
Tiaochang Xiaoyan extract tablets ameliorate chronic inflammation by activating macrophage lysosomes in chronic colitis rats
Introduction
Ulcerative colitis (UC) is a chronic and progressive colonic inflammatory disease with recurrent mucosal inflammation and mucosal damage. Recurrent inflammation in colonic mucosa is the most important pathological factor that leads to the occurrence of mucosal ulcers (1,2), and has been linked to increased risk of ulcer-associated colorectal cancer (3). It was found in previous studies that the occurrence of colonic mucosal inflammation in UC patients is closely related to mucosal hypoxia, abnormal activation of inflammatory cells, and autophagy disorder (4,5). Abnormal colonic mucosal inflammation is not only related to the toxin and pathogen translocation to submucosa that is caused by the increased permeability of UC colonic mucosa but is also closely related to the clearance of apoptotic body and necrotic fragments and abnormal immune mediators, and then destroy the immune homeostasis (6,7). Some studies have suggested that the accumulation of apoptotic fragments can lead to tissue damage and the destruction of immune homeostasis, which further leads to abnormal autoimmune and immune homeostasis (8,9). In addition, it was discovered that mucosal damage in colonic mucosa of UC is related to abnormal immune homeostasis (7,10), which is caused by the formation of an abnormal immune complex in colon mucosa. In this process, phagocytosis of macrophages plays an important role in removing pathogenic microorganisms, the clearance of apoptotic body, and other abnormal immune mediators, and maintaining the immune homeostasis of colonic mucosa (9). The lysosome is considered an important organelle in macrophages, which plays an important role in regulating the immune functions of macrophages. Previous studies have found that the maturation of macrophage lysosomes is important for the stabilization of macrophage-mediated autophagy, immune presentation, and other functions (11,12). However, the mechanism of lysosomal function in macrophages is unclear. The Toll-like receptor (TLR) signaling pathway plays an important role in the development of colonic mucosal inflammation in UC (13). Previous studies have found that the activation of the TLR9 signaling pathway in colonic lamina propria macrophages (LPM) can significantly inhibit macrophage autophagy, aggravate the inflammatory response, and increase the apoptosis of mucosal epithelial cells (13-16). Furthermore, the dysfunction of lysosomal activity in LPM has been found to be the main cause of macrophage dysfunction (14). In addition, the TLR9 signaling pathway is involved in the regulation of autophagy of LPM in UC (13), but the effect of the TLR9 signaling pathway on the regulation of lysosomal structure and the function of LPM is unclear. Therefore, the aim of the present study was to explore the role and mechanism of lysosomes in LPM on chronic inflammation.
UC belongs to the categories of diarrhea, dysentery, and intestinal wind of traditional Chinese medicine. Chronic recurrence belongs to the categories of rest dysentery and chronic dysentery of traditional Chinese medicine, which are characterized by long unhealing and easy recurrence. According to the theory of traditional Chinese medicine, it is generally believed that ulcerative colitis is mainly caused by spleen and kidney deficiencies and stagnation of dampness, heat, phlegm, and qi (17). The onset of UC is believed to be related to pathogenic toxins in the body (18). It is believed that the pathogenic toxins are the root of UC, which can cause UC. Therefore, in terms of treatment, detoxification is considered the main method of treatment. Detoxification is used throughout the whole process of UC and is supplemented by heat and damp clearance, qi activation, and blood circulation. TCXYT is derived from the Xianglian pill, which is a traditional Chinese medicine for treating chronic dysentery recorded in the Taiping Huimin Heji Bureau [1078–1085]. TCXYT consists of Radix Astragali, Lindera aggregata, Rhizoma coptidis, Oldenlandia diffusa and coix seed. Our previous research found that TCXYT can effectively inhibit the chronic inflammation of colonic mucosa and promote the repair of colonic ulcers in patients with ulcerative colitis; the mechanism may be related to inhibiting the autophagy function of colon cells by regulating the balance of pro-inflammatory and anti-inflammatory factors (19-21). Our findings indicated that TCXYT can regulate the lysosomal activity of macrophages in the colon of rats with chronic colitis induced by 2,4,6-trinitrobenzenesulfonic acid (TNBS). We conducted the following research.
