
A low proportion of regulatory T cells before chemoradiotherapy predicts better overall survival in esophageal cancer
Introduction
Esophageal cancer (EC) ranks eighth among all cancers in global incidence (1), with nearly half or more of these cases occurring in China (2). In China, squamous cell cancer (SCC) is the most common type of EC, accounting for about 90% of all the patients with EC (3), and most patients with esophageal squamous cancer (ESCC) are diagnosed as advanced and inoperable (4). Definite concurrent chemoradiotherapy (CCRT) is considered the standard treatment for locally advanced nonoperative patients, and can offer better improvement of overall survival (OS) than can radiotherapy alone (RT) (5). Based on the Radiation Therapy Oncology Group (RTOG) 94-05 study, 50.4 Gy has been made the standard dose in Western countries (6), but a global consensus regarding the exact CCRT radiation dose for EC world has not yet been reached.
In cancer treatment, ascertaining the prognosis of patients is essential, and one of the most critical factors affecting prognosis is whether or not the patient’s immune system is functional. The immune system functions either to eliminate cancer cells or to keep cancer cells in check by maintaining an equilibrium; if there is a malfunction in the immune system, immune escape may happen which then allows cancer cells to grow into clinically apparent tumors (7). Lymphocyte subsets are the most critical of the immune cells for providing immunity to patients (8). Therefore, analyzing these lymphocyte subsets may have clinical significance for the prognostic evaluation of patients with EC. The immune function of tumor patients primarily consists of T lymphocyte subsets, natural killer (NK) cells, and B Cells, and can be indicated by the proportions of CD3+, CD4+, CD8+ T cells, and CD4+CD8+ double-positive cells, the ratio of CD4+/CD8+, NK Cells, NKT Cells, and regulatory T cells, and the total B cell count. Thus far, most research on the immune status of EC cells has been based on T lymphocyte subsets and NK cells (9-11). Although many immunological markers have been discovered and used to predict the prognosis of patients with solid malignancies, there is no consensus on the relationship between these markers and the prognosis of EC. Consequently, we investigated the value of peripheral blood T lymphocyte subsets (the proportions of CD4+ helper T cells, the proportions of CD8+ cytotoxic T cells, the proportions of CD4+ helper T cells/the proportions of CD8+ cytotoxic T cells, the proportions of CD4+CD8+ double-positive T cells, and the proportions of CD4+CD25HICD127LOW regulatory T cells) in predicting OS in patients with EC before chemoradiotherapy. According to the ROC curve, regulatory T cells with the highest diagnostic value were selected from the peripheral blood T lymphocyte subsets for detailed analysis.
Labeling methods differ from laboratory to laboratory. Regulatory T cells (Treg) are widely accepted to play a key role in the immune tolerance and escape of cancer cells. Originally Tregs were identified as being CD4+ T cells expressing high levels of CD25 (IL2 receptor). The identification of the transcription factor FoxP3 resulted in defining Tregs as CD4CD25HIFoxP3+ T cells by staining permeablized cells. Recently Tregs have been identified as CD4CD25HI T cells with low levels of CD127 (IL7 receptor) expressing a CD4+CD25HICD127LOW cell surface phenotype (12). This similar report (13) is about regulatory T cells (CD45+CD4+CD25HIFOXP3+) detected by flow cytometry using Foxp3 + markers, and found that the density of the FOXP3+ regulatory T cell (Treg) infiltrate present in the residual tumor (or its scar) correlated with the pathological response (the less Tregs the more pronounced was the histological response) and predicted cancer-specific survival. Here, another marker (CD127LOW) was used to detect regulatory T cells (CD45+CD4+CD25HICD127LOW), and the proportion of regulatory T cells before treatment was used to predict the prognosis of OS. Compared with FOXP3 detection, CD127 detection is more convenient, the patient is easy to collect blood, the doctor is convenient to operate; in addition, the detected cells can still be used for cell culture and functional tests. Therefore, the selection of CD127LOW as a molecular marker in this study has practical significance (14,15). The novelty of this paper lies in the use of CD127-labeled Treg, aiming at the effect of pre-treatment Treg proportion on prognosis, while most T-cell-related articles observe the change of proportion before and after treatment.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-196).
Methods
Patients
Nonoperative chemoradiotherapy patients with EC diagnosed at Shanxi Cancer Hospital from January 2015 to January 2020 were retrospectively examined. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Based on the national laws in China, the study did not require the approval of an ethics committee or informed consent due to its retrospective nature.
