
Elevated high-sensitivity C-reactive protein levels predict poor outcomes among patients with acute cardioembolic stroke
Introduction
Cardioembolic stroke (CES) is a severe subtype of cerebral infarction, accounting for 20% of cerebral infarctions (1). This condition is characterized by high in-hospital mortality rates (27.3%) (2), neurological deficits at hospital discharge, and a high risk of stroke recurrence (26%) (3). However, the prognostic indicators for patients with CES remain unclear. Acute strokes may trigger an inflammatory response, leading to elevated C-reactive protein (CRP) levels (4), which may be associated with poor prognosis as they reflect inflammation or tissue damage (5). Therefore, CRP is a potential predictor of future cardiovascular and cerebrovascular events and prognostic markers after the event. However, compared with CRP, high-sensitivity CRP (hs-CRP) can be measured quantitatively and accurately detect low-level inflammation (6), and it can reflect minute changes in inflammation better than CRP can. Hs-CRP also has greater clinical significance in evaluating the relationship between acute inflammation and prognosis (7). Also, hs-CRP is more widely used in clinical practice, especially in the risk stratification of cardiovascular disease (CVD) (8) and stroke (9). To further clarify the role of hs-CRP in CES, we assessed hs-CRP levels as a predictor of CES prognosis. Therefore, the purpose of our study was to analyze the association between hs-CRP levels in the acute phase of CES and its outcomes.
Methods
Ethics
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Tianjin Huanhu Hospital (No. 2005012) and informed consent was taken from all individual participants.
Patient selection
We retrospectively analyzed the clinical biochemical indexes and outcomes of all patients with first ischemic stroke in the Department of Neurology of Tianjin Huanhu Hospital within 72 h after stroke onset from May 1, 2005 to December 31, 2015. Patients with thrombolysis or mechanical thrombectomy, concomitant infection, abnormal liver and kidney function, or cardiac insufficiency were not included in the study; thus, data from 9,746 patients were analyzed. Among them, there were 8,970 patients with non-CES, 776 patients with CES, and 298 patients with no data on hs-CRP levels on admission. Finally, 478 patients were included in our study.
All participants were screened according to rigorous procedures and treated according to current guidelines. A spreadsheet was used to collect and extract detailed baseline data, which included a complete medical history (100%), complete medication history (100%), complete neurological examination (100%), standard biochemical blood test (100%), head CT (100%), head MRI (89.7%), head CTA (10.9%), head MRA (26.4%), 12-lead electrocardiogram (100%), standard 12-lead dynamic electrocardiogram (61.9%), transthoracic echocardiography (100%), microemboli monitoring (13%), carotid ultrasound (100%), and transcranial Doppler ultrasound (100%).
Diagnostic criteria of CES
TOAST subtype classification, the most widely used classification of ischemic stroke, organizes ischemic stroke into five etiological subtypes (10), which include CES. Stroke classification was performed by two senior neurologists. The diagnostic criteria for CES were as follows (10): (I) symptoms appear as cortical or cerebellar dysfunction rather than lacunar syndrome; (II) infarct size (cortical, cerebellar, brain stem, or subcortical) is confirmed to be >1.5 cm through imaging (head CT and/or MRI); (III) identification of a recognized source of cardiac emboli; and (IV) no clinical evidence of extracranial internal carotid artery stenosis.