We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/apm-21-250).
Methods
Animals
Sprague-Dawley rats (140–160 g, certificate No. 4400210019232) were obtained from the Experimental Animal Center of Southern Medical University, Guangzhou, China (license No. scxk-Guangdong-2006-0015). The Institutional Animal Care and Use Committee of The Second Affiliated Hospital of Guangzhou University of Chinese Medicine approved all of the procedures involving the rats (animal ethics approval No. 2016021-2). Experiments were performed in compliance with the Institutional Animal Care and Use Committee of Guangzhou University of Chinese Medicine’s guidelines for the care and use of animals. We quantitatively analyzed the weight, fecal texture, and other metrics of the rats. The rats were housed in a pathogen-free environment and were allowed to acclimatize for 7 days before use. These rats were divided into the control group (n=6); model group; the high-, middle-, and low-dose TCXYT groups; and the hydroxychloroquine sulfate (HS) group. (The dosage of TCXYT in the trial is calculated based on the clinical dosage. For example, the dosage for rats is 60 mg/kg × 70 kg × 0.018/200 g =378 mg/kg. In the experiment, each rat weighed about 400 g, and given 2.5 mL of the drug solution with a concentration of 0.06 g/mL by gavage. Therefore, the dosage of each rat was 375 mg/kg. In order to facilitate the configuration of the drug, 0.4 g/kg of the trial drug was used as the medium dose, 1/2 times was the low dose, and 2 times was the high dose.)
Reagents
TNBS and lipopolysaccharide (LPS) were purchased from Sigma (St Louis, MO, USA). The Lysosome Staining Kit was purchased from Abnova (Taipei City, Taiwan, China). Enzyme-linked immunosorbent assay (ELISA) kits for interleukin (IL)-1β, IL-4, IL-10, and tumor necrosis factor-α (TNF-α) were from R&D Systems (Minneapolis, MN, USA). HS (an inhibitor of autophagy and TLR7/9) was obtained from Selleck Chemicals (Houston, TX, USA). Mouse interferon-γ (IFN-γ), IL-1R-associated kinase (IRAK) 1, and IRAK4 were from Cell Signaling Technology (Danvers, MA, USA). CD11c (ab11029), Microtubule Associated Protein 1 Light Chain 3 Beta (LC3B, ab192890), TLR9 (ab134368), and myeloid differentiation primary response 88 (MyD88) antibodies, goat anti-rabbit antibodies, and rabbit anti-mouse antibodies were from Abcam (Cambridge, UK).
Drugs
TCXYT is a herbal preparation that consists of Radix Astragali seu Hedysari, Radix Linderae, Rhizoma Coptidis, Herba Hedyotis and Semen Coicis (Table S1). In addition, all herbal medicines were purchased from Lingnan Traditional Chinese Medicine Co. Ltd. (Guangzhou, China) and provided by the Department of Pharmacy at The Second Affiliated Hospital of Guangzhou University of Chinese Medicine. Quality of herbal medicines was tested according to the standards of the Pharmacopoeia of the People’s Republic of China [2015] before the experiment.
Sample preparation and Q-Orbitrap high resolution LC/MS analysis
The methods and further details used have been described elsewhere (22,23). The extract of TCXYT was powdered and passed through 100-mesh sieves. An aliquot of 50 mg of powder was extracted in 10 mL of 70% methanol (v/v) for 30 min by ultrasonication (40 kHz, 300 W). The sample was maintained at room temperature for 5 min, and the supernatant was filtered through a 0.22-µm membrane before use. An aliquot of 1 µL was injected for analysis.
