
Effectiveness of vitamin D supplementation on lipid profile in polycystic ovary syndrome women: a meta-analysis of randomized controlled trials
Introduction
Polycystic ovary syndrome (PCOS), accounting for the majority of cases of anovulatory infertility, is the most common endocrine disorder among women, with a prevalence of 5−20% among countries (1). Besides, PCOS is associated with obesity, insulin resistance (IR), hyperinsulinemia, dyslipidemia and vitamin D deficiency (VDD) (2-6). Among cardiovascular risk factors, dyslipidemia is certainly the most persistent and highly prevalent. Up to 70% of women with PCOS have dyslipidemia based on the NCEP guidelines (7), even thin women (8). PCOS can affect lipid metabolism through excess androgen and IR (2,9,10). In addition, dyslipidemia may increase the risk of cardiovascular disease in PCOS patients (11). However, the most common lipid-lowering drugs are statins and fibrates, both of which have some adverse reactions, such as hepatotoxicity and myopathy (12-14). With this in mind, the identification of new strategies should be taken into consideration.
In recent years, study have reported that vitamin D can regulate lipid metabolism (15). Moreover, VDD is common in PCOS patients and several studies have demonstrated that vitamin D status could affect lipid metabolism (16,17), The study of Maidana P et al. found that decreased serum vitamin D levels are associated with metabolic and hormonal disorders in patients with PCOS (18). Thus, many researchers have explored the effect of vitamin D on the lipid profile. Studies have showed that vitamin D may reduce triglycerides (TG) by affecting calcium intake (19-21). Many observational studies have reported that the serum 25-hydroxycholesterol [25(OH)D] concentration is closely related to the blood lipid concentration.However, the lipid-lowering effect of vitamin D varied with the study population, and may be more obvious in patients with metabolic disorders (22-24). Some randomized controlled trials (RCTs) (25-35) have evaluated the effect of vitamin D supplementation on blood lipid levels, but the results are controversial. Therefore, we designed this meta-analysis to try and resolve the current controversy.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2492).
Methods
Protocol and registration
This study was designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (36).
Search strategy
This study was based on the Cochrane Handbook (37). Five databases were searched: Medline, the Cochrane Library, PubMed, and Web of Science, and all RCTs published before January 2020 were identified. Based on the PICOS (Participant, Intervention, Comparison, Outcome and Study design) principle (38), the search strategy was as follows: “vitamin D” OR “ergocalciferols” OR “cholecalciferol” OR “25-hydroxyvitamin D2” OR “hydroxycholecalciferols” OR “calcifediol” OR “calcitriol” OR “24,25-dihydroxyvitamin D3” OR “dihydrotachysterol” AND “polycystic ovary syndrome” OR “PCOS”. We also searched The ClinicalTrials.gov registry for unpublished trials. If necessary, we contacted the authors for more information. Relevant references from the included studies were searched to retrieve additional eligible studies. There were no restrictions on language.
Study selection
All data were independently reviewed by two auditors (Jiaqian Luo and Jialing Yuan), according to the following inclusion and exclusion criteria.
Inclusion criteria: (I) participants: women with PCOS, aged >18 years; (II) intervention measure: vitamin D in any dose and dosage form; (III) control group: placebo, positive drug or blank control; (IV) outcome indicators: serum lipid profile [TC, TG, LDL-cholesterol (LDL-C), very LDL-C (VLDL-C), and high-density lipoprotein cholesterol (HDL-C)]; (V) study type: all RCTs.
Exclusion criteria: (I) not RCT; (II) no assessing primary data; (III) letters to the editor; and (IV) duplicate publication or secondary research paper.
A third reviewer (Tao Li) was consulted to resolve any disagreements.
Data extraction
Using a predesigned data extraction table, the following information was collected: study author(s); year of publication; study design; sample size; mean age, body mass index (BMI), mean serum 25(OH)D (ng/mL) of study subjects; follow-up duration; doses of vitamin D, and outcomes (TG, TC, LDL-C, VLDL-C, HDL-C). In cases of disagreement, the differences were resolved through cross-checking, and discussion or consultation with the third researcher (Tao Li). If the original data were not provided directly in the text, reference was made to the tables or figures in the publication. If the relevant details were not fully reported in the study, the authors were contacted for further information.
Quality assessment
The quality of the included studies was evaluated according to the Cochrane Handbook for Systematic Reviews (version 5.3.0) (37). The two investigators (Jiaqian Luo and Jialing Yuan) independently evaluated and, if any, disagreements were resolved through discussion or consultation with a third investigator (Tao Li).