The inclusion criteria for patients were the following: (I) diagnosis of EC; (II) non-operative patients undergoing chemoradiotherapy in our hospital; (III) ≤80 years of age; (IV) first treatment in our hospital. Meanwhile, the exclusion criteria were the following: (I) history of another malignancy or mixed pathological types; (II) incomplete laboratory data; (III) dysfunction of significant organs; (IV) related concomitant diseases (such as significant organ dysfunction, diabetes, hypertension, moderate-to-severe heart disease, moderate-to-severe kidney disease, acute pneumonia or pancreatitis, etc.) that might have affected or potentially have affected the study parameters.
Analysis of prognostic factors and stratification
The laboratory and clinicopathological data of the included patients were extracted from electronic medical records. The collected data included general information (sex, age, Karnofsky Performance Status (KPS), body mass index (BMI), smoking history, family history of cancer), diagnostic information (pathological type, clinical staging, gastroscope description including position and partition), treatment-related information (radiation dose, number of radiotherapy/chemotherapy cycles), and immune function (proportion of CD4+, CD8+ T cells, and CD4+CD8+ double-positive cells, and the ratio of CD4+/CD8+ and regulatory T cells). Flow cytometry was used to detect the proportions of CD4+, CD8+, and regulatory T cells on the surface of peripheral blood T lymphocytes. All data were collected from the test taken closest to the time before the first treatment. Receiver operating characteristic (ROC) curves were used to determine the optimal boundary value of immune function data for continuous variables.
The primary endpoint of assessment was patient OS, which was defined as the time from pathologic diagnosis to the time of the last follow-up or death from any cause. For chemoradiotherapy treatment, patients were administered all radiation treatments as either three-dimensional conformal radiation therapy (3D-CRT) or intensity-modulated radiation therapy with standard fractionation (1.8/2.0 Gy fractions once daily for 5 days/week). Patients treated with a total dose of 50–70 Gy. The chemotherapy regimen mainly included a docetaxel and cisplatin (DP) regimen or a paclitaxel and cisplatin (TP) regimen.
Statistical analysis
First, cutoff values were determined by the ROC curves. All patients were then divided into two groups based on the proportions of regulatory T cells. The correlation between regulatory T cell proportion and clinicopathological characteristics was analyzed by chi-square test. Univariate survival analysis was performed using the Kaplan-Meier method, and the difference in the survival curve was evaluated by the log-rank test. Independent prognostic factors for OS were determined by univariate and multivariate Cox proportional regression models. All analyses were bilateral, and significance was set at a P value of 0.05. SPSS Statistics v.26 (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Results
Patient characteristics and grouping
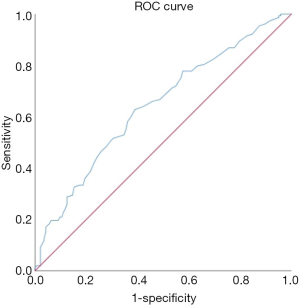
The mean pretreatment proportion of regulatory T cells (%) in the 297 patients was 5.47%±1.91%, with a median of 5.20% (range, 1.30–13.80%). We used the continuous variable of regulatory T cells as the test variable and OS as the state variable. When we studied the cutoff value of the regulatory T cells using the ROC curve, we calculated that the appropriate cutoff value of the regulatory T cells was 5.15% (the sensitivity was 62.7% and the specificity was 61.1%) (Figure 1). Therefore, we set 5.15% as the cutoff value and divided the patients into a high-regulatory T cell group (>5.15%; n=145, 48.8%) and a low-regulatory T cell group (≤5.15%; n=152, 51.2%) to predict prognosis. Of the clinicopathological features analyzed, the proportion of pretreatment regulatory T cells were significantly associated with tumor location and the proportion of CD4+CD8+ double-positive cells (Table 1).
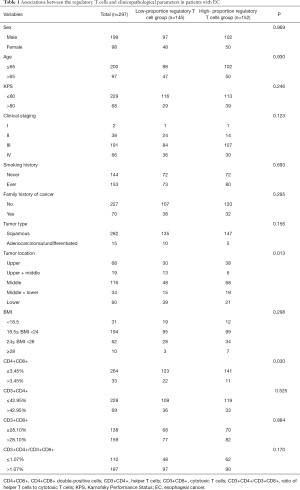
Full table
Intergroup comparison of patients’ OS
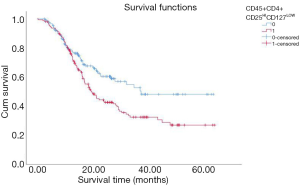
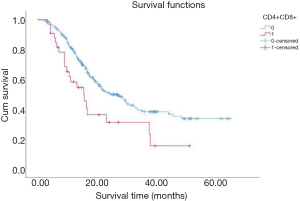
By the end of May, 2020, 153 out of the total 297 patients had died, with 135 dying due to cancer. To investigate whether high regulatory T cells reflected a poor prognosis, Kaplan-Meier analysis and log-rank tests were performed. The result showed that patients with a high proportion of regulatory T cells had a significantly worse OS than patients with a low proportion of regulatory T cells (Figure 2). Furthermore, the OS of patients with a low proportion of CD4+CD8+ double-positive cells was lower than that of patients with a high proportion of CD4+CD8+ double-positive cells (Figure 3).
