidus, cortex phellodendri, poria cocos, atractylodes, peach kernel, safflower, radix paeoniae rubra, and turtle shell. The herbs were purchased from Beijing Bencao Fangyuan Pharmaceutical Co., Ltd. (Beijing, China), lot No.: 20190320. Colchicine tablets (0.5 mg/tablet, lot No.: 190406-01) were purchased from Kunming Pharmaceutical Group Co., Ltd. (China).
Reagents and instruments
A DMEM medium and fetal bovine serum (FBS) were purchased from Gibco, cell counting kit-8 (CCK-8) reagents were purchased from Solabel company, a CO2 incubator was obtained from Sanyo, a Cell Cycle Detection Kit was purchased from Jiangsu KeyGEN BioTECH Corp., Ltd., a super clean workbench was obtained from Suzhou Purification Equipment Co., Ltd., a biological inverted microscope was purchased from Olympus, and a desktop low-speed centrifuge was obtained from Medical Equipment Plant of Shanghai Medical Device Co., Ltd.
Study methods
Preparation of the drug serum
Wistar rats were kept at room temperature of 21–25 °C with 50–60% relative humidity, living in a half day and half night environment. They were fed together and given free access to water. The 27 Wistar rats were randomly divided (by drawing lots) into the blank control group (BC group, n=3), positive control group (n=3), and TCM groups (n=3, 0.24 g/mL, 0.73 g/mL, 1.22 g/mL, 2.45 g/mL, 7.34 g/mL, 12.24 g/mL and 24.48 g/mL). The rats in the BC group were fed 1 mL/100 g distilled water. The rats in the positive control group were given 1 mL/100 g colchicine solution (0.05 mg/mL). The different fold dosages for the rats were converted according to the actual clinical dosages of children in each TCM group based on the third edition of Experimental Methodology of Pharmacological, namely 1 mL/100 g each time for drench, twice a day, with an interval of 8 h, administered for 7 consecutive days (4). The rats were anesthetized with 2% pentobarbital sodium by intraperitoneal injection 1 h after the final administration. Blood was taken from the abdominal aorta under aseptic conditions. After standing for four hours at 4°C, the blood was centrifuged for 20 min at 3,000 r/min. The supernatant was absorbed and inactivated in a water bath for 30 min at 56 °C, filtered with a 0.22 um micropore filter, and refrigerated at –80 °C standby.
HSC-T6 culture
HSC-T6 were cultured in a DMEM medium containing 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin in a constant-temperature incubator with 5% CO2 and saturated humidity at 37 °C. The solution was changed every 2–3 days. Digestive passage was conducted when the cells were covered to 80%. A serum-free starved culture was performed for 24 h to synchronize the cell cycle. According to the concentration required in the experiment, the samples were hatched in a 6-well plate and a 96-well plate and divided into drug serum groups (0.24 g/mL, 0.73 g/mL, 1.22 g/mL, 2.45 g/mL, 7.34 g/mL, 12.24 g/mL, and 24.48 g/mL groups), a positive control group, and a BC group with drug-free serum culture medium. The supernatant was discarded after the cells were attached to the wall. The 10% drug serum culture solution and DMEM were added for inoculation according to the groups. The cells were collected for testing after being cultured for the length of time required by the experiment.
Cell viability tested by cell counting kit-8 (CCK-8)
The logarithmic growth cells were collected, and the cell suspension (100 µL/well) was inoculated in a 96-well plate. The culture plate was placed in an incubator and hatched for 24 h at 37 °C with 5% CO2. The culture solution was changed to a DMEM medium without serum and continued to culture in the incubator for 24 h. The control group was changed to a serum-free medium, and the other groups were changed to a TGF-β1 serum-free medium to continue culturing for 24 h. The corresponding medium containing 10% drug serum was added to each group and continuously cultured for 24 h. A total of 10 µL CCK-8 10 reaction solution was added to each well. The culture plate was placed in the incubator and incubated for 2 h at 37 °C. The absorbance (OD value) was measured at 450 nm by ELISA and repeated three times. The cell proliferation inhibition rate (%) = (absorbance of 1-test/blank control group) ×100%.
Apoptosis rate tested by flow cytometry
The cells in the tube were transferred to the flow cytometry (FCM) tube and washed with cold PBS twice. Following this, 1× binding buffer was added to resuspend the cells. The cell concentration was adjusted to 2×105/mL. Subsequently, 2 µl Annexin VFITC and 2 µL propidium iodide staining solution were added, and the tube was mixed gently and incubated for 15 min at 4 °C without light. FCM was used for testing (Q2 zone: advanced apoptotic cells; Q3 zone: early apoptotic cells), and Cell Quest software was used to analyze the results. The experiment was repeated three times.