Data analysis
Using Revman 5.3 software for data analysis, we calculated the standardized mean difference (SMD) and 95% confidence interval (CI) of all outcomes (TG, TC, LDL-C, VLDL-C, HDL-C). The Q test and I2 test were used to analyze heterogeneity. When the included studies had large heterogeneity (P<0.10 or I2 >50%), the random-effects model was used to pool the estimations of SMD across studies. Otherwise, a fixed-effects model was used. Sensitivity analysis was used to find the source of heterogeneity and evaluate the stability and reliability of the meta-analysis results. Subgroup analyses were performed based on vitamin D doses, intervention duration, and type of supplementation. A funnel plot was constructed to determine publication bias. P values <0.05 were considered to be statistically significant.
Results
Search results and study characteristics
The flow diagram of our study selection process is shown in Figure 1. In total, we identified 954 citations with 339 duplicates. After the preliminary screening of the titles and abstracts, 43 studies were selected for full-text review and 32 studies were excluded because 6 were not RCTs, 13 included research providing no quantitative outcomes, and the remainder were either conference abstracts, reviews, or animal experiments. Finally, we enrolled 11 studies (25-35) in our meta-analysis.
As shown in Table 1, we summarized the characteristics of the 11 studies involving 677 subjects included in the meta-analysis, among which 391 patients were randomly distributed into a vitamin D group and the rest were the control group. The sample size ranged from 28 to 180. The doses of vitamin D varied from 400 to 12,000 IU/day with duration from 8 to 24 weeks.
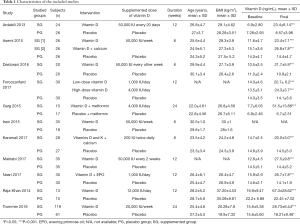
Full table
Quality assessment
The results of quality assessment of the RCTs included in our meta-analysis are shown in Figure 2. Randomization was performed according to a computer-generated random list or by means of a randomly generated number pattern in nine trials (25-29,31,32,34,35); two RCTs did not provide details about the method of randomization (30,33). With respect to allocation, eight trials were categorized as low risk (25-28,31,32,34,35) with the appropriate use of allocation concealment, and three trials were unclear (29,30,33). Regarding to blinding of participants and personnel and outcome assessment, all RCTs were low risk (25-35). Nine of the RCTs included in our study were characterized by a low risk of blinding of incomplete outcome data (25-29,31-34), and two trials were categorized as high risk (30,35). Selective reporting was evaluated: three RCTs were categorized as high risk (29,30,32), and the rest were classified as low risk (25-28,31,33-35). Moreover, six trials (25-28,31,34) were classified as low risk and the remaining five were classified as unclear (29,30,32,33,35) for other bias.
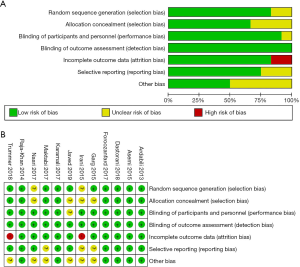
Pooled analysis
Effect of vitamin D on serum TC
All 677 subjects had data on the effect of vitamin D on serum TC (25-35). Results demonstrated that vitamin D statistically reduced TC in patients with PCOS (Figure 3A: −0.35 mg/dL, 95% CI: −0.63 to −0.07 mg/dL, P=0.01, I2 =66%).
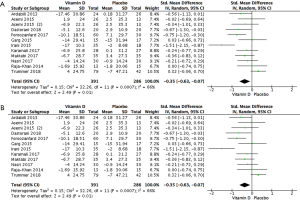
Sensitivity analysis was performed to find the sources of heterogeneity and most of the heterogeneity belonged to the studies by Trummer et al. (35), and Irani et al. (30). We therefore re-analysed the data after excluding those studies. The heterogeneity among the studies was clearly reduced (I2 =0%, P=0.55) and the results demonstrated that vitamin D statistically reduced serum TC (Figure 3B: −0.36 mg/dL, 95% CI: −0.54 to −0.18 mg/dL, P<0.0001).
