
Camrelizumab combined with lenvatinib in the treatment of gastric cancer with liver metastasis: a case report
Introduction
As one of the most common malignant tumors, gastric cancer poses a serious threat to patients’ lives. It not only directly affects the nutritional intake of patients, but also may lead to digestive tract obstruction, perforation, and even massive bleeding and other complications. Although the incidence of gastric cancer has decreased in western countries in recent years, it is still the most common malignant tumor of the digestive tract in China. Because there is no screening of gastric cancer, advanced gastric cancer is mostly discovered at the time of treatment, which reduces the efficacy of treatment. For patients with advanced gastric cancer, it is currently recognized that comprehensive treatment based on systemic drug therapy should be adopted. At present, drug therapies for gastric cancer in China, including chemotherapeutic drugs and molecular targeted drugs, have a sufficient evidence base and extensive clinical practice experience. Immunotherapies for gastric cancer are widely used in China and abroad, though it remains in question whether immunotherapy is a better option for patients who are intolerant or unwilling to receive chemotherapy and are HER2 negative. In this case, we selected immunotherapy combined with anti-angiogenesis therapy based on the needs of patients, and achieved relatively good clinical effects. This provides some ideas for the treatment of gastric cancer. Of course, this requires large-scale clinical trials to confirm. Camrelizumab is a new humanized immunoglobulin G4 (IgG4) type monoclonal antibody (mAb). It can bind programmed death molecule 1 (PD-1) and block its binding with programmed death molecule ligand 1 (PD-L1), so as to restore the immune function of the body and achieve the anti-tumor effect. Lenvatinib is a multi-target tyrosine kinase inhibitor (TKI), can inhibit many important pathways affecting angiogenesis and cell proliferation, including vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR), platelet-derived growth factor receptor α, and proto oncogenes RET and KIT. These signaling pathways have been confirmed to be related to the occurrence, development and poor prognosis of a variety of malignant tumors in previous studies. Lenvatinib can inhibit tumor growth by blocking these pathways.
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2572).
Case presentation
A 53-year-old man presented to West China Hospital of Sichuan University with upper abdominal pain in August 2019. An enhanced CT scan showed scattered nodules and masses in the liver. The pathological results of liver biopsy revealed malignant tumors with necrosis. In August 2019, the patient was diagnosed with liver cancer at another hospital, and underwent the first transcatheter arterial chemoembolization (TACE) treatment. The whole body PET/CT examination in September 2019 showed: (I) scattered space occupying can be seen in the liver, with necrosis and flaky high-density shadows, and metabolism was unevenly increased. Many liver cancers with intrahepatic metastases changed after interventional therapy. (II) Increased metabolism of lymph nodes on the right side of the sternum, right side of the inferior trachea, the side of the esophagus, the posterior diaphragm, hilar area, hepatogastric ligament area, posterior pancreas, and para-aortic lymph nodes, all of which had tumor metastases, and some of the lymph nodes were fused. The boundary between the stomach wall, portal vein, and pancreas was unclear. (III) Slight inflammation in the lower lobe of the right lung. Compared with the older films suggested that the tumor was shrinking. After the operation, lenvatinib was given orally. But after these treatments, the patient did not relieve abdominal pain and developed progressive emaciation. Subsequently, the liver biopsy of the patient showed adenocarcinoma by immunohistochemistry. It was recommended to check the digestive tract, biliary pancreatic system, and exclude primary lesions such as the internal bile ducts that are under consideration after metastasis. A gastroscopy was later performed, which showed gastric ulcer-type lesions (Figure 1). The stomach had multiple mucosal bulges. A biopsy revealed adenocarcinoma, and a tumor thrombus was found in the vessel. After consultation with the pathology department, combined with the patient’s medical history and imaging examinations, the revised diagnosis was gastric adenocarcinoma with liver metastasis (cTxN3M1, stage IV).