Hs-CRP and clinical assessment
Blood samples were withdrawn from all participants in the fasting state within 24 h of admission. Serum hs-CRP assays were measured using immunoturbidimetric assays. We analyzed all variables in an accredited central laboratory. Hs-CRP levels were classified according to quartiles (<2.31, 2.31 to <6.09, 6.09 to <22.30, and ≥22.30 mg/L). The basic demographic information of each patient was recorded at admission. Biochemical indicators, such as cholesterol and hemoglobin A1c (HbA1c), were recorded in the spreadsheets. The variables of interest are defined in the following section. Hypertension was defined as having a history of hypertension, having used any antihypertensive drug, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg (11). Diabetes was defined as having a history of diabetes, or having used any glucose-lowering drugs, or having a fasting glucose level of >126 mg/dL (12). Dyslipidemia was defined as having a history of dyslipidemia, having used any lipid-lowering drug, or having total cholesterol (TC) levels ≥200 mg/dL, triglycerides (TG) levels ≥150 mg/dL, high-density lipoprotein cholesterol (HDL-C) levels ≤40 mg/dL, and low-density lipoprotein cholesterol (LDL-C) levels ≥130 mg/dL (13). Current smokers were defined as those who had been smoking daily for more than 1 year. Current drinkers were defined as those who had been drinking at least once a week for more than a year. Obesity was defined as a body mass index of ≥30 kg/m2 (14) measured at admission. All patients obtained an index of severity on the National Institute of Health Stroke Scale (NIHSS) on admission.
Follow-up
We followed up all patients with face-to-face interviews or telephone calls at 3 months and 1 year after stroke and recorded the results in the stroke database. One week before the deadline, we called the patients or their authorized agents to remind them about the review and to set up a follow-up appointment. Most patients were assessed through outpatient services, while others who refused to go to the hospital were assessed through telephone interviews. We reclassified stroke subtypes at follow-up to ensure accurate diagnosis, and the subtype of stroke did not change during follow-up.
Study outcome
Stroke severity was assessed using the modified Rankin scale (mRS), with mRS scores of 0 to 2 classified as a good outcome, and scores of 3 to 6 as a poor outcome (15,16). Composite endpoints included poor outcomes, vascular death, myocardial infarction (MI), and recurrent stroke (ischemic or hemorrhagic). The primary outcome was determined by separating patients into two groups according to the mRS score. The secondary outcome was based on dividing patients into good outcomes and composite endpoints (17,18).
Statistical analysis
Continuous variables were presented as median (25th, 75th percentiles). The Kruskal-Wallis test or Mann-Whitney U-test was used to evaluate the significance of intergroup differences. Categorical variables were presented as counts and percentages. The chi-square test was used to analyze differences between groups. Confounder variables identified as significant in the univariate analyses (P<0.05) were entered into logistic regression analyses to determine the association between hs-CRP levels and outcomes, and results were presented as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). P<0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS 24.0).
Results
Detailed demographic and baseline data
The detailed demographic and baseline data are presented in Table 1. A total of 478 CES patients [270 men (56.5%); median age: 71 years] were enrolled in our study. There were 398 patients with atrial fibrillation (chronic: 288 cases, paroxysmal: 82 cases, persistent: 28 cases) and four patients with atrial flutter. The patients with higher hs-CRP levels were mostly male and elderly, compared with those with lower hs-CRP levels (P<0.05). While obesity differed across the four groups, there was no significant trend. Hypertension, diabetes, dyslipidemia, smoking, alcohol consumption, and HbA1c were evenly distributed in the four groups. There were statistically significant differences in hs-CRP levels and NIHSS scores at admission among the four groups, whereby higher hs-CRP levels corresponded to a higher NIHSS score (P<0.001). Furthermore, the NIHSS score at discharge was also positively correlated with baseline CRP levels (P<0.001). Details are provided in Table 1.