Subgroup analyses were also performed based on vitamin D dosage, intervention duration and type of supplementation (Table 2). Regarding the dose of intervention, vitamin D in doses ≤5,000 IU/day statistically reduced serum TC in patients with PCOS (−0.29 mg/dL, 95% CI: −0.58 to −0.01 mg/dL, P=0.04). Regarding the duration of intervention, in the duration of ≤12 weeks statistically reduced serum TC in patients with PCOS (−0.39 mg/dL, 95% CI: −0.57 to −0.20 mg/dL, P<0.0001). For the type of supplementation, vitamin D alone statistically reduced serum TC (−0.39 mg/dL, 95% CI: −0.76 to −0.01 mg/dL, P=0.04).
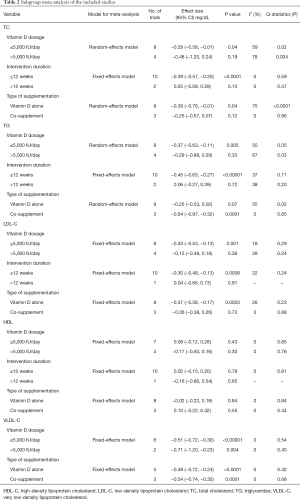
Full table
Effect of vitamin D on serum TG
Results for the 677 participants (25-35) showed that vitamin D statistically reduced TG in patients with PCOS (Figure 4A: −0.35 mg/dL, 95% CI: −0.59 to −0.12 mg/dL, P=0.003, I2 =52%). Sensitivity analysis demonstrated that most of the heterogeneity belonged to the studies by Trummer et al. (35), and Raja-Khan et al. (34). The re-analysed data demonstrated that vitamin D statistically reduced serum TG as well after excluding those studies (Figure 4B: −0.50 mg/dL, 95% CI: −0.68 to −0.32 mg/dL, P<0.00001, I2 =0%).
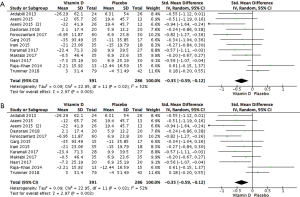
Subgroup analyses indicated that vitamin D in doses ≤5,000 IU/day statistically reduced serum TG (−0.37 mg/dL, 95% CI: −0.63 to −0.11 mg/dL, P=0.005). For a duration ≤12 weeks, vitamin D statistically reduced serum TG (−0.45 mg/dL, 95% CI: −0.63 to −0.27 mg/dL, P<0.00001). Regarding the type of supplementation, co-supplement statistically reduced serum TG (−0.64 mg/dL, 95% CI: −0.97 to −0.32 mg/dL, P=0.0001).
Effect of vitamin D on serum LDL-C
Meta-analysis of 10 studies with 556 participants demonstrated that vitamin D statistically reduced LDL-C (Figure 5: −0.28 mg/dL, 95% CI: −0.45 to −0.11 mg/dL, P=0.001, I2 =19).
The results of subgroup analyses are shown in Table 2. Subgroup analyses showed that vitamin D in doses ≤5,000 IU/day statistically reduced serum LDL-C in patients with PCOS (−0.33 mg/dL, 95% CI: −0.53 to −0.13 mg/dL, P=0.001). Administration of vitamin D for at ≤12 weeks statistically reduced the serum LDL-C in the patients (−0.30, 95% CI: −0.48 to −0.13, P=0.0008). Regarding the type of supplementation, vitamin D alone statistically reduced serum LDL-C (−0.37 mg/dL, 95% CI: −0.58 to −0.17 mg/dL, P=0.0003).
Effect of vitamin D on serum HDL-C
The meta-analysis of 10 RCTs with 556 patients showed that vitamin D did not statistically change serum HDL in patients with PCOS (Figure 6: 0.01 mg/dL, 95% CI: −0.16 to −0.18 mg/dL, P=0.89, I2 =0%).
Subgroup analyses showed that the effect of vitamin D on TG did not depend on vitamin D dosage, intervention duration, or type of supplementation (Table 2).
Effect of vitamin D on serum VLDL-C
Meta-analysis of 7 RCTs with 443 patients showed that vitamin D statistically changed serum VLDL-C in patients with PCOS (Figure 7: −0.54 mg/dL, 95% CI: −0.74 to −0.35 mg/dL, P<0.00001, I2 =0%).
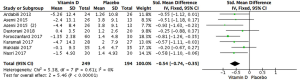
According to subgroup analyses (Table 2), any vitamin D dosage and type of supplementation could statistically reduce serum VLDL-C in patients with PCOS (≤5,000 IU/day: −0.51 mg/dL, 95% CI: −0.72 to −0.30 mg/dL, P<0.00001; >5,000 IU/day: −0.71 mg/dL, 95% CI: −1.20 to −0.23 mg/dL, P=0.004; vitamin D alone: −0.48 mg/dL, 95% CI: −0.72 to −0.24 mg/dL, P<0.0001; co-supplement: −0.54 mg/dL, 95% CI: −0.74 to −0.35 mg/dL, P=0.0001).
