
Associations of serum cryptococcal antigen with different of clinical characteristics: a comprehensive analysis of 378 pulmonary cryptococcosis patients
Introduction
Cryptococcosis is a global invasive mycosis associated with significant morbidity and mortality. Cryptococcosis is a potentially serious fungal disease, typically caused by inhalation of Cryptococcus neoformans or Cryptococcus gatti, which tend to form aerosol (1,2). Pulmonary cryptococcosis (PC) refers to acute or subacute infections of the lungs caused by Cryptococcus (3). PC occurs not only in immunocompromised hosts (ICH), such as people with acquired immune deficiency syndrome (AIDS), but also in some non-immunocompromised hosts (NICH). Treatment for PC needs to be tailored according to the immune status of the host site of infection, access to health care facilities and availability of antifungal drugs. Antifungal agents with activity against Cryptococcus include polyenes (amphotericin B), flucytosine, and azoles. The clinical manifestations of PC are variable and lack specificity, and can include cough, expectoration, fever, chest pain, or an absence of symptoms. The computer tomography (CT) findings of PC may easily be confused with tumor or tuberculosis, leading to misdiagnosis. Cryptococcal antigens (CRAGs) can be detected by latex agglutination (LA) tests, and the Cryptococcus LA test is commonly used in clinical practice as a serological detection method for PC, demonstrating high sensitivity and specificity (4). However, some PC patients show negative LA test results. This study thus aimed to analyze and identify the potential associations of clinical manifestations, CT findings, and host immune status with the LA test results in PC patients. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/apm-21-127).
Methods
Study subjects
From January 2002 to December 2013, patients with proven diagnosis or clinical diagnosis of PC at Shanghai Pulmonary Hospital (Shanghai, China) were retrospectively reviewed. The following data were obtained from the medical records: sex, age, hospitalization time, occupation, exposure history, preliminary diagnosis, basic diseases, symptoms and signs, host immune status, laboratory examination, imaging data, etc. The information from patients’ relevant follow-up was obtained on regular clinic visits and by telephone follow-up. The study was approved by the institutional research ethics committee of Shanghai Pulmonary Hospital (No. K14-168). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from all healthy donors for inclusion in the study. Patients without complete, detailed medical records were excluded. Finally, the records of 378 patients were considered for this analysis.
Diagnostic criteria of PC
According to the international consensus obtained by the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) and the Chinese consensus obtained by the Chinese Thoracic Society, the diagnostic strategy of PC includes proven diagnosis and clinical diagnosis (5-10). The proven diagnosis must meet one of the following statements: (I) Cryptococcus were detected in pathological lung tissue specimens with histochemical staining or cell chemical dyeing methods; (II) Cryptococcus were found in sterile pathological lung tissue and pleural effusion specimens by culture or microscopy; (III) Cryptococcus were found in blood by culture or microscopy consistent with the cultivation of the lower respiratory tract specimens or microscopy results. Clinical diagnosis is considered in conjunction with medical history, respiratory symptoms, and chest radiographic evidence, and should meet one of the following criteria: (I) Cryptococcus were found in qualified sputum or bronchoalveolar lavage fluid (BALF) by culture or microscopy; (II) positive CRAG tests in lung tissue fluid, serum, BALF, cerebrospinal fluid (CSF), and pleural effusion specimens (8,10).
The specimens were inoculated on Sabouraud Dextrose Agar and cultured at 25 or 37 °C for one week to identify whether there was a growth of cryptococcus. CRAG was detected by LA test using CryptoTrol (Immuno-Mycologics Inc., Norman, OK, USA). The result of LA test was considered positive if the titer ≥1:8. The patient was defined as ICH when he or she suffered from one of the following conditions: malignancy, organ transplantation, diabetes, liver cirrhosis, connective tissue disease, low peripheral blood cluster of differentiation 4 (CD4)+ cell count or CD4+/CD8+ <1.5. If the patient did not meet any of the above criteria, the patient was defined as NICH (4,11).