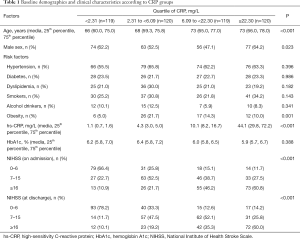
Full table
Results at 3-month follow-up
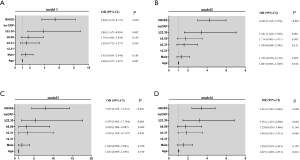
At the 3-month follow-up, 75 patients had experienced vascular death, MI, and recurrent stroke (ischemic or hemorrhagic), and were included in the composite endpoint event. Thirty-three patients died due to other reasons, and one patient did not attend the follow-up review. Therefore, data from 444 patients were analyzed (Figure 1). Univariate analyses demonstrated that age, sex, hs-CRP, and NIHSS scores were associated with poor outcomes (Table 2). After adjusting for confounding factors such as diabetes, hypertension, dyslipidemia, alcohol consumption, smoking, obesity, and HbA1c, the primary outcomes in only the fourth quartile of hs-CRP (hs-CRP ≥22.30 mg/L) and NIHSS scores were associated with poor outcomes [OR: 3.862, 95% CI: (1.675–8.904), P=0.00; and OR: 5.438, 95% CI: (3.619–8.172), P<0.001, respectively] (Figure 2, model 1). The secondary outcomes were similar to the primary outcomes: the fourth quartile of hs-CRP (≥22.30 mg/L) and NIHSS scores had a significant tendency to increase the risk of composite endpoints [fourth quartile of hs-CRP: OR: 3.381, 95% CI: (1.620–7.058), P=0.001 and NIHSS score: OR: 4.246, 95% CI: (3.015–5.978), P<0.001] (Figure 2, model 2). There was a significant difference in outcomes among patients grouped by hs-CRP quartile at 3-month follow-up, with higher levels of hs-CRP (≥22.30 mg/L) being significantly correlated with a poor outcome and composite endpoints after adjusting for confounders (Figure 2).

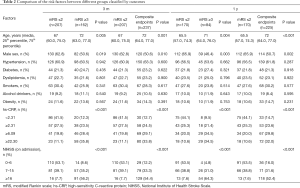
Full table
Results at 1-year follow-up
At the 1-year follow-up, 141 patients had vascular death, MI, and recurrent stroke (ischemic or hemorrhagic), and were included in the composite endpoint event. Fourteen patients died due to other reasons, and 35 patients did not attend the follow-up. Finally, data from 395 patients were analyzed (Figure 1). Univariate analyses demonstrated that age, sex, hs-CRP, and NIHSS scores were associated with poor outcomes (Table 2). After adjusting for confounding factors, such as diabetes, hypertension, dyslipidemia, alcohol consumption, smoking, obesity, and HbA1c, the three higher quartiles of hs-CRP and NIHSS scores were associated with poor outcomes for the primary endpoint (P=0.043, P=0.049, P=0.005, and P<0.001, respectively) (Figure 2, model 3). Furthermore, the fourth quartile of hs-CRP (≥22.30 mg/L) was significantly associated with poor outcomes [OR: 5.479, 95% CI: (1.692–17.744), P=0.005] in the multivariate analysis (Figure 2, model 3). Regarding the secondary outcome, the fourth quartile of hs-CRP (≥22.30 mg/L) and NIHSS scores were positively correlated with the composite endpoints (OR: 3.181, 95% CI: (1.475–6.860), P=0.003; and OR: 3.416, 95% CI: (2.407–4.848), P<0.001, respectively) (Figure 2, model 4). At the 1-year follow-up, higher hs-CRP levels (≥22.30 mg/L) had a significant correlation with poor outcomes and composite endpoints.
Discussion
In our study, patients with higher hs-CRP levels were mostly elderly (P<0.001), and a previous study found that people over 56 had significantly higher CRP levels, but this only applied to healthy women (19). This is consistent with our findings. However, studies with other groups have not been reported. In the current study, CES accounted for 7.96% of ischemic strokes, which was significantly lower than that in China (about 10%) (20). The main possible reason for this is the exclusion of thrombolytic patients from our study population. Second, the average age of our study population, 71 years, was low, and CES is the most common stroke subtype in the elderly (21).