Univariate and multivariate analysis
Two factors were found to be associated with better OS on univariate analysis: CD4+CD8+ double-positive cell proportion >3.45% (P<0.05) and CD45+CD4+CD25HICD127LOW regulatory T cell proportion ≤5.15% (P<0.05). Conversely, multivariate analysis indicated that KPS, tumor location, and proportion of CD4+CD8+ double-positive and proportion of regulatory T cells, were independent risk factors for poor OS (Table 2).
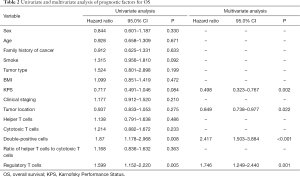
Full table
Discussion
In our study, a high proportion of CD4+CD8+ (3.5%) double-positive cells and a low proportion of CD45+CD4+CD25HICD127LOW regulatory T cells independently predicted that 297 cases of EC would have better OS before chemoradiotherapy. To our knowledge, this is the first study to examine the correlation of CD4+CD8+ double-positive T cells and regulatory T cells on the prognosis of patients with EC before non-operative chemoradiotherapy.
T cells are universally considered to be the most important immune cells in the field of antitumor action. Based on their functions and surface markers, they can be classified into four categories: cytotoxic T cell (Tc), helper T cell (Th), regulatory/inhibitory T cell (Treg/Ts), and memory T cells. In general, CD3 cells represent mature lymphocytes and are the primary active cells in cellular immunity, while CD4+ and CD8+T cells can be divided into two subgroups according to their different surface marker functions (16). CD4+T cells mainly refer to helper T cells, which are critical for regulating the immune response of the body. They can regulate the immune response of other T cells, assist B cells in secreting antibodies, and play a vital role in antitumor immunity (17). CD8+T cells are another group of T cells. CD8+ T cells, which often show cytotoxic activity, are the cytotoxic effector cells (CD8+ CTL) and the primary T cells involved in antitumor activities (18). The most commonly studied indicators of immune function in the literature are CD4+ T cells, CD8+T cells, and the ratio of CD4+ T to CD8+ T cells.
In recent years, CD4+CD8+ double-positive T lymphocytes have garnered intense interest in the field of HIV infection, but studies regarding these cells’ relation to cancer are rare, with the role of CD4+CD8+ double-positive T cells in cancer prognosis remaining largely unexamined. Mature CD4+CD8+ double-positive T cells exist in peripheral blood and tissues in various environments, including in human cancers (19), and they have been reported to play a suppressive role in cancers, including metastatic colorectal cancer (20). The proportion of CD4+CD8+ double-positive T cells in the tumor-infiltrating lymphocyte pool has been found to be elevated relative to single-positive CD4+ T cells or CD8+ T cells (20); however, to our knowledge, no literature related to EC has been published. Regulatory T cells control immune responses to foreign antigens and antigens associated with the altered self, such as in the case of tumors. Studies have shown that the immune system of the body is immune to tumor cells through the immunosuppressive effect of CD4+CD25+ regulatory T cells Low or no response level of epidemic response leads to immune escape of tumors, which is conducive to the occurrence and development of tumors. With the progress of the disease, the number of CD4+CD25+ regulatory T cells will also increase, affecting the prognosis of tumor patients. Increased frequencies of tumor regulatory T cells are often correlated with the poor prognosis of patients with cancer, which is likely to be a consequence of regulatory T cell-mediated suppression of antitumor immunity (21). In this study, the presence of a high proportion of regulatory T cells predicted worse OS. Further studies of this indicator are needed to fully elucidate their contribution to the immune response in the prognosis of EC.