Cell cycle detection by FCM
The cells were washed once with precooled PBS and centrifuged at a speed of 1,000 r/min for 5 min. The supernatant was subsequently discarded. RNase A was mixed with propidium iodide at a ratio of 1:4 and blended. A total of 500 µL was added to each sample and incubated for 30 min at 37 °C. The DNA stages were analyzed by FCM. The red fluorescence at 488 nm of the excitation wavelength and the results of Modfit software analysis were recorded. The experiment was repeated three times.
Statistical analysis
Data were statistically analyzed using SPSS 22.0 and expressed as mean ± standard deviation (). Comparisons between the groups and time points were conducted using one-way analysis of variance (ANOVA). P<0.05 was considered statistically significant.
Results
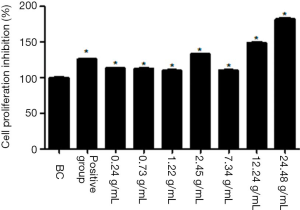
As shown in Figure 1, the cellular proliferation inhibition rate in the positive control group and all the TCM groups increased significantly 24 h after the drug serum had acted on the cells. The difference in each group was statistically significant compared with the BC group (P<0.01). In addition, the inhibition rate of the 24.48 g/mL TCM group was the highest, which showed that the TCM could inhibit proliferation of HSCs. It was observed that by promoting circulation, removing stasis, and dredging collaterals, the prescription could inhibit the activity of the HSCs.
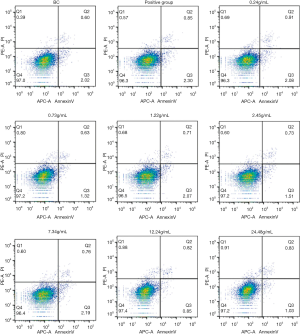
As shown in Table 1 and Figure 2, the cellular apoptosis rate was <3% in the Q2 and Q3 zones. The apoptosis rate was not clear, and the difference between the two groups was not significant (P>0.05). This indicated that by promoting circulation, removing stasis, and dredging collaterals, the prescription did not alleviate or reverse liver fibrosis through an HSC apoptosis mechanism.
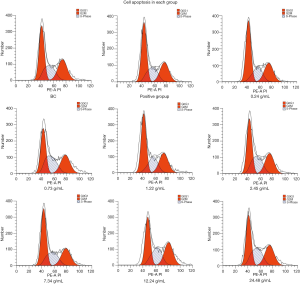
As shown in Table 2 and Figure 3, the number of cells in the positive control group, 0.24 g/mL TCM group, 1.22 g/mL TCM group, and 7.34 g/mL TCM group increased significantly in the G0/G1 phase compared with the BC group, and the difference was statistically significant (P<0.05). The number of cells in the positive control group and all the TCM groups increased significantly in the S phase. The difference was statistically significant in the 0.73 g/mL TCM group, 2.45 g/mL TCM group, 12.24 g/mL TCM group, and 24.48 g/mL TCM group compared with the BC group (P<0.05). The number of cells in the positive control group and all the TCM groups decreased significantly in the G2/M phase. The difference was statistically significant in the positive group, 0.24 g/mL TCM group, 0.73 g/mL TCM group, 1.22 g/mL TCM group, and 2.45 g/mL TCM group compared with the BC group (P<0.05). Based on these results, it is thought that by promoting circulation, removing stasis, and dredging collaterals, TCM therapy based on the theory of liver collateral disease can inhibit the activation of HSCs by blocking the cell cycle in the G0/G1 and S phases.