In the present study, the median hs-CRP was 6.09 mg/L, which was quite higher than what was observed in our previous studies on small-artery occlusion (1.54 mg/L) and large-artery arteriosclerosis (2.49 mg/L) (16,22). Several other studies also found higher plasma CRP levels in patients with CES than in other TOAST subtypes (23,24), and den Hertog et al. (5) reported that CES was more often observed in patients with CRP ≥7 mg/L. There are several potential mechanisms underlying this increased CRP in patients with CES. First, CRP is generally elevated in patients with cardiac disease (25), especially in patients with atrial fibrillation (26), which is one of the major causes of CES (27). This might explain the pre-stroke and elevated baseline CRP levels in CES. Second, in the acute phase of cerebral infarction, the extent of brain injury and blood-brain barrier (BBB) disruption may determine the degree of acute-phase inflammatory response. It has been confirmed that the degree of BBB destruction in CES patients is more serious than that in patients with other stroke subtypes (28), and this could be related to CRP levels (29). While these mechanisms may play a role in the increased CRP we observed, two studies have reported no differences in CRP levels among stroke subtypes (30,31). These conflicting results may be related to differences in the race, age, or gender of the subjects (32,33).
In this study, regarding the primary outcome, we concluded that elevated hs-CRP levels in acute CES patients can predict poor functional outcomes at 3 months and 1 year post-stroke; however, this association was only evident in the fourth quartile of hs-CRP (≥22.30 mg/L). Inflammation plays a critical role not only in the pathogenesis of stroke, but also in the deterioration following the stroke. Previous studies have found that increased CRP levels can predict the risk of poor prognosis with acute ischemic stroke (34,35), and lower CRP levels may be related to clinical improvement and better prognosis at 3 months (36). In contrast, Modrego (37) failed to observe any relationship between CRP levels and prognosis of acute ischemic cerebrovascular disease. The explanation for these differences may be the small sample size of the study and not including the stroke subtypes in the analysis. In this study, patients with higher hs-CRP levels were mostly male, elderly, and with high NIHSS at the time of admission. Patients with high NIHSS do worse than patients with low NIHSS, those with large infarcts do worse than small infarcts, and elderly patients do worse than younger patients, all of which might explain the poor long-term outcomes.
In our present study, the findings observed for our secondary outcomes mirrored those found for our primary outcome, whereby, in acute patients with CES, hs-CRP ≥22.30 mg/L significantly correlated with composite endpoint events, which predicted new vascular events and poor functional outcome. A previous study found that elevated CRP levels in patients with acute ischemic stroke independently predicted recurrent vascular events and mortality (38). Furthermore, Christensen et al. (39) found that CRP +10 mg/L was independently correlated with 1-year mortality. Inflammation plays an important causal role in both vascular injury and coagulation (40); therefore, this may explain why the activation of individual inflammatory processes increases the risk of future cardiovascular events (41).
There are some limitations to the present study. First, CRP levels are usually related to the size of tissue damage; however, the relationship between hs-CRP levels and infarct size was not analyzed. Second, genotype analysis for CRP was lacking in this study. Serum CRP levels are affected by CRP gene polymorphism (9,42). A previous study found that the CRP gene SNP rs1130864 was an independent predictor of poor short-term prognosis in patients with first ischemic stroke (43). Third, the sampling time following stroke onset may have influenced our results. Di Napoli et al. (44) found that CRP levels at discharge were a better predictor of prognosis. Although more well-designed studies are needed, our study clearly shows the relationship between hs-CRP levels and the outcomes of CES.
Conclusions
In this study, we can conclude that elevated hs-CRP in patients with CES is an independent predictor of poor outcomes; this association is particularly evident when hs-CRP ≥22.30 mg/L. Hs-CRP may serve as a predictor of stroke disability as well as recurrent stroke (ischemic or hemorrhagic), MI, or vascular death in patients with CES. Potential mechanisms have not yet been identified, and our findings need to be confirmed in other populations.
Acknowledgments
We would like to thank Editage for English language editing.
Funding: This study was supported by the National Natural Science Foundation of China (81671169 to JW); and the Tianjin Municipal Science and Technology Commission (17JCZDJC36500 to JW).