Publication bias
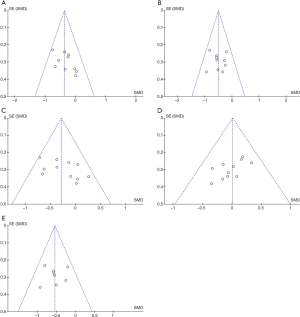
According to the funnel plots, there was no obvious publication bias on TG, TC, LDL-C, VLDL-C, HDL-C. (Figure 8A,B,C,D,E).
Discussion
The present meta-analysis of 11 RCTs with 677 participants assessed the effectiveness of vitamin D supplementation on the serum lipid profiles of women with PCOS. The pooled results from the current study revealed that vitamin D supplementation had statistical effects on TC, TG, LDL-C, and VLDL-C, but the changes in serum HDL-C were not satisfactory. Sensitivity analysis further verified the conclusions. It must be kept in mind that following vitamin D supplementation alone, TC and LDL-C concentrations statistically decreased compared with co-supplementation of vitamin D, while the result for TG concentrations was contrary. TC, TG and LDL-C concentrations were statistically reduced only at the lower dosage (≤5,000 IU/day) of vitamin D and duration ≤12 weeks. Circulating concentrations of VLDL-C were not affected by the type of intervention or the dosage of vitamin D used for supplementation.
VDD is a worldwide problem affecting half of the general adult population, but especially in women with PCOS (39). About 67−85% of women with PCOS have serum concentrations of 25(OH)D <20 ng/mL (40). What’s more, there is a close relationship between vitamin D status and metabolic dysfunction in women with PCOS (16-18,41,42). Many studies have explored the effect of vitamin D supplementation on metabolic disorders in patients with PCOS, but the results have been controversial. The study of Ardabili et al. indicated that vitamin D supplements could decrease serum total cholesterol, triglyceride, and VLDL levels significantly, but it did not affect serum HDL-cholesterol and LDL-cholesterol. Asami et al. showed that calcium plus vitamin D supplementation for eight weeks among vitamin D deficient women with PCOS had beneficial effects on serum triglycerides and VLDL-cholesterol levels, but it did not affect other lipid profiles. Dastorani et al. found that 50,000 IU vitamin D supplementation every other week for 8 weeks had beneficial effects on lipid profile in women with PCOS. Karamali et al. found calcium, vitamins D and K co-supplementation for 8 weeks among vitamin D-deficient women with PCOS had beneficial effects on serum triglycerides and VLDL cholesterol levels. Nasri et al.showed that vitamin D and EP co-supplementation for 12 weeks among vitamin D-deficient women with PCOS significantly improved triglycerides, VLDL cholesterol. However, vitamin D supplementation had no significant effect on serum lipid profile in PCOS in the studies of Garget al., Irani et al., Raja-Khan et al., and Trummer et al. (25-35). Recently, a meta-analysis by Łagowska et al. suggested that vitamin D supplementation had a positive effect on IR in women with PCOS (43). In our meta-analysis, we investigated the effects of vitamin D supplementation on blood lipid levels in patients with PCOS. Our results showed that PCOS patients treated with vitamin D had associated improvements in TC, TG, LDL and VLDL-C levels, but no effect on HDL was observed. This finding is in line with the results of a meta-analysis by Akbari et al., which underlined that vitamin D supplementation statistically decreased LDL-C concentrations in gestational diabetes patients, though other lipid profile parameters did not change (44). In addition, a meta-analysis by Tina et al. also demonstrated that vitamin D supplementation improved serum concentrations of TC, TG, and LDL-C, but negligible changes in serum HDL-C, in patients with T2DM (45). However, in 2012, a meta-analysis conducted by Wang et al. concluded that vitamin D supplementation could increase LDL-C and suggested that the lipid-lowering effects of vitamin D supplementation might be seen more apparently in patients with metabolic disorders such as T2DM or PCOS (46).