A UHPLC Ultimate 3000 instrument coupled with a Q-Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) was used. Samples were separated on a Welch Ultimate Polar reversed-phase C18 column (150×2.1 mm 1.8 µm). Mobile phase A is an aqueous solution containing 5% methanol-0.1% formic acid and 2 mmol/L ammonium formate; and mobile phase B was a methanol solution containing 15% isopropanol+0.1% formic acid. A gradient elution program was used as follows: 0–5 min, 20% A; 5–10 min, 20–50% A; 10–15 min, 50–80% A; and 15–25 min, 80–95% A. The flow rate was 0.30 mL/min, and the column temperature was maintained at 35 °C. The mass spectrometer was operated in the (+/−) electrospray ionization (ESI) mode. The parameters were as follows: spray voltage: 3.8kV, sheath gas pressure: 40 arb, Aux gas pressure: 10 arb, capillary temperature: 350 °C, heater temperature: 300 °C, scan mode: full MS (Mass spectrometry) (resolution 70,000), and scan range: m/z 100–1,500. The data collected by high-resolution liquid quality were collected by CD2.1 (Thermo Fisher), and then the database was retrieved and compared (mzCloud, mzVault, ChemSpider).
Lysosome staining
RAW64.7 cells and LPM (100 µL/well, 1.0×105/mL) were cultured in 96-well plates for 24 h. Lysosome staining was detected with the lysosome staining kit (Abnova, KA4111, Taibei City, Taiwan,China). When cells were properly fused, the cells were transferred to the corresponding culture medium and 100 µL of Lyso Green working solution (20 µL of 500× Lyso Green stock solution in 10 mL of live cell staining buffer) was added as described previously (8). The cells were incubated at 37 °C in an atmosphere containing 5% CO2 for 1 h. Finally, the cells were visualized under a fluorescence microscope with a fluorescein isothiocyanate (FITC) filter set (excitation and emission at 490 and 525 nm, respectively).
Lysosome activity
Lysosome activity was assayed as described previously (24). Briefly, RAW264.7 cells were solubilized in 25 µL of 0.1% Triton X-100. Next, the lysates were incubated with 150 µL of 10 mM p-nitrophenyl phosphate (Sigma, USA) for 1 h at 37 °C. The reaction was stopped by adding 50 µL of 0.2 M borate buffer, and absorbance of the mixture at 405 nm was determined using a spectrophotometer. Relative lysosome activity (%) was calculated as the ratio of the absorbance at 405 nm of TCXYT-treated cells to that of control cells multiplied by 100%.
Immunofluorescence
Colonic tissue or treated cells were fixed with 4% (w/v) paraformaldehyde (Sigma, USA), and blocked and incubated with an anti-CD11c antibody (1:100) overnight at 4 °C. The cells were then washed in phosphate-buffered saline (PBS). After incubation with a secondary FITC-conjugated antibody and 4',6-diamidino-2-phenylindole (DAPI; Sigma, USA), the cells were rewashed in PBS, mounted in anti-fade reagent, and observed under an Olympus microscope, as described previously (25,26).
Statistical analysis
Data were analyzed using IBM SPSS version 21.0 (IBM Corp., Armonk, NY, USA) and were presented as mean ± standard error of mean. One-way analysis of variance or a general linear model with repeated measures was used to analyze the data of ≥3 groups, and the least significant difference post-hoc test was used for multiple comparisons. Student’s t-test was used to analyze differences between two groups. P<0.05 indicated statistical significance.
TNBS-induced chronic colitis, the disease activity index (DAI), ELISA, histological analysis and Western blot are described elsewhere (27,28).
The isolation of colonic LPM, cell culture, cell viability, and proliferation have been described elsewhere (29-31).
Results
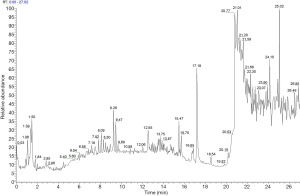
UPLC/UV chromatograms of the TCXYT extract
The TCXYT extract was separated within 20 min on a C18 column (150×2.1 mm 1.8 µm) eluted with acetonitrile, methanol, and water containing 0.1% formic acid. The compounds were detected in the (–) ESI mode, and their MS/MS spectra were analyzed in an untargeted manner. Although TCXYT exhibited only a few major peaks in the HPLC/UV analysis, a number of minor compounds could be observed in the enlarged chromatogram (Figure 1). A total of 174 compounds were identified from TCXYT (Table S2). The chemical analysis of TCXYT extract served as the quality control for the reproducibility of the animal experiment.