The main limitations of the study include its retrospective design, single-center scope, and small sample size. Furthermore, there were several underlying confounders that we could not control, such as: radiotherapy time interval, the physician’s ability in radiotherapy techniques. Therefore, a prospective, multicenter, clinical, large-scale trial is needed to confirm our results. Moreover, for patients with unoperated EC, information on the pathological stage could not be obtained, and the subjective judgment of the clinical staging might not have been accurate. Thus, further research is needed to provide a more comprehensive and accurate analysis.
In summary, regulatory T cells play a role in predicting the prognosis of patients with EC before chemoradiotherapy, and a low proportion of pretreatment regulatory T cells were demonstrated to independently predict a better OS.
Acknowledgments
The authors would like to thank Hou Ru for providing support: submission suggestions.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-196
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-196
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-196). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Based on the national laws in China, the study did not require the approval of an ethics committee or informed consent due to its retrospective nature.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Zeng H, Zheng R, Zhang S, et al. Esophageal cancer statistics in China, 2011:estimates based on 177 cancer registries. Thorac Cancer 2016;7:232-7. [Crossref] [PubMed]
- Vaghjiani RG, Molena D. Surgical management of esophageal cancer. Chin Clin Oncol 2017;6:47. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation therapy oncology group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (radiation therapy oncology group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature 2007;450:903-7. [Crossref] [PubMed]
- Talvas J, Garrait G, Goncalves-Mendes N, et al. Immunonutrition stimulates immune functions and antioxidant defense capacities of leukocytes in radiochemotherapy-treated head & neck and esophageal cancer patients: a double-blind randomized clinical trial. Clin Nutr 2015;34:810-7. [Crossref] [PubMed]
- Xie J, Wang J, Cheng S, et al. Expression of immune checkpoints in T cells of esophageal cancer patients. Oncotarget 2016;7:63669-78. [Crossref] [PubMed]
- Nakajima M, Kato H, Miyazaki T, et al. Prognostic significance of heat shock protein 110 expression and T lymphocyte infiltration in esophageal cancer. Hepatogastroenterology 2011;58:1555-60. [PubMed]
- Xu B, Chen L, Li J, et al. Prognostic value of tumor-infiltrating NK cells and macrophages in stage II+III esophageal cancer patients. Oncotarget 2016;7:74904-16. [Crossref] [PubMed]
- Clifton GT, Patil R, Clive K, et al. Abstract LB-329: Defining regulatory T-cells as CD4+CD25hiCD127- or as CD4+CD25hiFoxP3+ in immune monitoring of cancer vaccine clinical trials. Cancer Research 2010.70-Abstract LB-329.
- Vacchelli E, Semeraro M, Enot DP, et al. Negative prognostic impact of regulatory T cell infiltration in surgically resected esophageal cancer post-radiochemotherapy. Oncotarget 2015;6:20840-50. [Crossref] [PubMed]
- Islas-Vazquez L, Prado-Garcia H, Aguilar-Cazares D, et al. LAP TGF-Beta Subset of CD4(+)CD25(+)CD127(-) Treg Cells is Increased and Overexpresses LAP TGF-Beta in Lung Adenocarcinoma Patients. Biomed Res Int 2015;2015:430943. [Crossref] [PubMed]
- Wang J, Yang J. Identification of CD4+CD25+CD127- regulatory T cells and CD14+HLA-DR-/low myeloid-derived suppressor cells and their roles in the prognosis of breast cancer. Biomed Rep 2016;5:208-12. [Crossref] [PubMed]
- Sistrunk WE, Macearty WC. Life expectancy following radical amputation for carcinoma of the breast: a clinical and pathologic study of 218 cases. Ann Surg 1922;75:61-9. [PubMed]
- Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science 1991;254:279-82. [Crossref] [PubMed]
- Hu Z, Zhang W, Usherwood EJ. Regulatory CD8(+)T Cells Associated with Erosion of Immune Surveillance in Persistent Virus Infection Suppress In Vitro and Have a Reversible Proliferative Defect. J Immunol 2013;191:312-22. [Crossref] [PubMed]
- Overgaard NH, Jung JW, Steptoe RJ, et al. CD4+/CD8+ double-positive T cells: more than just a developmental stage? J Leukoc Biol 2015;97:31-8. [Crossref] [PubMed]
- Sarrabayrouse G, Corvaisier M, Ouisse LH, et al. Tumor-reactive CD4+ CD8αβ+ CD103+ αβT cells: a prevalent tumor-reactive T-cell subset in metastatic colorectal cancers. Int J Cancer 2011;128:2923-32. [Crossref] [PubMed]
- deLeeuw RJ, Kost SE, Kakal JA, et al. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res 2012;18:3022-9. [Crossref] [PubMed]
(English Language Editor: J. Gray)