The exact mechanism by which vitamin D affects lipid markers has not been elucidated. It has been demonstrated that vitamin D increases LPL gene expression in muscles and adipose tissue, and activation of LPL increases the clearance of circulating lipoprotein particles (20). Cho et al. indicated that vitamin D may decrease hepatic TG production or secretion by its effects on calcium intake (19). And other researchers attribute it to the effects of vitamin D in reducing parathyroid hormone and improving insulin sensitivity (40,46,47). Moreover, the serum TC concentration is affected by cholesterol absorption from the gut and endogenous biosynthesis of cells. Kane et al. suggested that vitamin D might reduce intestinal cholesterol absorption (48).
Based on our subgroup results, the administration of vitamin D ≤5,000 IU/day statistically reduced the serum concentrations of TC, TG, LDL-C, and VLDL-C, which according with the results of the meta-analysis by Xue et al. (49), who suggested that supplementation with a low dose of vitamin D was enough to reduce TG concentration, whereas supplementation with high doses was not beneficial. Besides, Sanders et al. pointed out that bolus doses of vitamin D3 >500,000 IU might increase the risk of fracture, altered biochemical markers, and cause issues with tolerability (50). Thus, we concluded that supplying vitamin D could improve the serum lipid profile, and supplementation of vitamin D ≤5,000 IU/day seemed to have a statistical effect compared with higher doses.
In the subgroup analysis performed according to the type of supplementation, statistical reductions of serum TC, LDL-C, and VLDL-C concentrations were observed in the vitamin D alone group, and statistical reductions of serum TG and VLDL-C concentrations were observed in the co-supplementation group. It is worth noting that vitamin D supplementation alone had a tendency to reduce serum TG concentration, but had no statistical significance (P=0.07). By contrast, previous studies observed a statistical reduction in TG affected by 4,500 IU/day of vitamin D supplementation for 2 months in females with T2DM (51), and by 50,000 IU/day of vitamin D supplementation for 16 weeks in subjects with metabolic syndrome (52). In our study, we also found a tendency to improved TG levels with vitamin D alone supplementation among these women with PCOS. Therefore, we speculate that vitamin D supplementation alone is likely to have TG-lowering effect, but larger samples and high-quality studies are needed to definitely identify this effect. Moreover, all three studies included in co-supplementation group showed a statistical reduction in serum TG concentration after vitamin D plus other nutrient supplementation (26,31,33). Two of the studies were combined with calcium (26,31) and one with evening primrose oil (EPO) (33). Previous studies have shown that calcium intake may lead to reduced absorption of fatty acids and increased fecal fatty acid content by forming insoluble calcium-fatty soaps in the gut, thereby reducing the serum TG concentration (53). In addition, increased intracellular calcium in the liver results in stimulating microsomal TG transfer protein, and thus reduced serum TG concentration (19). EPO is rich in gamma-linolenic acid, which would have a TG-lowering effect by inhibiting synthesis of hepatic TG (54). As mentioned before, it is possible that co-supplementation with vitamin D and calcium or EPO may be more effective than single supplementation in reducing the serum TG concentration.
We also demonstrated that serum TC, TG, LDL-C, and VLDL-C concentrations were statistically reduced after ≤12 weeks. This result, however, needs to be interpreted with caution because there was little literature for the >12 weeks subgroup, in which at most two RCTs were included. Larger prospective studies are needed to further validate our results.
The Endocrine Society guidelines (55) recommend that people should maintain blood levels of 25(OH)D >20 ng/mL to prevent rickets and osteomalacia. Moreover, to maximize the effects of vitamin D on calcium, bone and muscle metabolism, the blood level of 25(OH)D should >30 ng/mL, and 25(OH)D at this concentration may have additional health benefits in reducing the risk of common cancers, autoimmune diseases, T2DM and Cardiovascular Disease (CVD). Considering that VDD is very common and that few foods contain vitamin D, the guidelines also suggest that increased dietary intake of vitamin D for patients at risk of VDD is necessary. As stated earlier, a relatively high prevalence of VDD is observed in women with PCOS (40). Additionally, there is an association between vitamin D status and metabolic dysfunction in women with PCOS, and there are positive associations of VDD with some comorbidities of PCOS, including T2D and CVD (41,42). All of the studies included in this meta-analysis showed that vitamin D supplementation helped restore physiological serum levels of 25(OH)D in PCOS women with VDD (25-35), and a beneficial effect of vitamin D supplementation on glucose metabolism was reported as well (43). Our study also indicated that vitamin D supplementation (alone or combined with other nutrients) may help improve the lipid profile. Although our study found that vitamin D supplementation showed a statistical reduction effect on lipid metabolism in PCOS patients, we can not directly prove the clinical significance of vitamin D supplementation in improving lipid metabolism because only a slight lipid-lowering effect was seen in this meta-analysis. But considering the high-prevalence of VDD and dyslipidemia in PCOS patients, and the recommendation for vitamin D supplementation in populations at high-risk for VDD, vitamin D supplementation may be a simple and low-risk add-on therapy for PCOS patients with VDD and dyslipidemia.