TCXYT significantly ameliorates inflammation and colonic mucosal injury
After the colitis model was established, TCXYT administration was initiated at day 3 for 7 consecutive days (Figure 2A). Compared with the control group, colonic mucosal injury was aggravated in the model group, as evidenced by increased DAI, IL-1β, and TNF-α serum levels, and decreased IL-4 and IL-10 levels (Figure 2B,C,D,E,F,G). Treatment with TCXYT for 7 days significantly ameliorated injury to the colonic mucosa (Figure 2G). TCXYT was also found to significantly decrease the DAI and the level of serum pro-inflammatory cytokines in a dose-dependent manner (Figure 2B,C,D), and increase the level of anti-inflammatory cytokines (Figure 2E,F).
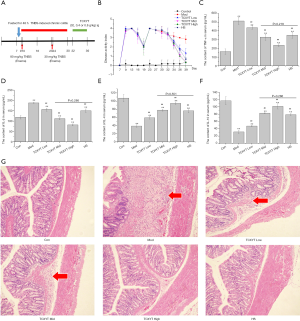
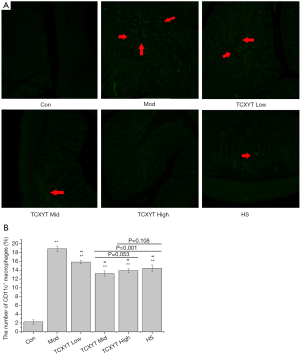
TCXYT reduces the infiltration of CD11c+ macrophages in colonic mucosa
Compared with the control group, the degree of CD11c+ macrophage infiltration in the model group was more severe (Figure 3A), and the number of CD11c+ cells in the lamina propria was greater (Figure 3B). Compared with the model group, the infiltration degree and number of CD11c+ macrophages in the high-, middle-, and low-dose TCXYT groups and the HS group decreased. The TCXYT group was dose dependent, and there was no significant difference between the middle-dose group and the high-dose group (Figure 3A,B). In short, TCXYT can reduce the infiltration of CD11c+ macrophages and the number of CD11c+ cells in the lamina propria in rats with chronic colitis.
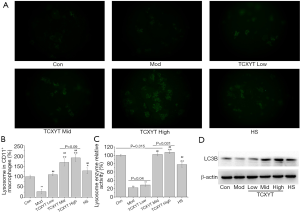
TCXYT promoted the activation of lysosome in LPM
The number and activity of lysosomes in LPM in the experimental colitis groups decreased (Figure 4A,B,C). After 7 days of treatment with TCXYT, the number and activity of lysosomes in LPM significantly increased compared with those in the model group (Figure 4A,B,C). In addition, TCXYT regulated the lysosomal activity in LPM in a dose-dependent manner (Figure 4C). The expression of LC3B in LPM significantly decreased in the colitis groups compared with the control group (Figure 4D). However, after 7 days of treatment with TCXYT, the expression of LC3B in LPM significantly increased compared with the model group. These effects were more significant in the medium- and high-dose groups than in the low-dose group (Figure 4A,B,C,D).
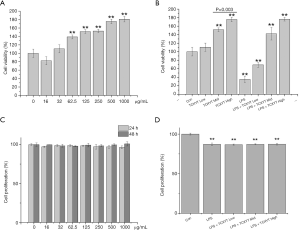
TCXYT increases the viability, but not the proliferation, of RAW264.7 cells
The viability of RAW264.7 cells was reduced, and their proliferation was reduced significantly, by LPS+IFN-γ (Figure 5A,B). However, the viability of RAW264.7 cells increased significantly with TCXYT in a dose-dependent manner (Figure 5C), and their proliferation was unaffected by treatment with TCXYT for 24 and 48 h (Figure 5D). TCXYT also significantly increased the viability of RAW264.7 cells stimulated with LPS (10 µg/mL) plus IFN-γ (10 ng/mL) in a dose-dependent manner, but did not influence their proliferation. On the basis of this, we used TCXYT at 0.12, 0.06, and 0.03 g/mL in subsequent experiments.