Statistical analysis
All data were independently entered by three individuals into an approved research database, after verification with SPSS 25.0 statistical analysis software (IBM Corp., Armonk, NY, USA) for data processing. All measurement data are presented as mean ± SD. All numeration data are presented as counts and percentages. The chi-squared test was used for ordinal data, the unpaired t-test was used for numerical data, and the nonparametric test was used for ranked data. Significance was set at a P value <0.05 for all statistical analyses.
Results
Demographic information
In this study, 171 patients with LA-positive CRAG results and clinical manifestations had an integrated diagnosis of pulmonary cryptococcosis, 207 cases had a proven diagnosis of PC, 11 of which were confirmed by cryptococcus microscopy or culture of sterile specimens. Pathological diagnosis occurred in 196 cases; among these cases, 7 were confirmed with transbronchial lung biopsy (TBLB), 47 were confirmed by percutaneous lung biopsy, 83 were confirmed by video-assisted thoracic surgery (VATS) lung biopsy, and the other 59 cases were confirmed by open thoracic operation. The demographic data of the 378 PC patients, including age, sex, smoking and drinking history, occupation, basis of disease, immune status, and pet ownership, collected from 2002 to 2013, are shown in Table 1. Among the 378 PC patients, 244 patients had positive serum LA findings and 134 patients had negative findings. The average age of the LA-positive group was 44.32±13.64, with 51.23% showing no basis of disease, and 41.8% being ICH. Meanwhile, the average age in the LA-negative group was 50.93±10.99, with 78.36% showing no basis of disease, and only 17.91% being ICH.
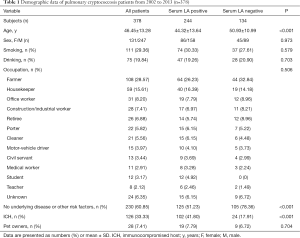
Full table
PC is an opportunistic infection and many host factors may contribute to its emergence. The host factors of the 378 patients are summarized in Table 2. The data showed that 39.15% of PC patients had comorbidities and 33.33% of PC patients were ICH, which included patients with CD4+/CD8+ <1.5, diabetes mellitus, glucocorticoid treatment, malignant tumor, AIDS, etc.
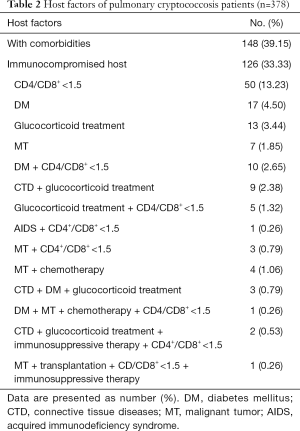
Full table
Clinical manifestations
The clinical manifestations of PC were non-specific and variable. More than 257 cases had cough, sputum, fever, chest pain, and shortness of breath as the main symptoms. Other rare symptoms included weight loss, hemoptysis, and headache. There were no abnormal signs in 235 cases. As shown in Table 3, all patients were categorized into two groups according to the serum LA test result. In the LA-positive group, 15.98% of patients had no symptoms; however, in the LA-negative group, 61.19% of patients had no symptoms, which was a significant difference between the two groups (P<0.001). Furthermore, physical examination on admission revealed 52.46% of the LA-positive patients had no signs, while 79.85% of LA-negative patients had no signs, which was also a significant difference (P<0.001). These differences combined with other results (Figure 1) indicate that LA-positive PC patients might be more likely to have symptoms and signs than LA-negative patients.
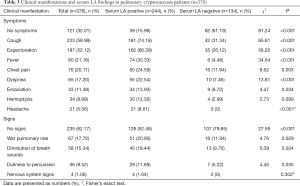
Full table

Laboratory investigations
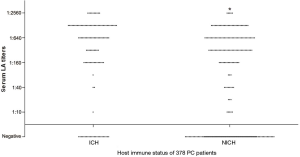
All patients underwent a serum LA test, with 64.55% showing positive results (titer from 1:10 to 1:2,560). In relation to immune status, 56.35% of the NICH patients and 80.95% of the ICH patients had positive LA test results, with this difference being significant (P<0.001). In addition, the ICH group had higher titers of serum LA test than did the NICH group (Figure 2).