According to ASCO’s guidelines, we recommend that patients take chemotherapy, but the patient refuse to do chemotherapy in the hope of seeking other treatments. The Next-generation sequencing technology (NGS) testing indicated that the tumor mutational burden (TMB) of the liver tissue and stomach tissue samples was 12.7 mutations/MB, which indicated high TMB. Therefore, we suggest that patients with immunotherapy, based on the previous camrelizumab in the treatment of gastric cancer has shown a good effect, we recommend patients to use camrelizumab. Inform the patient in detail of the immunotherapy’s side effects, costs and prognosis. After informed consent, the patient requested the addition of camrelizumab 200 mg every 2 weeks on the basis of lenvatinib. CT re-examinations were performed after 6 cycles of camrelizumab treatment. The scans showed that there were multiple low-density nodules and tumors in the liver, with iodized oil deposits, and many liver cancers with intrahepatic metastases changed after treatment. The hepatogastric ligament, hepatic hilum, hepatoduodenal ligament, portal space, and swollen lymph nodes around the abdominal aorta were mostly metastasized. Intrahepatic lesions, and abdominal cavity and retroperitoneal lymph nodes shrank. Efficacy was evaluated by partial response (PR). The patient continued to complete the 7–27 cycle of camrelizumab immunotherapy. During October 2019, January 2020 and April 2020, hepatic artery superselective angiography + chemotherapy perfusion embolization was performed three times under local anesthesia. In August 2020, gastroscopy showed that the ulcer disappeared (Figure 2). The enhanced CT was reviewed in March, June, July and October 2020, and the efficacy evaluation was continuous partial response (Figures 3,4). High levels of the tumor markers carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) at the time of onset continued to drop to normal ranges after treatment (Figures 5,6). At present, the patient’s abdominal pain is relieved, the disease is under control, and the patient’s quality of life is good. The original treatment is being continued.
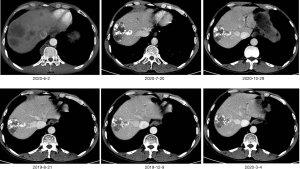
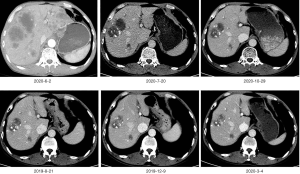
In addition, the patient tolerated the treatment well. After using camrelizumab, natriuretic peptide tests and echocardiography were repeated several times, and there were no abnormalities, and no immune myocarditis occurred. Only a slight increase in the concentration of thyroid stimulating hormone (TSH) was found, and the remaining thyroid hormones were normal. The patient did not experience reactive cutaneous capillary endothelial proliferation (RCCEP) during the treatment cycle, and his body was in good condition. The survival period has reached 14 months at the present time.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
Tumor immune escape is an important mechanism in the occurrence and development of malignant tumors, and immune checkpoints play a key role in this mechanism (1). The interaction of PD-1 and PD-L1 also plays an important role in tumor immune escape (2). PD-1 is an immunosuppressive molecule in the CD28 and CDB7 family. It is a membrane protein containing 268 amino acid residues. It is widely expressed on T cells, macrophages, B cells, and other immune cell membranes. Its ligands are PD-L1 and PD-L2. PD-L1 is a protein encoded by the CD274 gene and is mainly expressed on the membranes of tumor cells, dendritic cells, and macrophages. PD-L2 is mainly expressed on the membranes of dendritic cells, macrophages, and B cells, and is associated with inflammation and autoimmune disease (3). PD-1/PD-L1 inhibitors inhibit the activation and proliferation of T cells by blocking the PD-1/PD-L1 signal transduction pathway, thereby mediating the process of negative immune regulation (4).