TCXYT activates lysosomes in RAW264.7 cells
The number and activity of lysosomes significantly increased with TCXYT compared with the control group in a dose-dependent manner (Figure 6A,B,C). The effects in the middle- and high-dose groups were similar and were superior to those of the low-dose group (Figure 6A,B). However, the expression of LC3B in RAW264.7 cells did not differ markedly among the treatment groups (Figure 6D).
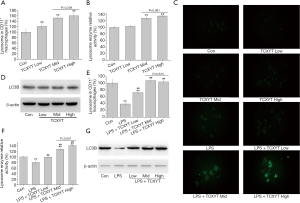
TCXYT also significantly increased the number and activity of lysosomes in RAW264.7 cells stimulated with LPS+IFN-γ in a dose-dependent manner compared with cells treated with only LPS+IFN-γ (Figure 6C,E,F). The expression of LC3B in RAW264.7 cells treated with LPS+IFN-γ was lower than that in the control group, but increased with TCXYT treatment (Figure 6G). However, there was no significant difference between the medium- and high-dose groups.
Therefore, TCXYT ameliorated inflammation in rats with chronic colitis, possibly by increasing the number and activity of lysosomes in macrophages.
TCXYT regulates the TLR9/MyD88/IRAK signaling pathway
The TLR9 signaling pathway plays a central role in the regulation of mucosal innate immunity, particularly of macrophage autophagy, which is implicated in the pathogenesis of UC (13). In the present study, the TLR9, MyD88, IRAK1, and IRAK4 protein levels in LPM were significantly increased in the groups with colitis compared with the control group. TCXYT significantly decreased the TLR9, MyD88, IRAK1, and IRAK4 protein levels in LPM in rats with colitis in a dose-dependent manner. In addition, the effects in the medium- and high-dose groups were superior to those in the low-dose group (Figure 7A).
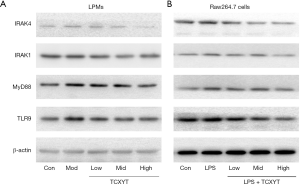
The TLR9, MyD88, IRAK1, and IRAK4 protein levels in RAW264.7 cells treated with LPS+IFN-γ were significantly increased compared with those in the control group. TCXYT significantly decreased the TLR9, MyD88, IRAK1, and IRAK4 protein levels in a dose-dependent manner (Figure 7B).
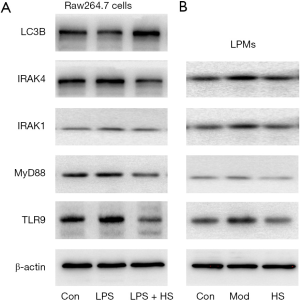
Inhibition of the TLR9 signaling pathway ameliorates inflammation and activates lysosomes
HS, an inhibitor of autophagy and TLR7/9, is an anti-malarial agent that is also used to treat inflammatory conditions (32). When RAW264.7 cells stimulated with LPS+IFN-γ were treated with HS for 24 h, the TLR9, MyD88, IRAK1, and IRAK4 protein levels significantly decreased, and the LC3B protein level increased, compared with cells stimulated with LPS+IFN-γ (Figure 8A). Similarly, after treatment with HS for 7 days, the TLR9, MyD88, IRAK1, and IRAK4 protein levels in LPM significantly reduced compared with those in the model group (Figure 8B). HS treatment also increased the expression of LC3B and the lysosomal number and activity in LPM (Figure 4A,B,C,D). Inflammation and colonic mucosal injury were also significantly ameliorated compared with the model group (Figure 2C,E,F,G).
Discussion
TCXYT is a herbal preparation that suppresses inflammation in the colonic mucosa of patients with UC (19). The bioactive components of TCXYT and their contents vary in composition and extraction methods. To clarify the chemical composition and to evaluate the quality of TCXYT, we used HPLC/UV to analyze the composition of TCXYT used in the present study. The components of TCXYT were detected, indicating that TCXYT is a mixture of compounds. The components and their contents are in line with the provisions of Pharmacopoeia of the People’s Republic of China and meet the requirements of good clinical practice in China.