Chest imaging findings
Chest CT was performed in all patients, and the characteristics of the images are summarized in Table 4, including solitary nodule or mass, multiple nodules and masses, patchy consolidation opacity, patchy mixed nodular shadows, interstitial infiltrates and diffuse granular shadows. Round or oval opacities <3 cm in diameter were considered to be nodules. Masses were defined as opacities ≥3 cm in diameter. Lung lesions of 63.23% patients were located mostly in the peripheral lung field (outer third of the lung), close to the pleura. In the LA-positive group, 35.65% of patients presented a nodule/mass in CT findings and 15.57% of patients had a solitary nodule or mass. In the LA-negative group, 79.10% of patients presented with a nodule/mass and 65.67% of patients had a solitary nodule or mass. Furthermore, patients with negative serum LA results were more likely to form solitary nodule/mass lesion than those with positive LA results (P<0.001). Meanwhile, patients with positive serum LA results were more likely to form patchy consolidation opacity lesions or combined patchy and nodular shadows than those with negative LA results (P<0.001). Also, patients with positive serum LA results were more likely to have lesions in the combined lung fields (P<0.001).
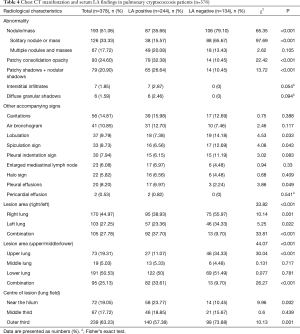
Full table
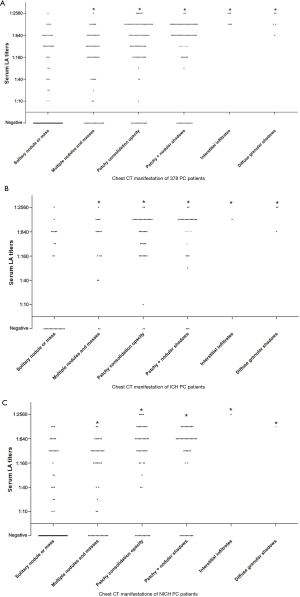
After analysis of the LA titers and the CT findings of all the PC patients (Figure 3), it was revealed that those with a solitary nodule or mass had lower titers than patients with other CT manifestations (P<0.001). The same analysis was performed on ICH and NICH PC patients (Figure 3), respectively, which clearly showed that in both the ICH (P<0.001) and NICH (P<0.001) group, patients with a solitary nodule or mass had lower titers than patient with the other five manifestation types (Table 4).
In our study, fluorodeoxyglucose positron emission tomography (FDG-PET) was performed in 169 cases, including 97 with serum-positive LA tests and 72 with serum-negative LA tests: anomalous radioactive concentrations or standardized uptake values (SUV) >2.5 were found in 66 cases (39.05%), in whom lung cancer was suspected; mild radioactive concentrations was found in 74 cases (43.79%), indicating the presence of inflammation and other benign diseases; and no obvious radiological signs were present in 29 cases (17.16%). The rate of mild and normal FDG uptake in the LA-positive group was significantly higher than in the LA-negative group (P<0.05). The FDG-PET results of the PC patients are shown in Table 5.

Full table
Discussion
Cryptococcus neoformans is an encapsulated budding yeast with a worldwide distribution. It is found in the soil, particularly soil contaminated with poultry droppings, and also within decaying plants or vegetables (12,13). In the environment, C. neoformans has a thin capsule and can easily form aerosol. However, within an organism, it can form a thick and adherent capsule. Cryptococcus infection occurs through the inhalation of desiccated yeast cells or spores, and is considered to be a primary pulmonary infection. Direct transmission between people or animals appears to be rare, but a previous study showed that symptoms of Cryptococcus infection can occur after birth from an asymptomatic mother, possibly indicating that placental infection can lead to a potentially serious condition (14). PC occurs commonly in ICH individuals, such as in AIDS patients, but can occur in NICH individuals as well (15-17). In the present study, there was only 1 AIDS case, while the others were non-HIV patients. There are two main human pathogenetic species of Cryptococcus: C. neoformans and C. gattii and both are associated with PC. It is well known that C. neoformans is found primarily in pigeon guano, is globally distributed, and is a common cause of infection in ICH; meanwhile C. gattii is linked mainly with eucalyptus trees and afflicts mostly immunocompetent individuals in tropical and subtropical regions (18-21).