Discussion
The effect of TCM in the treatment of liver fibrosis has been affirmed by the medical community, clinical common single Chinese medicine, compound medicine, effective ingredients of Chinese medicine and acupuncture and moxibustion treatment, Chinese medicine compound W multi-component, multi-directional, multi-channel, multi-level advantages, has obtained an outstanding curative effect in the treatment of fibrosis (5). The theory of liver collateral disease is one of the important components of TCM collateral disease theories. The collaterals of the human body are widely distributed, and collateral disease is a series of symptoms that occur under collateral damage, which is characterized by blood stasis and qi stagnation and manifesting in visceral dysfunction. As the liver belongs to the yin in physique and yang in function, unobstructed liver collaterals can play a role in liver catharsis and regulate changes in qi and the blood (6). The liver acts through liver collaterals. Liver collateral malnutrition in children can be induced by various means, such as qi transportation and distribution disorders, collateral qi stagnation, and qi-blood-body fluid movement disorders in the liver collaterals. Qi is a commander of the blood, and stagnation in qi leads to poor blood flow, blood stasis, and collateral obstruction, resulting in masses under the ribs, qi-blood-body fluid retention, and accumulation of turbid phlegm in the liver collaterals, finally leading to liver fibrosis and cirrhosis. Liver collateral stasis and a deficiency in qi and blood may lead to the failure of transportation, distribution, and irrigation. The liver and gallbladder lose nourishment and become deficient in yin and yang, and qi, while blood in the collaterals causes a deficiency in qi, blood in the liver collaterals, and a loss of nourishment of the liver and gallbladder, which results in prolonged and repeated illness. Therefore, from the perspective of liver collateral disease theory, the treatment of liver fibrosis can be guided by the basic pathogenesis of qi and blood imbalance and the principle of unobstructed collaterals. Our previous study confirmed that children with postoperative biliary atresia treated with TCM had increased blood circulation, which removed blood stasis and softened hardness to dissipate stagnation. Furthermore, removing blood stasis and dredging collaterals improved the liver function more rapidly, smoothly, and effectively after a biliary atresia operation to effectively delay the progress of liver fibrosis (3).
In the TCM prescription mentioned above, raw malt was reused as a monarch to strengthen the spleen and soothe the liver. The use of raw materials takes on the meaning of growing and developing, which accords with the physiological characteristics of liver vitality. The administered drugs were combined with radix paeoniae rubra, lycopus lucidus, and salvia miltiorrhiza. These medicines are gentle and mainly affect the liver meridian and blood system, working to promote circulation, remove stasis, and dredge collaterals, as well as improving liver collateral stasis and stagnation. Furthermore, lycopus lucidus can enter the spleen meridian. Its scent refreshes the spleen, aids in the excretion of water, and reduces swelling, though its effect is slow. In combination with tetrapanax papyriferus, it can prevent and cure liver ascites resulting from water and blood stasis. Moreover, combining the treatment with other administered drugs, such as herba artemisiae capillariae, longhairy antenoron herb, and cortex phellodendri, helps clear the spleen and stomach and remove the dampness-heat of the liver and gallbladder. In addition, these drugs are important for treating jaundice; regardless of whether it is yang jaundice or yin jaundice, they are all compatible applications. Assistant with poria cocos and atractylodes, it takes the meaning of the four characters and can replenish qi and tonify the middle-jiao, strengthen the spleen, and nourish the stomach (7). Peach kernel and safflower work synergistically to activate the blood circulation and dredge the collaterals. The turtle shell is salty-cold in nature and heavy in substance. It not only nourishes the liver and kidney, but also softens hardness by dissipating stagnation. It can be used to treat the symptoms of lumps below the costal region, abdominal mass, and accumulation. The combination clears liver collateral stagnation, smooths qi and the blood of the liver collaterals, tonifies the deficiency of and damage to the liver collaterals, and therefore plays a role in anti-fibrosis and anti-cirrhosis. The anti-fibrosis and anti-cirrhosis mechanisms of the compound drugs have been studied. Through animal experiments, Niu and Wu (8) found that total flavonoids of artemisia stellariae can effectively protect the liver function and hydroxyproline content in liver tissues of experimental rats and block the progression of liver fibrosis at the same time. Furthermore, radix paeoniae rubra can restrict matrix hyperplasia by inhibiting collagen proliferation (9). Ye and Cao (10) confirmed that the mechanism of salvia containing serum to inhibit the proliferation of hepatic stellate cells down-regulates TGF-1 and CyclinD1 and up-regulates RXR- to control the development of HF. Therefore, the above-mentioned herbs were selected for the experimental study to protect the liver based on the theory of liver collateral disease.
HSCs are the main cells in the liver that synthesize the extracellular matrix (11). They are in a state of rest in a normal liver. However, when the liver is damaged by inflammation or mechanical stimulation, activation and proliferation of HSCs can drive the occurrence of liver fibrosis. Therefore, inhibiting HSC proliferation and promoting HSC apoptosis are key factors in the treatment of liver fibrosis. Both cellular proliferation and apoptosis are closely related to the regulation of the cell cycle (12). The cell cycle is divided into the DNA synthetic prophase (G1), DNA synthetic phase (S), DNA synthetic anaphase (G2), and cell division phase (M). The G0/G1 and G2/M phases of the cell cycle have cell cycle control checkpoints that can ensure the function of cell mitosis synchronization and ensure that the cell cycle begins downstream events when upstream events are completed correctly (13). This study used FCM to determine the different periodic phases of cells according to the DNA content in the G0/G1, S, and G2/M phases. The results revealed that promoting blood circulation, removing blood stasis, and dredging collaterals could block the G0/G1 and S phases of HSC-T6 based on the theory of liver collateral disease; in addition, it could prevent them from entering the G2/M phase normally, thereby reducing the number of cells in the G2/M phase. Therefore, this method may block HSC-T6 in the G0/G1 and S phases and inhibit the proliferation of HSC-T6 to achieve anti-hepatic fibrosis.