Macrophages, a type of myeloid cell, play a pivotal role in the innate immune response against pathogens. In patients with UC, a heterogeneous population of inflammatory cells is present in colon tissues, particularly CD11c+ macrophages (33,34). Although the role of macrophages in the development of UC has been explored, the effect and its mechanism are unclear. However, it is well known that increased macrophage infiltration in colon tissue and abnormal polarization are related to the development of inflammation in UC (33). In the present study, compared with the control group, the infiltration of CD11c+ macrophages in the colon of rats with chronic colitis was significantly increased, and the mucosal damage was aggravated. After 7 days of TCXYT treatment, the degree of CD11+ macrophage infiltration and tissue injury in the colon tissue of the high-, middle-, and low-dose TCXYT groups improved at different degrees. Therefore, TCXYT alleviates inflammation and promotes the repair of colon mucosal injury by reducing the infiltration of CD11c+ macrophages in the colon.
Lysosomes in macrophages are not only degradative organelles but also play a central role in nutrient sensing, metabolism, and cell-growth regulation (35). Lysosomal number and activity, two important elements of lysosome function, are related to the activity and polarization of macrophages (36,37). The lysosomal activity of CD11c+ macrophages in the colon of patients with UC was significantly inhibited (38), but the mechanism is unknown. The number and activity of lysosomes in LPM isolated from the colon tissue of rats with chronic colitis significantly decreased, the level of pro-inflammatory cytokines increased, and anti-inflammatory cytokines decreased. In addition, TCXYT significantly increased the number and activity of lysosomes in CD11c+ macrophages. However, TCXYT increased the viability, but not the proliferation, of CD11c+ macrophages in vitro, suggesting that its anti-inflammatory activity was mediated by effects on lysosomes in macrophages, rather than on macrophage proliferation directly; however, the mechanism is unclear.
TLRs play central roles in the regulation of macrophage-mediated mucosal innate immunity and in the pathogenesis of UC (31). TLR9 is a conserved transmembrane receptor that recognizes pathogen-associated molecular patterns, and initiates an immune response by modulating lysosomal activity in macrophages (39). TLR9 is an important component of the TLR9/MyD88/IRAK signaling pathway, and its activation triggers the production of cytokines and chemokines, which are important in the development of inflammation in UC (31,40). In addition, the lysosomal activity in macrophages is regulated by the TLR9/MyD88 signaling pathway in macrophages (41). IRAK1 and IRAK4 are important factors in the TLR9/MyD88 signaling pathway and play a key role in the activation of macrophages (42,43). However, the role of IRAKs (i.e., IRAK1 and IRAK4) in macrophages in the development of UC is unknown. We found that lysosomal activity in macrophages was inhibited in RAW264.7 cells stimulated with LPS+IFN-γ, and in LPM from the colons of rats with chronic colitis, the IRAK1 and IRAK4 protein levels increased significantly and the TLR9/MyD88 signaling pathway was activated. However, the IRAK1 and IRAK4 protein levels significantly decreased and lysosomal activity in macrophages significantly increased with HS-mediated suppression of the TLR9/MyD88 signaling pathway. Therefore, the lysosomal activity in macrophages may be inhibited by activating the TLR9/MyD88/IRAK signaling pathway. In addition, TCXYT reduced the infiltration of CD11c+ macrophages in colon tissue and increased lysosomal activity in macrophages by inhibiting the TLR9/MyD88/IRAK signaling pathway.
Conclusions
The composition and stability of TCXYT are elevated by chromatography. In the study, the results showed that TCXYT can be used as a qualified clinical drug which had stable drug composition and physical-chemical properties. In addition, TCXYT is promising for the treatment of UC, as it ameliorates inflammation and CD11c+ macrophage infiltration in the colon of rats with chronic colitis. TCXYT may promote the activation of lysosomes in macrophages by inhibiting the TLR9/MyD88/IRAK signaling pathway. However, several issues warrant further study, including how lysosomes regulate the differentiation of macrophages and determination of the exogenous regulator of macrophages in the development of UC. These issues must be resolved if TCXYT is to be used to ameliorate inflammation in the colon mucosa of patients with UC.
Acknowledgments
The authors thank the technical staff for their assistance with the study and Dr. Zhaoyu Lu who reviewed the article.