In this case report, the patient’s liver, stomach, and blood samples underwent a panoramic cancer genetic test. The TMB results were all TMB-high (TMB-H). A variety of immunotherapy drugs have been approved for the treatment of TMB-H (≥10 muts/Mb) solid tumor patients (5). The high TMB of this patient contributed to our use of camrelizumab treatment. Camrelizumab is a humanized IgG4κ anti-PD-1 monoclonal antibody that binds to PD-1 to block the PD-1/PD-L1 pathway, thereby activating T cells and producing sustained anti-tumor effects and inhibiting tumour growth (6). Camrelizumab is expressed in the Chinese hamster ovary cell (CHO) cell line using recombinant technology. Since camrelizumab demonstrated good survival benefits in a single-arm phase II clinical trial of classical Hodgkin’s lymphoma, the drug was approved by the State Food and Drug Administration (CFDA) on May 29, 2019. It is approved for the treatment of patients with relapsed or refractory classical Hodgkin’s lymphoma that have undergone at least second-line chemotherapy (7). Camrelizumab is also currently approved for the treatment of other malignant tumors, including esophageal squamous cell carcinoma, gastric/gastroesophageal junction cancer, hepatocellular carcinoma (HCC), nasopharyngeal carcinoma, and non-squamous non-small cell lung cancer. For the treatment plan of this patient, we also referred to some studies on camrelizumab in the treatment of gastric cancer combined with apatinib. For example, camrelizumab was studied in a phase I trial of second-line and higher treatment of advanced gastric cancer and esophagogastric junction cancer (8). Camrelizumab combined with apatinib has also been studied in a phase II trial as a second-line and higher treatment for HCC and gastric cancer/esophagogastric junction cancer (GC/EGJC) Ia/b (9), and pembrolizumab + lenvatinib were studied in the clinical phase II EPOC1706 trial (10). With the deepening of immunotherapy for gastric cancer, more and more data continue to surprise us. We think that for patients with liver metastasis from advanced gastric cancer, tumor mutation burden (TMB) test, PD-L1 immunohistochemical detection, tissue microsatellite (MSI) detection can be carried out, immunotherapy can be used as an option, of course, this also needs to be confirmed by large-scale clinical trials.
Camrelizumab has demonstrated a good safety and efficacy profile in a variety of solid tumors. It will be used in chemotherapy, radiotherapy, targeted therapy, traditional Chinese medicine, and other anti-tumor therapies in the future. Combined application may further improve its efficacy. Preclinical data show that compared with similar drugs, camrelizumab has considerable in vivo efficacy and safety, and clinical studies of this drug have shown that it is effective in esophageal cancer, stomach cancer, liver cancer, lung cancer, and nasopharyngeal cancer. We look forward to further developments in domestic anti-tumor immunotherapy drugs, which will bring new hope to cancer patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2572
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2572). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Umansky V, Blattner C, Fleming V, et al. Myeloid- derived suppressor cells and tumor escape from immune surveil- lance. Semin Immunopathol 2017;39:295-305. [Crossref] [PubMed]
- Li Y, Fan ZY, You WY, et al. The role and clinical significance of PD-1/PD-L1 signaling pathway in tumor immune escape. Journal of PLA Medical College 2015;36:762-5.
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 2017;8:561. [Crossref] [PubMed]
- Zak KM, Kitel R, Przetocka S, et al. Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure 2015;23:2341-8. [Crossref] [PubMed]
- Chung HC, Ros W, Delord JP, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol 2019;37:1470-8. [Crossref] [PubMed]
- Mo H, Huang J, Xu J, et al. Safety, anti-tumour activity, and pharmacokinetics of fixed- dose SHR- 1210, an anti- PD- 1 antibody in advanced solid tumours: a dose- escalation, phase 1 study. Br J Cancer 2018;119:538-45. [Crossref] [PubMed]
- Markham A, Keam SJ. Camrelizumab: first global approval. Drugs 2019;79:1355-61. [Crossref] [PubMed]
- Huang J, Mo H, Zhang W, et al. Promising efficacy of SHR-1210, a novel anti-programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer 2019;125:742-9. [Crossref] [PubMed]
- Xu J, Zhang Y, Jia R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res 2019;25:515-23. [Crossref] [PubMed]
- Kawazoe A, Fukuoka S, Nakamura Y, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Oncology 2020;21:1057-65. [PubMed]
(English Language Editor: C. Betlazar-Maseh)