In this study, PC was found to mainly affect middle-aged individuals, and occurred more often in males than in females. The male-female ratio (1.89:1) was similar to that in previous reports in China or other Western countries (13,22). The possible explanation for the skewed distribution is associated with the differences of the immune system and physiology between males and females. Although the C. neoformans extracted from pigeon guano is regarded as the most important source of infection (23,24), our study only had 14 patients with a history of direct exposure to pigeon droppings. However, 194 patients were farmers, construction/industrial workers, porters, or cleaners, who might have had a history of indirect exposure to soil or dust contaminated with pigeon or other poultry droppings.
Clinical manifestations of PC are variable and mostly associated with the host’s immune status. It was reported that approximately one-third of immunocompetent patients with PC were asymptomatic (25). Our study revealed that 38.4% of NICH were asymptomatic, while symptomatic patients presented with cough, expectoration, fever, chest pain, and dyspnea. These symptoms may also manifest in other common diseases, including lung cancer, pneumonia, and pulmonary tuberculosis. Therefore, PC is likely to be misdiagnosed. Furthermore, 21 (5.56%) patients presented with headache, 8 of whom had cryptococcal meningitis as a complication. Thus, headache often suggests an increased risk for cryptococcal meningitis, but occurs too rarely with concurrent meningeal irritation to be noticeable.
Routine laboratory investigations, such as peripheral white blood cell counts and C-reactive protein, were generally nonspecific in this study, which is consistent with a previous study (26). Moreover, 36.14% (73/202) of patients presented with serum CD4+/CD8+ <1.5 suggesting that PC may be associated with cellular immune function, especially T lymphocyte cell immunity. Several studies have shown that Cryptococcus LA tests can be used not only for auxiliary diagnosis of deep cryptococcosis, but also for semi-quantitative detection of CRAG in serum, CSF, BALF, and urine to evaluate the treatment efficacy and monitor the outcome of disease (27-29). In patients with cryptococcal meningitis, the positive rate of CRAG is as high as 94.1–100% in CSF and 86–93.6% in serum (30-32), but the serum-positive rate in HIV-negative isolated PC patients without meningitis is only about 25–56% (33), while the positive rate in BALF or lung tissue fluid is as high as 100% (34-36). In our study, the positive LA serum rates in BALF, pleural fluid, lung tissue fluid, and CSF were 64.55%, 80%, 85%, 95%, and 100%, respectively. The positive rate was much higher in ICH patients than in NICH patients, and the ICH group generally had a high titer of serum LA test, which is similar to the findings of Hung et al. (37). In this study, symptoms and signs were much more likely to appear in LA-positive patients than in LA-negative patients, suggesting that patients with serum LA-positive results had a more severe and higher load of Cryptococcus infection. The results also act as a reminder to clinicians that even in the absence of symptoms or serum LA indications, the diagnosis of PC still cannot be ruled out, and further LA testing in other specimens should be performed. The proportion of negative LA tests in our patients is noteworthy and could possibly be attributed to a concentration of CRAG below the kit detectability limit, a masking effect by unknown non-specific proteins in vivo, a prozone phenomenon arising from high concentrations of CRAG, or a poorly encapsulated strain with low production of polysaccharide (38,39).