Several strengths of the current study are as follows. Firstly, this meta-analysis used an up-to-date literature search representing the most available data on this topic. Secondly, all included studies were placebo-controlled RCTs with acceptable methodological quality and the least probable chance of bias. Thirdly, the design of the study was analyses of a specific population (PCOS patients) instead of pooling populations with different health conditions. There are also some limitations. Firstly, we found few eligible studies and none of them were sufficiently powered because they had relatively small numbers of participants. Secondly, the results of subgroup analyses of duration of therapy and type of vitamin D supplementation need to be interpreted with caution as there was little literature included, especially in the >12-week’ duration subgroup, in which only two RCTs were included. Thirdly, patients might have had different status of PCOS. Finally, the history of using antidyslipidemic drugs (e.g., statins) was not clear.
Conclusions
To date, the evidence from RCTs indicated that vitamin D supplementation (alone or with co-supplementation) could statistically improve lipid metabolism, but the effect is small. However, considering the high-prevalence of VDD and dyslipidemia in PCOS patients, and the recommendation of vitamin D supplementation in populations at high-risk for VDD, vitamin D supplementation may be a simple and low-risk add-on therapy for PCOS patients with VDD and dyslipidemia. Vitamin D dosage, the duration of intervention and type of vitamin D supplementation (alone or with co-supplementation) may influence the effects of vitamin D supplementation on the lipid profile. The lipid-modulating effects of vitamin D supplement should be further investigated through large-scale, randomized trials with adequate doses, duration, and types of supplementation.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2492
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2492). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 2013;6:1-13. [Crossref] [PubMed]
- Patel S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol 2018;182:27-36. [Crossref] [PubMed]
- Broskey NT, Tam CS, Sutton EF, et al. Metabolic inflexibility in women with PCOS is similar to women with type 2 diabetes. Nutrition Metabolism 2018;15:75. [Crossref] [PubMed]
- Li X, Zhang T, Li S, et al. Correlation between glucose metabolism and serum steroid hormones in patients with polycystic ovary syndrome. Clin Endocrinol (Oxf) 2020;92:350-7. [Crossref] [PubMed]
- Ho CW, Chen HH, Hsieh MC, et al. Hashimoto’s thyroiditis might increase polycystic ovary syndrome and associated comorbidities risks in Asia. Ann Transl Med 2020;8:684. [Crossref] [PubMed]
- Zhu S, Zhang B, Jiang X, et al. Metabolic disturbances in non-obese women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertility Sterility 2019;111:168-77. [Crossref] [PubMed]
- Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med 2001;111:607. [Crossref] [PubMed]
- Macut D, Bjekić-Macut J, Savić-Radojević A. Dyslipidemia and Oxidative Stress in PCOS. Front Horm Res 2013;40:51-63. [Crossref] [PubMed]
- Macut D, Bjekić-Macut J, Rahelić D, et al. Insulin and the polycystic ovary syndrome. Diabetes Res Clin Pract 2017;130:163-70. [Crossref] [PubMed]
- Pirwany IR, Fleming R, Greer IA, et al. Lipids and lipoprotein subfractions in women with PCOS: relationship to metabolic and endocrine parameters. Clin Endocrinol (Oxf) 2001;54:447-53. [Crossref] [PubMed]
- Blagojevic IP, Eror T, Pelivanovic J, et al. Women with polycystic ovary syndrome and risk of cardiovascular disease. J Med Biochem 2017;36:259-69. [Crossref] [PubMed]
- Barter PJ, Rye KA. New Era of Lipid-Lowering Drugs. Pharmacological reviews 2016;68:458-475. [Crossref] [PubMed]
- Harper CR, Jacobson TA. The broad spectrum of statin myopathy: from myalgia to rhabdomyolysis. Curr Opin Lipidol 2007;18:401-8. [Crossref] [PubMed]
- Golomb BA, Evans MA. Statin adverse effects. Am J Cardiovasc Drugs 2008;8:373-418. [Crossref] [PubMed]
- Muscogiuri G, Altieri B, Annweiler C, et al. Vitamin D and chronic diseases: the current state of the art. Arch Toxicol 2017;91:97-107. [Crossref] [PubMed]
- Menichini D, Facchinetti F. Effects of vitamin D supplementation in women with polycystic ovary syndrome: a review. Gynecol Endocrinol 2020;36:1-5. [Crossref] [PubMed]