Apoptosis is a programmed cell death pattern distinguished from the concept of necrosis (14). HSC apoptosis is an important method for eliminating activation, that is apoptotic processes can reduce the number of activated HSCs without causing damage to organelles, such as lysosomes, or inflammatory damage to the microenvironment (15). In the present study, the apoptosis of HSCs was detected by FCM Annexin V FITC/PI double staining. The apoptosis rate of each group was not clear based on the results. This suggests that activating blood circulation, removing blood stasis, and dredging collaterals may not play an antagonistic role in HF by inducing the apoptosis of HSCs.
Conclusions
The mechanism of liver fibrosis is highly complex and involves numerous cytokines, regulatory proteins, and signaling pathways (16). Therefore, it is necessary to determine the target of anti-liver fibrosis in rats by exploring the activation of the blood circulation, removal of blood stasis, and dredging of collaterals in suppressing the activation of HSCs based on the theory of liver collateral disease. The present study indicates that TCM can inhibit the proliferation of HSCs and block the cell cycle in the G0/G1 and S phases based on the theory of liver collateral disease and can therefore play a role in inhibiting HSC activation and anti-hepatic fibrosis. The application of TCM demonstrated a concentration-dependent trend. However, the present study did not confirm whether this method achieved its anti-liver fibrosis effects by suppressing HSC apoptosis. Therefore, further research into methods for inhibiting HSC activation is needed.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff that implemented the intervention and evaluation components of the study.
Funding: This study was funded by the Capital’s Funds for Health Improvement and Research (CFH 2020-2-2092). The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1545
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1545
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1545). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No.: 1103241911038659) granted by ethics committee of Beijing Children’s Hospital, in compliance with the Guide for the Use of Laboratory Animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elpek GO. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: an update. World J Gastroenterol 2014;20:7260-76. [Crossref] [PubMed]
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature 2008;453:314-21. [Crossref] [PubMed]
- Chen L, Hu Y, Yang M, et al. Intervention effect of Chinese medicine on infants after biliary atresia surgery and its long-term efficacy. Chinese Acute Medicine 2016;25:353-6.
- Xu SY, Bian RL, Chen X. Pharmacological Laboratory Methodology (Third Edition). Beijing: People's Health Press, 2002, 180.
- Xie JZ, Zhao R, Wang L, et al. Effects of sinomenine on proliferation and apoptosis of human hepatic stellate cells in vitro. Zhonghua Gan Zang Bing Za Zhi 2019;27:146-8. [PubMed]
- Xu G. Connotation and definition of the extension of Luo disease. Journal of Chinese Medicine 2005;1:96-8.
- Hu Y. Pei Xueyi Paediatric Clinical Evidence Hundred Cases. Beijing: People's Health Publishing House, 2013, 21.
- Niu S, Wu Z. Hepatoprotective effect of Artemisia capillaris Thumb flavones extract on chronic liver injury induced by carbon tetrachloride in rats. Med J Chin PAPF 2015;26:162-5.
- Zhang J, Han Y. Advances in the experimental study of anti-hepatic fibrosis with single drug. Asia-Pacific Traditional Medicine 2014;10:38-42.
- Ye F, Cao W. Mechanism of Danshen drug-containing serum on TGF-β1 and CyclinD1 in hepatic stellate cells. Journal of Chongqing Medical University 2016;41:369-73.
- Wan Z, Hu G. The relationship between Wnt signaling pathway and liver fibrosis. World Journal of Chinese Digestion 2011;19:1761-6. [Crossref]
- Wu D, Gu QH, Li ZW. Cyclin-dependent kinase, cell cycle regulation and liver fibrosis. World Chinese Digestive Magazine 2013;21:2158-63. [Crossref]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science 1989;246:629-34. [Crossref] [PubMed]
- Shackelford RE, Kaufmann WK, Paules RS. Cell cycle control, checkpoint mechanisms, and genotoxic stress. Environ Health Perspect 1999;107:5-24. [PubMed]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972;26:239-57. [Crossref] [PubMed]
- Fan EY, He SQ, Wen B, et al. Effects of BieJlajian Pill on Proliferation and Apoptosis of Hepatic Stellate Cells in Mice. Chinese Journal of Integrative Medicine 2018;36:960-4.