The radiologic manifestations of PC are variable, and include nodular shadow, mass-like opacity, patchy consolidation opacity, diffuse infiltration, hilar adenopathy, and pleural effusions (4). Nodular lesions are the most common of these findings in immunocompetent patients, whereas immunosuppressed patients are more likely to have alveolar or interstitial opacities and evidence of cavitation. In the present study, 13 patients had interstitial pneumonia or diffuse granular shadows, 11 of them (included the AIDS patient) were ICH. Of the non-AIDS patients, 51.06% had nodules or masses, and the high frequency of these lesions was consistent with other studies (21,37,40). Of the nodular shadows found, 65.28% were a solitary pulmonary nodule (SPN), and were usually accompanied by lobulation, spiculation sign, or pleural indentation sign, with most being mistaken for evidence of lung cancer. Most patients had lesions mainly in subpleural areas, involving the unilateral lung, and lesion were more frequently located in the right lung or the lower lung than in the left lung or the upper lung. These results are consistent with Fox’s findings (41). However, the lesion patterns had some relevance to the result of the serum LA tests. Interstitial pneumonia or diffuse granular shadows were seen in the LA-positive patients. Patchy consolidation opacity or mixed lesion were the most common findings in the LA-positive patients, whereas LA-negative patients mainly showed nodules and/or masses. This is perhaps because the lesions of the nodules/masses were so limited that the associated CRAG released was also minute. The incidence of pleural effusion in the LA-positive group was greater than that in the LA-negative group, and the incidence of lobulations or spiculation sign in the LA-positive group was lower. Thus, serum LA testing is helpful for the diagnosis of PC, especially in the patients with radiological findings of multiple lesions or non-nodules/mass shadows. However, if the radiologic manifestations include a solitary pulmonary nodule and the serum LA test is negative, PC still cannot be excluded until more conclusive evidence can be found.
It is common for 18FDG-PET to be performed for detection of lung malignant lesions with high sensitivity and low specificity. PC patients can show higher FDG uptake, misleading physicians to presumptively diagnose lung cancer (42-44). In our study, 66 (39.05%) of the 169 patients that underwent 18FDG-PET scans had high FDG uptake or SUV >2.5. Moreover, the positive rate of 18FDG-PET in the LA-negative group was higher than that in the LA-positive group. The high positive rate might be associated with lesions in LA negative group being mainly nodules or masses.
The present study had some limitations. First, there was only 1 AIDS patient in our cohort; however, medical care is organized in China in such a way that known HIV-positive patients are preferentially referred to infectious diseases hospitals and centers, whereas our institution is specialized in pulmonary-focused diseases. Second, the rate of sputum culture positivity in this study was only 2.65% (10 out of 378), and our clinical laboratory does routinely identify Cryptococcus to the species level. Furthermore, this study was a single-center retrospective analysis, and some selection bias was inevitable. Thus, further multicenter, large sample, prospective research will be performed to improve the diagnosis and treatment strategies of PC in the future.
Conclusions
PC is more frequently found in middle-aged male non-immunocompromised hosts, whose clinical features and radiographic features are non-specific; thus, misdiagnosis or negligence of this condition is possible. Serum CRAG tests by LA may be helpful for accurate diagnosis, and our study found significantly different clinical characteristics between serum LA-positive and serum LA-negative patients. The serum LA titers were associated with clinical manifestations, CT findings, and host immune status of PC patients.
Acknowledgments
The authors thank all study participants for their cooperation, technical help, and sample collection.
Funding: This study was funded by Foundation of Shanghai Municipal Commission of Health and Family Planning (No. 20144Y0244).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/apm-21-127
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-21-127
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-21-127). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the institutional research ethics committee of Shanghai Pulmonary Hospital (No. K14-168). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from all healthy donors for inclusion in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li SS, Mody CH. Cryptococcus. Proc Am Thorac Soc 2010;7:186-96. [Crossref] [PubMed]
- Núñez M, Peacock JE, Chin R. Pulmonary cryptococcosis in the immunocompetent host. Therapy with oral fluconazole: a report of four cases and a review of the literature. Chest 2000;118:527-34. [PubMed]
- Ye F, Xie JX, Zeng QS, et al. Retrospective analysis of 76 immunocompetent patients with primary pulmonary cryptococcosis. Lung 2012;190:339-46. [Crossref] [PubMed]
- Huang HR, Fan LC, Rajbanshi B, et al. Evaluation of a new cryptococcal antigen lateral flow immunoassay in serum, cerebrospinal fluid and urine for the diagnosis of cryptococcosis: a meta-analysis and systematic review. PLoS One 2015;10:e0127117. [Crossref] [PubMed]
- Chinese Society of Respiratory Diseases, Editorial Board of Chinese Journal of Tuberculosis and Respiratory Diseases. Consensus on the diagnosis and treatment of invasive pulmonary mycosis. Chin J Tuberc Respir Dis 2007;30:821-34.