- Dastorani M, Aghadavod E, Mirhosseini N, et al. The effects of vitamin D supplementation on metabolic profiles and gene expression of insulin and lipid metabolism in infertile polycystic ovary syndrome candidates for in vitro fertilization. Reprod Biol Endocrinol 2018;16:94. [Crossref] [PubMed]
- Maidana P, Fritzler A, Mocarbel Y, et al. Association Between Vitamin D and Adrenal Parameters with Metabolic and Inflammatory Markers in Polycystic Ovary Syndrome. Sci Rep 2019;9:3968. [Crossref] [PubMed]
- Cho HJ, Kang HC, Choi SA, et al. The possible role of Ca2+ on the activation of microsomal triglyceride transfer protein in rat hepatocytes. Biol Pharm Bull 2005;28:1418-23. [Crossref] [PubMed]
- Querfeld U, Hoffmann MM, Klaus G, et al. Antagonistic effects of vitamin D and parathyroid hormone on lipoprotein lipase in cultured adipocytes. J Am Soc Nephrol 1999;10:2158-64. [PubMed]
- Garbossa SG, Folli F. Vitamin D, sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Rev Endocr Metab Disord 2017;18:243-58. [Crossref] [PubMed]
- Tepper S, Shahar DR, Geva D, et al. Identifying the threshold for vitamin D insufficiency in relation to cardio-metabolic markers. Nutr Metab Cardiovasc Dis 2014;24:489-94. [Crossref] [PubMed]
- Muscogiuri G, Mitri J, Mathieu C, et al. Mechanisms in endocrinology: vitamin D as a potential contributor in endocrine health and disease. Eur J Endocrinol 2014;171:R101-10. [Crossref] [PubMed]
- Savastano S, Valentino R, Di Somma C, et al. Serum 25-Hydroxyvitamin D Levels, phosphoprotein enriched in diabetes gene product (PED/PEA-15) and leptin-to-adiponectin ratio in women with PCOS. Nutrition Metabolism 2011;8:84. [Crossref] [PubMed]
- Rahimi-Ardabili H, Pourghassem Gargari B, Farzadi L. Effects of vitamin D on cardiovascular disease risk factors in polycystic ovary syndrome women with vitamin D deficiency. J Endocrinol Invest 2013;36:28-32. [PubMed]
- Asemi Z, Foroozanfard F, Hashemi T, et al. Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr 2015;34:586-92. [Crossref] [PubMed]
- Dastorani M, Aghadavod E, Mirhosseini N, et al. The effects of vitamin D supplementation on metabolic profiles and gene expression of insulin and lipid metabolism in infertile polycystic ovary syndrome candidates for in vitro fertilization. Reprod Biol Endocrinol 2018;16:94. [Crossref] [PubMed]
- Foroozanfard F, Talebi M, Samimi M, et al. Effect of Two Different Doses of Vitamin D Supplementation on Metabolic Profiles of Insulin-Resistant Patients with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm Metab Res 2017;49:612-7. [Crossref] [PubMed]
- Garg G, Kachhawa G, Ramot R, et al. Effect of vitamin D supplementation on insulin kinetics and cardiovascular risk factors in polycystic ovarian syndrome: a pilot study. Endocr Connect 2015;4:108-16. [Crossref] [PubMed]
- Irani M, Seifer DB, Grazi RV, et al. Vitamin D Supplementation Decreases TGF-1 Bioavailability in PCOS: A Randomized Placebo- Controlled Trial. J Clin Endocrinol Metab 2015;100:4307-14. [Crossref] [PubMed]
- Karamali M, Ashrafi M, Razavi M, et al. The Effects of Calcium, Vitamins D and K co-Supplementation on Markers of Insulin Metabolism and Lipid Profiles in Vitamin D-Deficient Women with Polycystic Ovary Syndrome. Exp Clin Endocrinol Diabetes 2017;125:316-21. [Crossref] [PubMed]
- Maktabi M, Chamani M, Asemi Z. The Effects of Vitamin D Supplementation on Metabolic Status of Patients with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm Metab Res 2017;49:493-8. [Crossref] [PubMed]
- Nasri K, Akrami S, Rahimi M, et al. The effects of vitamin D and evening primrose oil co-supplementation on lipid profiles and biomarkers of oxidative stress in vitamin D-deficient women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Endocr Res 2018;43:1-10. [Crossref] [PubMed]
- Raja-Khan N, Shah J, Stetter CM, et al. High-dose vitamin D supplementation and measures of insulin sensitivity in polycystic ovary syndrome: a randomized, controlled pilot trial. Fertil Steril 2014;101:1740-6. [Crossref] [PubMed]
- Trummer C, Schwetz V, Kollmann M, et al. Effects of vitamin D supplementation on metabolic and endocrine parameters in PCOS: a randomized-controlled trial. Eur J Nutr 2019;58:2019-28. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009;151:264-9. [Crossref] [PubMed]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011;5:S38. Available online: handbook. cochrane.org.
