
Taurine attenuates the damage of lupus nephritis mouse via inactivation of the NF-κB pathway
Introduction
Lupus nephritis (LN) is one of the most common and devastating manifestations of the chronic autoimmune disease systemic lupus erythematosus (SLE) (1). LN usually appears at least 3 years after the onset of SLE (2). SLE patients can pathologically form anti-double-stranded DNA immune complexes and deposit them onto regions of the kidney, and as a consequence, inflammatory responses and the subsequent chain reactions of abnormal and destructive immune activities can be generated, leading to irreversible kidney injury (1). Immunosuppressive therapies (3) that are commonly used for LN treatment have been confirmed to improve the condition of LN and patient outcomes (4,5), however, the existing irreversible damage to the kidneys cannot be reversed. For severe and end-stage LN patients, a novel therapy that involves the recovery of renal functions is urgently required.
Taurine, a sulfur-containing β-amino acid, was first isolated from ox bile in 1827, and identified in human bile in 1846. It is a natural compound widely distributed in the human body and in foods. In some species, taurine is an essential nutrient, however, it is considered a semi-essential nutrient in humans (6-9). Several animal models and clinical studies have elucidated its therapeutic functions in many types of diseases (10,11), concerning motor function (12,13), the cardiovascular system (14,15), and the central nervous system (16,17), as well as mitochondrial related disorders (18). In addition, metabolic diseases, such as diabetes (19-21), and inflammatory diseases, such as arthritis (22), can also benefit from the therapeutic effects of taurine. Furthermore, in vitro studies have also revealed the diversity of its cytoprotective activities. Publications show no known side effects of taurine when taken in reasonable doses (6), which renders it superior to other types of therapeutic drugs.
At present, no research on taurine treatment for LN has been reported, however, the role that taurine plays in other nephrosis has been extensively described (23). Taurine has been proposed to promote renal function in a variety of renal disorders, including immune- and toxicity-induced forms of chronic renal failure, diabetic nephropathy, glomerulonephritis, as well as acute kidney injury (23). Investigations into the mechanisms underlying how taurine and its derivatives improve renal dysfunction have also been carried out. In animal models (3,24), evidence suggests the ischemia/reperfusion injury of the kidney can be attenuated (25), and renal blood flow can also be influenced by taurine (26). Moreover, taurine has been shown to act as a regulator in biological oxidation reactions (27), stress responses, the cell cycle, as well as apoptosis (22). Interestingly, recent reports have described a long non-coding RNA (lncRNA), taurine-upregulated gene 1 (TUG1), which had been originally identified in a genomic screen for genes upregulated in response to taurine treatment in mouse retinal cells (28), and may be a potential therapeutic target for LN treatment (29). It should be noted that most of the studies were performed at the molecular, cellular, and animal levels, but not yet in clinical trials.
Given the above evidence, we carried out an exploratory study of taurine treatment on LN using the spontaneous development autoimmune disease MRL/lpr mouse model, and C57BL/6 mice were used as controls. Our results indicated that taurine can indeed rescue kidney injury, improve impaired tissues in LN, relieve peroxidation stress in nephrocytes, and even reverse the apoptosis-promoting conditions in LN mice. Furthermore, we explored the signaling pathways related to the renal protection conferred by taurine, and the results suggest that taurine regulates the NF-κB pathway. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2087).
Methods
Ethical compliance
All animal procedures and experiments in this study were approved by the Animal Ethics Committee of the First Affiliated Hospital of Hebei North University, and were conducted strictly in accordance with the guidelines of International Guiding Principles for Animal Research.
Animal experiments
In this study, we used 5 female C57BL/6 mice and 45 female MRL/lpr mice to conduct animal experiments. All mice were purchased from the Shanghai SLAC Laboratory Animal Company Limited (Shanghai, China). The animal facility was temperature controlled at 25±1 °C with a light cycle of 12 h light/12 h dark in flow sterile conditions. The mice had free access to food and water. At the age of 12 weeks, MRL/lpr mice were separated into five groups and administered 0, 50 and 100 mg/kg body weight of taurine for 5 days, while 5 C57BL/6 mice were given PBS as the negative control group (30). Asatone, a specific activator of NF-κB p65, was used to further verify the results of this study. After taurine treatment, urine and blood samples were taken for kidney function analysis, then all the animals were then sacrificed under chloral hydrate anesthesia. Mice kidneys were dissected for protein, RNA extraction, hematoxylin and eosin (HE) staining and TUNEL staining. The positive drug in this experiment was Enalapril, with a concentration of 25 mg/mL (31).
Histology
Tissue specimens were harvested from the kidneys of sacrificed mice, then fixed for 48 hours with 10% formalin. Samples were dehydrated in graded ethanol and embedded in paraffin wax. The tissue was cut into 4 to 7 µm sections and stained with HE for histopathological analysis.
ELISA
Serum inflammatory factors were analyzed using iNOS (LifeSpan BioSciences), TNF-α, IL-4, and IL-10 (R&D system) ELISA kits. Sample preparation and assays were performed according to the manufacturer’s instructions.
Levels of glutathione peroxidase (GPx), superoxide dismutase (SOD), and malondialdehyde (MDA) were all detected using commercial kits from Abcam (Cambridge, UK), and sample preparation and the assays were performed according to the manufacturer’s instructions.