- De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813-21. [Crossref] [PubMed]
- Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020;71:1367-76. [Crossref] [PubMed]
- Huston SM, Mody CH. Cryptococcosis: an emerging respiratory mycosis. Clin Chest Med 2009;30:253-264. vi. [Crossref] [PubMed]
- Editorial Board of Chinese Journal of Mycology. Chinese expert consensus statement on management of cryptococcal infection. Chin J Mycol 2007;5:65-68.
- Shirley RM, Baddley JW. Cryptococcal lung disease. Curr Opin Pulm Med 2009;15:254-60. [Crossref] [PubMed]
- Suwatanapongched T, Sangsatra W, Boonsarngsuk V, et al. Clinical and radiologic manifestations of pulmonary cryptococcosis in immunocompetent patients and their outcomes after treatment. Diagn Interv Radiol 2013;19:438-46. [Crossref] [PubMed]
- Chowdhary A, Rhandhawa SH, Prakash A, et al. Environmental prevalence of Cryptococcus neoformans and Cryptococcus gattii in India: an update. Crit Rev Microbiol 2012;38:1-16. [Crossref] [PubMed]
- Zhang Y, Li N, Zhang Y, et al. Clinical analysis of 76 patients pathologically diagnosed with pulmonary cryptococcosis. Eur Respir J 2012;40:1191-200. [Crossref] [PubMed]
- Castro G, Cervi MC, Martinez R. Vertical transmission of Cryptococcus neoformans from a mother coinfected with human immunodeficiency virus: case report. Rev Soc Bras Med Trop 2006;39:501-3. [Crossref] [PubMed]
- Panigrahi MK, Kumar NN, Jaganathan V, et al. Pulmonary cryptococcosis with cryptococcal meningitis in an immunocompetent host. Lung India 2014;31:152-4. [Crossref] [PubMed]
- Sun L, Chen H, Shao C, et al. Pulmonary cryptococcosis with trachea wall invasion in an immunocompetent patient: a case report and literature review. Respiration 2014;87:324-8. [Crossref] [PubMed]
- Saeed N, Ansari HA, Khan N, et al. Disseminated cryptococcosis in an immunocompetent child. BMJ Case Rep 2016;2016:bcr2016217195. [Crossref] [PubMed]
- Dixit A, Carroll SF, Qureshi ST. Cryptococcus gattii: An Emerging Cause of Fungal Disease in North America. Interdiscip Perspect Infect Dis 2009;2009:840452. [Crossref] [PubMed]
- Kronstad JW, Attarian R, Cadieux B, et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol 2011;9:193-203. [Crossref] [PubMed]
- Walsh NM, Botts MR, McDermott AJ, et al. Infectious particle identity determines dissemination and disease outcome for the inhaled human fungal pathogen Cryptococcus. PLoS Pathog 2019;15:e1007777. [Crossref] [PubMed]
- Diaz JH. The Disease Ecology, Epidemiology, Clinical Manifestations, and Management of Emerging Cryptococcus gattii Complex Infections. Wilderness Environ Med 2020;31:101-9. [Crossref] [PubMed]
- McClelland EE, Hobbs LM, Rivera J, et al. The role of host gender in the pathogenesis of Cryptococcus neoformans infections. PLoS One 2013;8:e63632. [Crossref] [PubMed]
- Nielsen K, De Obaldia AL, Heitman J. Cryptococcus neoformans mates on pigeon guano: implications for the realized ecological niche and globalization. Eukaryot Cell 2007;6:949-59. [Crossref] [PubMed]
- Chitty JL, Edwards DJ, Robertson AAB, et al. Quantitation of Purines from Pigeon Guano and Implications for Cryptococcus neoformans Survival During Infection. Mycopathologia 2019;184:273-81. [Crossref] [PubMed]
- Goldman JD, Vollmer ME, Luks AM. Cryptococcosis in the immunocompetent patient. Respir Care 2010;55:1499-503. [PubMed]
- Yu JQ, Tang KJ, Xu BL, et al. Pulmonary cryptococcosis in non-AIDS patients. Braz J Infect Dis 2012;16:531-9. [Crossref] [PubMed]