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81573786 and 81904106) and Young Creative Talents Project of Guangdong Province Universities and Colleges (No. 2018KQNCX044).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-250
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-250
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-250). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Institutional Animal Care and Use Committee of The Second Affiliated Hospital of Guangzhou University of Chinese Medicine approved all of the procedures involving the rats (animal ethics approval No. 2016021-2). Experiments were performed in compliance with the Institutional Animal Care and Use Committee of Guangzhou University of Chinese Medicine’s guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ulcerative colitis. Lancet 2017;389:1756-70. [Crossref] [PubMed]
- Mucosal Healing in Ulcerative Colitis: A Comprehensive Review. Drugs 2017;77:159-73. [Crossref] [PubMed]
- Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet 2020;395:123-31. [Crossref] [PubMed]
- Hypoxia ameliorates intestinal inflammation through NLRP3/mTOR downregulation and autophagy activation. Nat Commun 2017;8:98. [Crossref] [PubMed]
- The role of hypoxia in intestinal inflammation. Mol Cell Pediatr 2016;3:1. [Crossref] [PubMed]
- Killing Is Not Enough: How Apoptosis Hijacks Tumor-Associated Macrophages to Promote Cancer Progression. Adv Exp Med Biol 2016;930:205-39. [Crossref] [PubMed]
- Natural resolution of inflammation. Periodontol 2000 2013;63:149-64. [Crossref] [PubMed]
- Recombinant Buckwheat Trypsin Inhibitor Improves the Protein and Mitochondria Homeostasis in Caenorhabditis elegans Model of Aging and Age-Related Disease. Gerontology 2019;65:513-23. [Crossref] [PubMed]
- Mechanobiology of Macrophages: How Physical Factors Coregulate Macrophage Plasticity and Phagocytosis. Annu Rev Biomed Eng 2019;21:267-97. [Crossref] [PubMed]
- The Sound of Silence: Signaling by Apoptotic Cells. Curr Top Dev Biol 2015;114:241-65. [Crossref] [PubMed]
- SNAPIN is critical for lysosomal acidification and autophagosome maturation in macrophages. Autophagy 2017;13:285-301. [Crossref] [PubMed]
- Lysosomal storage and impaired autophagy lead to inflammasome activation in Gaucher macrophages. Aging Cell 2016;15:77-88. [Crossref] [PubMed]
- Excessive endosomal TLR signaling causes inflammatory disease in mice with defective SMCR8-WDR41-C9ORF72 complex function. Proc Natl Acad Sci U S A 2018;115:E11523-E11531. [Crossref] [PubMed]
- The macrophage-specific V-ATPase subunit ATP6V0D2 restricts inflammasome activation and bacterial infection by facilitating autophagosome-lysosome fusion. Autophagy 2019;15:960-75. [Crossref] [PubMed]
- Rapid Downregulation of DAB2 by Toll-Like Receptor Activation Contributes to a Pro-Inflammatory Switch in Activated Dendritic Cells. Front Immunol 2019;10:304. [Crossref] [PubMed]
- Matharu KS, Mizoguchi E, Cotoner CA, et al. Toll-like receptor 4-mediated regulation of spontaneous Helicobacter-dependent colitis in IL-10-deficient mice. Gastroenterology 2009;137:1380-90 e1-3.
- The Mexican consensus on the diagnosis and treatment of ulcerative colitis. Rev Gastroenterol Mex 2018;83:144-67. [Crossref] [PubMed]
- Ping weisan alleviates chronic colitis in mice by regulating intestinal microbiota composition. J Ethnopharmacol 2020;255:112715. [Crossref] [PubMed]
- Beiping Z, Feng l, Suiping H, et al. Clinical study on treatment of Ulcerative colitis with Tiaochang Xiaoyan Tables combined with Changdiqing liquid enema. Pharmaceutical Industry Information 2005:61-2.
- Effect of Tiaochang Xiaoyan Tablets on Serum Cytokines in Patients with Mild-to-moderate Ulcerative Colitis. Journal of Guangzhou University of Traditional Chinese Medicine 2020;37:226-33.
- Effects of Tiao Chang Xiao Yan tables on serum cytokines and LC3B in rats with Ulcerative colitis. Acta Medica Mediterranea 2020;36:571-8.