- O’Connor D, Green S, Higgins JP. Cochrane Book Series. In Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons 2008:81-94.
- Selimoglu H, Duran C, Kiyici S, et al. The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J Endocrinol Invest 2010;33:234-8. [Crossref] [PubMed]
- Wehr E, Pieber TR, Obermayer-Pietsch B. Effect of vitamin D3 treatment on glucose metabolism and menstrual frequency in polycystic ovary syndrome women: a pilot study. J Endocrinol Invest 2011;34:757-63. [PubMed]
- Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr 2005;94:483-92. [Crossref] [PubMed]
- Grundmann M, von Versen-Höynck F. Vitamin D - roles in women's reproductive health? Reprod Biol Endocrinol 2011;9:146. [Crossref] [PubMed]
- Łagowska K, Bajerska J, Jamka M. The Role of Vitamin D Oral Supplementation in Insulin Resistance in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2018;10:1637. [Crossref] [PubMed]
- Akbari M, Mosazadeh M, Lankarani KB, et al. The effects of vitamin D supplementation on glucose metabolism and lipid profiles in patients with gestational diabetes: a systematic review and meta- analysis of randomized controlled trials. Horm Metab Res 2017;49:647-53. [Crossref] [PubMed]
- Jafari T, Fallah AA, Barani A. Effects of vitamin D on serum lipid profile in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Clin Nutr 2016;35:1259-68. [Crossref] [PubMed]
- Wang H, Xia N, Yang Y, et al. Influence of vitamin D supplementation on plasma lipid profiles: A meta-analysis of randomized controlled trials. Lipids Health Dis 2012;11:42. [Crossref] [PubMed]
- Kaplan M, Kerry R, Aviram M, et al. High glucose concentration increases macrophage cholesterol biosynthesis in diabetes through activation of the sterol regulatory element binding. Protein 1 (SREBP1): inhibitory effect of insulin. J Cardiovasc Pharmacol 2008;52:324-32. [Crossref] [PubMed]
- Kane L, Moore K, Lütjohann D, et al. Vitamin D3 effects on lipids differ in statin and non-statin-treated humans: superiority of free 25-OHD levels in detecting relationships. J Clin Endocrinol Metab 2013;98:4400-9. [Crossref] [PubMed]
- Xue Y, Xu P, Xue K, et al. Effect of vitamin D on biochemical parameters in polycystic ovary syndrome women: A meta-analysis. Arch Gynecol Obstet 2017;295:487-96. [Crossref] [PubMed]
- Sanders KM, Nicholson GC, Ebeling PR. Is high dose vitamin D harmful? Calcif Tissue Int 2013;92:191-206. [Crossref] [PubMed]
- Mohamad MI, El-Sherbeny EE, Bekhet MM. The Effect of Vitamin D Supplementation on Glycemic Control and Lipid Profile in Patients with Type 2 Diabetes Mellitus. J Am Coll Nutr 2016;35:399-404. [Crossref] [PubMed]
- Salekzamani S, Mehralizadeh H, Ghezel A, et al. Effect of high-dose vitamin D supplementation on cardiometabolic risk factors in subjects with metabolic syndrome: a randomized controlled double-blind clinical trial. J Endocrinol Invest 2016;39:1303-13. [Crossref] [PubMed]
- Reid IR. Effects of calcium supplementation on circulating lipids: potential pharmacoeconomic implications. Drugs Aging 2004;21:7-17. [Crossref] [PubMed]
- Coleman MP, Key TJ, Wang DY, et al. A prospective study of obesity, lipids, apolipoproteins and ischaemic heart disease in women. Atherosclerosis 1992;92:177-85. [Crossref] [PubMed]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911-30. [Crossref] [PubMed]
(English Language Editor: K. Brown)