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1927
Peer Review File: Available at http://dx.doi.org/10.21037/apm-20-1927
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1927). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study conformed to the Helsinki Declaration (as revised in 2013). The study was approved by the Ethics Committee of Tianjin Huanhu Hospital (No. 2005012) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ornello R, Degan D, Tiseo C, et al. Distribution and temporal trends from 1993 to 2015 of ischemic stroke subtypes: a systematic review and meta-analysis. Stroke 2018;49:814-9. [Crossref] [PubMed]
- Arboix A, Alio J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev 2010;6:150-61. [Crossref] [PubMed]
- Nouh A, Hussain M, Mehta T, et al. Embolic strokes of unknown source and cryptogenic stroke: implications in clinical practice. Front Neurol 2016;7:37. [Crossref] [PubMed]
- McColl BW, Allan SM, Rothwell NJ. Systemic inflammation and stroke: aetiology, pathology and targets for therapy. Biochem Soc Trans 2007;35:1163-5. [Crossref] [PubMed]
- den Hertog HM, van Rossum JA, van der Worp HB, et al. C-reactive protein in the very early phase of acute ischemic stroke: association with poor outcome and death. J Neurol 2009;256:2003-8. [Crossref] [PubMed]
- VanGilder RL, Davidov DM, Stinehart KR, et al. C-reactive protein and long-term ischemic stroke prognosis. J Clin Neurosci 2014;21:547-53. [Crossref] [PubMed]
- Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499-511. [Crossref] [PubMed]
- Andersson J, Johansson L, Ladenvall P, et al. C-reactive protein is a determinant of first-ever stroke: prospective nested case-referent study. Cerebrovasc Dis 2009;27:544-51. [Crossref] [PubMed]
- Kong H, Qian YS, Tang XF, et al. C-reactive protein (CRP) gene polymorphisms, CRP levels and risk of incident essential hypertension: findings from an observational cohort of Han Chinese. Hypertens Res 2012;35:1019-23. [Crossref] [PubMed]
- Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993;24:35-41. [Crossref] [PubMed]
- Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation 2000;101:329-35. [Crossref] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes--2010. Diabetes Care 2010;33:S11-61. [Crossref] [PubMed]
- Guptha S, Gupta R, Deedwania P, et al. Cholesterol lipoproteins and prevalence of dyslipidemias in urban Asian Indians: a cross sectional study. Indian Heart J 2014;66:280-8. [Crossref] [PubMed]
- Wilterdink JL, Bendixen B, Adams HP Jr, et al. Effect of prior aspirin use on stroke severity in the trial of Org 10172 in acute stroke treatment (TOAST). Stroke 2001;32:2836-40. [Crossref] [PubMed]
- Winter Y, Wolfram C, Schaeg M, et al. Evaluation of costs and outcome in cardioembolic stroke or TIA. J Neurol 2009;256:954-63. [Crossref] [PubMed]
- Qiu R, Gao Y, Hou D, et al. Association between hs-CRP levels and the outcomes of patients with small-artery occlusion. Front Aging Neurosci 2016;8:191. [Crossref] [PubMed]
- Zhu B, Pan Y, Jing J, et al. Neutrophil counts, neutrophil ratio, and new stroke in minor ischemic stroke or TIA. Neurology 2018;90:e1870-8. [Crossref] [PubMed]
- Freeman WD, Aguilar MI. Prevention of cardioembolic stroke. Neurotherapeutics 2011;8:488-502. [Crossref] [PubMed]
- Caparević Z, Kostić N. The influence of age and the beginning of menopause on the lipid status, LDL oxidation, and CRP in healthy women. Srp Arh Celok Lek 2007;135:280-5. [Crossref] [PubMed]
- Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol 2019;18:394-405. [Crossref] [PubMed]
- Chen RL, Balami JS, Esiri MM, et al. Ischemic stroke in the elderly: an overview of evidence. Nat Rev Neurol 2010;6:256-65. [Crossref] [PubMed]