- A targeted strategy to analyze untargeted mass spectral data: Rapid chemical profiling of Scutellaria baicalensis using ultra-high performance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J Chromatogr A 2016;1441:83-95. [Crossref] [PubMed]
- HPLC/UV analysis of chlorfenapyr residues in cabbage and soil to study the dynamics of different formulations. Sci Total Environ 2005;350:38-46. [Crossref] [PubMed]
- Cytopiloyne, a polyacetylenic glucoside from Bidens pilosa, acts as a novel anticandidal agent via regulation of macrophages. J Ethnopharmacol 2016;184:72-80. [Crossref] [PubMed]
- Influence of iRoot SP and mineral trioxide aggregate on the activation and polarization of macrophages induced by lipopolysaccharide. BMC Oral Health 2018;18:56. [Crossref] [PubMed]
- Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol 2006;7:868-74. [Crossref] [PubMed]
- Effects of initiating time and dosage of Panax notoginseng on mucosal microvascular injury in experimental colitis. World J Gastroenterol 2017;23:8308-20. [Crossref] [PubMed]
- Proanthocyanidins from grape seeds modulates the nuclear factor-kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int Immunopharmacol 2011;11:1620-7. [Crossref] [PubMed]
- The Different Effects of VEGFA121 and VEGFA165 on Regulating Angiogenesis Depend on Phosphorylation Sites of VEGFR2. Inflamm Bowel Dis 2017;23:603-16. [Crossref] [PubMed]
- Novel anti-inflammatory agent 3-[(dodecylthiocarbonyl)-methyl]-glutarimide ameliorates murine models of inflammatory bowel disease. Inflamm Res 2016;65:245-60. [Crossref] [PubMed]
- A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol Gastrointest Liver Physiol 2009;296:G1167-79. [Crossref] [PubMed]
- Evaluation of TLR9 expression on PBMCs and CpG ODN-TLR9 ligation on IFN-alpha production in SLE patients. Immunopharmacol Immunotoxicol 2017;39:11-8. [Crossref] [PubMed]
- Lactobacillus brevis KB290 With Vitamin A Ameliorates Murine Intestinal Inflammation Associated With the Increase of CD11c+ Macrophage/CD103- Dendritic Cell Ratio. Inflamm Bowel Dis 2018;24:317-31. [Crossref] [PubMed]
- Human intestinal pro-inflammatory CD11c(high)CCR2(+)CX3CR1(+) macrophages, but not their tolerogenic CD11c(-)CCR2(-)CX3CR1(-) counterparts, are expanded in inflammatory bowel disease. Mucosal Immunol 2018;11:1114-26. [Crossref] [PubMed]
- Probing labeling-induced lysosome alterations in living cells by imaging-derived mean squared displacement analysis. Biochem Biophys Res Commun 2018;503:2704-9. [Crossref] [PubMed]
- Physiological difference in autophagic flux in macrophages from 2 mouse strains regulates cholesterol ester metabolism. Arterioscler Thromb Vasc Biol 2013;33:903-10. [Crossref] [PubMed]
- Inhibition of lysosomal function in macrophages incubated with elevated glucose concentrations: a potential contributory factor in diabetes-associated atherosclerosis. Atherosclerosis 2012;223:144-51. [Crossref] [PubMed]
- Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut 2010;59:1192-9. [Crossref] [PubMed]
- Autophagosome-lysosome fusion triggers a lysosomal response mediated by TLR9 and controlled by OCRL. Nat Cell Biol 2016;18:839-50. [Crossref] [PubMed]
- Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med 2005;201:915-23. [Crossref] [PubMed]
- The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 2008;456:658-62. [Crossref] [PubMed]
- IRAK4 as a molecular target in the amelioration of innate immunity-related endotoxic shock and acute liver injury by chlorogenic acid. J Immunol 2015;194:1122-30. [Crossref] [PubMed]
- Toll-like receptors and their adaptors are regulated in macrophages after phagocytosis of lipopolysaccharide-coated titanium particles. J Orthop Res 2011;29:984-92. [Crossref] [PubMed]
(English Language Editor: R. Scott)