- Sivertsen EA, Torfoss D. Cryptococcal meningitis. Tidsskr Nor Laegeforen 2004;124:28-30. [PubMed]
- Qazzafi Z, Thiruchunapalli D, Birkenhead D, et al. Invasive Cryptococcus neoformans infection in an asplenic patient. J Infect 2007;55:566-8. [Crossref] [PubMed]
- Lin TY, Yeh KM, Lin JC, et al. Cryptococcal disease in patients with or without human immunodeficiency virus: clinical presentation and monitoring of serum cryptococcal antigen titers. J Microbiol Immunol Infect 2009;42:220-6. [PubMed]
- Antinori S, Radice A, Galimberti L, et al. The role of cryptococcal antigen assay in diagnosis and monitoring of cryptococcal meningitis. J Clin Microbiol 2005;43:5828-9. [Crossref] [PubMed]
- Min J, Huang K, Shi C, et al. Pulmonary Cryptococcosis: comparison of Cryptococcal antigen detection and radiography in Immunocompetent and Immunocompromised patients. BMC Infect Dis 2020;20:91. [Crossref] [PubMed]
- Singh N, Alexander BD, Lortholary O, et al. Pulmonary cryptococcosis in solid organ transplant recipients: clinical relevance of serum cryptococcal antigen. Clin Infect Dis 2008;46:e12-18. [Crossref] [PubMed]
- Pappas PG, Perfect JR, Cloud GA, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis 2001;33:690-9. [Crossref] [PubMed]
- Baughman RP, Rhodes JC, Dohn MN, et al. Detection of cryptococcal antigen in bronchoalveolar lavage fluid: a prospective study of diagnostic utility. Am Rev Respir Dis 1992;145:1226-9. [Crossref] [PubMed]
- Liaw YS, Yang PC, Yu CJ, et al. Direct determination of cryptococcal antigen in transthoracic needle aspirate for diagnosis of pulmonary cryptococcosis. J Clin Microbiol 1995;33:1588-91. [Crossref] [PubMed]
- Senghor Y, Guitard J, Angoulvant A, et al. Cryptococcal antigen detection in broncho-alveolar lavage fluid. Med Mycol 2018;56:774-7. [Crossref] [PubMed]
- Hung MS, Tsai YH, Lee CH, et al. Pulmonary cryptococcosis: clinical, radiographical and serological markers of dissemination. Respirology 2008;13:247-51. [Crossref] [PubMed]
- Engler HD, Shea YR. Effect of potential interference factors on performance of enzyme immunoassay and latex agglutination assay for cryptococcal antigen. J Clin Microbiol 1994;32:2307-8. [Crossref] [PubMed]
- McMullan BJ, Halliday C, Sorrell TC, et al. Clinical utility of the cryptococcal antigen lateral flow assay in a diagnostic mycology laboratory. PLoS One 2012;7:e49541. [Crossref] [PubMed]
- Galanis E, Macdougall L, Kidd S, et al. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999-2007. Emerg Infect Dis 2010;16:251-7. [Crossref] [PubMed]
- Fox DL, Müller NL. Pulmonary cryptococcosis in immuocompetent patient: CT findings in 12 Patients. AJR Am J Roentgenol 2005;185:622-6. [Crossref] [PubMed]
- Ghimire P, Sah AK. Pulmonary cryptococcosis and tuberculoma mimicking primary and metastatic lung cancer in 18F-FDG PET/CT. Nepal Med Coll J 2011;13:142-3. [PubMed]
- Lee CH, Tzao C, Chang TH, et al. Case of pulmonary cryptococcosis mimicking hematogeneous metastases in an immunocompetent patient: value of absent 18F-fluorodeoxyglucose uptake on positron emission tomography/CT scan. Korean J Radiol 2013;14:540-3. [Crossref] [PubMed]
- Wang J, Ju HZ, Yang MF. Pulmonary cryptococcosis and cryptococcal osteomyelitis mimicking primary and metastatic lung cancer in (18)F-FDG PET/CT. Int J Infect Dis 2014;18:101-3. [Crossref] [PubMed]
(English Language Editor: J. Gray)