- Hou D, Liu J, Feng R, et al. The role of high-sensitivity C-reactive protein levels in functional outcomes in patients with large-artery atherosclerosis and small-artery occlusion. Neurol Res 2017;39:981-7. [Crossref] [PubMed]
- Rajeshwar K, Kaul S, Al-Hazzani A, et al. C-reactive protein and nitric oxide levels in ischemic stroke and its subtypes: correlation with clinical outcome. Inflammation 2012;35:978-84. [Crossref] [PubMed]
- Luo Y, Wang Z, Li J, et al. Serum CRP concentrations and severity of ischemic stroke subtypes. Can J Neurol Sci 2012;39:69-73. [Crossref] [PubMed]
- Blake GJ, Ridcker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med 2002;252:283-94. [Crossref] [PubMed]
- Anderson JL, Allen Maycock CA, Lappé DL, et al. Frequency of elevation of C-reactive protein in atrial fibrillation. Am J Cardiol 2004;94:1255-9. [Crossref] [PubMed]
- Zhang J, Yang Y, Sun H, et al. Hemorrhagic transformation after cerebral infarction: current concepts and challenges. Ann Transl Med 2014;2:81. [PubMed]
- Liu C, Shi FN, Chen ZC, et al. Severe blood-brain barrier disruption in cardioembolic stroke. Front Neurol 2018;9:55. [Crossref] [PubMed]
- Kuhlmann CR, Librizzi L, Closhen D, et al. Mechanisms of C-reactive protein-induced blood-brain barrier disruption. Stroke 2009;40:1458-66. [Crossref] [PubMed]
- Dewan KR, Rana PV. C-reactive protein and early mortality in acute ischemic stroke. Kathmandu Univ Med J (KUMJ) 2011;9:252-5. [Crossref] [PubMed]
- Yokokawa H, Goto A, Terui K, et al. Prevalence of metabolic syndrome and serum marker levels in patients with four subtypes of cerebral infarction in Japan. J Clin Neurosci 2008;15:769-73. [Crossref] [PubMed]
- Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem 2008;54:1027-37. [Crossref] [PubMed]
- Ahonen TM, Kautiainen HJ, Keinänen-Kiukaanniemi SM, et al. Gender difference among smoking, adiponectin, and high-sensitivity C-reactive protein. Am J Prev Med 2008;35:598-601. [Crossref] [PubMed]
- Ishikawa J, Tamura Y, Hoshide S, et al. Low-grade inflammation is a risk factor for clinical stroke events in addition to silent cerebral infarcts in Japanese older hypertensives: The Jichi Medical School ABPM Study, wave 1. Stroke 2007;38:911-7. [Crossref] [PubMed]
- Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke 2001;32:133-8. [Crossref] [PubMed]
- Tiainen M, Meretoja A, Strbian D, et al. Body temperature, blood infection parameters, and outcome of thrombolysis‐treated ischemic stroke patients. Int J Stroke 2013;8:632-8. [Crossref] [PubMed]
- Modrego PJ, Boned B, Berlanga JJ, et al. Plasmatic B-type natriuretic peptide and C-reactive protein in hyperacute stroke as markers of CT-evidence of brain edema. Int J Med Sci 2008;5:18-23. [Crossref] [PubMed]
- Di Napoli M, Schwaninger M, Cappelli R, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Poolin Project members. Stroke 2005;36:1316-29. [Crossref] [PubMed]
- Christensen H, Boysen G. C-reactive protein and white blood cell count increases in the first 24 hours after acute stroke. Cerebrovasc Dis 2004;18:214-9. [Crossref] [PubMed]
- Gussekloo J, Schaap MC, Frolich M, et al. C-reactive protein is a strong but nonspecific risk factor of fatal stroke in elderly persons. Arterioscler Thromb Vasc Biol 2000;20:1047. [Crossref] [PubMed]
- Winbeck K, Poppert H, Etgen T, et al. Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke 2002;33:2459-64. [Crossref] [PubMed]
- Ladenvall C, Jood K, Blomstrand C, et al. Serum C-reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke 2006;37:2018-23. [Crossref] [PubMed]
- Guo J, Yu L, Zhang J, et al. CRP gene polymorphism predicts post-stroke functional outcome in Han Chinese. Acta Neurol Scand 2014;129:263-8. [Crossref] [PubMed]
- Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke 2001;32:917-24. [Crossref] [PubMed]