Kidney function measurements
Blood urea nitrogen (BUN) and serum creatinine (CRE) were measured using specific reagents through the use of the automatic analyzer (Fujifilm Ltd., Japan). Total urinary protein concentrations were measured using the pyrogallol red-molybdate (PRM) protein dye-binding assay as previously described (32).
Dichlorofluorescein (DCF) staining
In our study, DCF staining was used to detect reactive oxygen species (ROS) generation. The DCF staining process was adapted from previous reports (33). Kidney tissues were firstly incubated in Hank’s balanced salt solution for 1 hour with 5 mM H2DCFDA. Then, the non-fluorescent H2DCFDA oxidized to the highly fluorescent 2',7'-dichlorofluorescein (DCF). DCF fluorescence was observed at 488 nm excitation wavelength and 515–540 nm emission wavelength under an Olympus confocal microscope (Shinjuku, Tokyo, Japan). The images were obtained using digital interference contrast (DIC) microscopy. The data were analyzed by Image J software (HyClone, Los Angeles Utah, USA).
TUNEL staining
Firstly, the paraffin sections were dewaxed with xylene, then were hydrated in graded ethanol. Next, 20 µM proteinase K and 3% methanol peroxide were used to clean the sections. Sections were then washed in PBS, then incubated with a mixture of fluorescein reagent for 60 minutes at 37 °C. The nuclei of the sections were stained with DAPI. Apoptosis was observed and imaged under an Olympus fluorescence microscope.
Western blot
Isolated frozen kidneys were homogenized in RIPA buffer with protease inhibitors using liquid nitrogen grinding. After sonication, tissues were incubated on ice for 20 minutes and occasionally resuspended. The samples were centrifuged thoroughly and supernatants were used for experiments. Protein concentrations were measured using the BCA Protein Assay Kit (Pierce), and 20 mg of total protein for each sample were loaded on gradient Bis/Tris gels (Invitrogen, Karlsruhe, Germany). They were run in NuPage running buffer (MOPS; Invitrogen), then transferred onto PVDF membranes (Millipore). After blocking in 10% tris-buffered saline-tween (TBST) skim milk solution, membranes were probed with primary antibodies followed by 3 TBST washes, then incubated with secondary antibodies and washed. Protein bands were visualized using a chemiluminescent substrate kit (Pierce). Quantification was conducted on Image J. The primary antibodies used were β-actin (1:3,000; Sigma-Aldrich), p65 and phosphorylated p65 (1:1,000; Cell Signaling), Bax (1:1,000; Abcam), Bcl-2 (1:1,000; Abcam), caspase-9 (1:2,000; Abcam), and caspase-3 (1:500; Abcam). The secondary antibodies were horseradish peroxidase (HRP) conjugated goat anti-rabbit and anti-mouse (1:5,000; Abcam), and donkey anti-goat (1:5,000; Abcam).
Statistics
Results are represented as the mean ± standard deviation (SD). All experiments were carried out in triplicates and repeated at least once. Student’s t-tests were performed to compare mean values within 1 experimental series. A significant difference was determined by P values of less than 0.05.
Results
Taurine treatment improves renal function
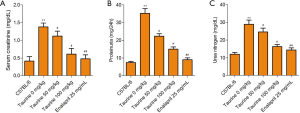
To investigate whether taurine could rescue LN, we measured 3 renal function indicators: CRE, BUN, and proteinuria. In the LN model group, CRE, BUN, and proteinuria were much higher than the control group. Surprisingly, upon taurine administration, all 3 of these renal function parameters were significantly reduced (Figure 1, P<0.05). The effect of 100 mg/kg taurine on the 3 renal function parameters are close to that of the positive drug group. It is suggested that taurine treatment can indeed improve impaired renal function in an MRL/lpr mouse model (Figure 1A,B,C).
Taurine treatment mitigates renal inflammation in LN mice
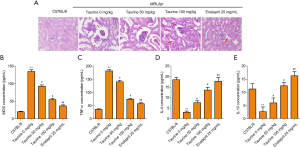
To explore why taurine can reverse kidney injury in MRL/lpr mice, we first examined if there was abnormal histological alterations in the kidneys of MRL/lpr mice. HE staining showed that compared with the control group, renal tissues of the LN mice showed mesangial matrix dilation and glomerular hypertrophy. However, taurine and positive drug treatment reduced this histopathological alteration (Figure 2A). Furthermore, to check whether the immunological aberrations in the MRL/lpr mouse model were also mitigated by taurine, serum biomarkers iNOS, TNF-α, IL-4, and IL-10 were measured using ELISA kits. TNF-α and iNOS concentrations in MRL/lpr mice reduced after taurine treatment in a dose-dependent manner, almost reaching positive drug treatment (Figure 2B,C). Contrastingly, IL-4 and IL-10 levels increased dose-dependently after taurine administration (Figure 2D,E). Unexpectedly, IL-10 concentration in the 5 days 50 mg/kg treatment group equalled control group levels, and the concentration in the 100 mg/kg treatment group exceeded control levels (Figure 2E). These data suggest that taurine can relieve inflammation in LN mice.
Taurine treatment improves oxidative stress in LN mice
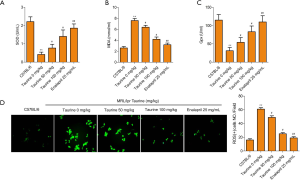
Oxidative stress-induced cytotoxicity was also tested by assaying SOD, MDA, and GPx concentrations (Figure 3A,B,C) using commercial kits. SOD and GPx were abundantly expressed, whereas the content of toxic MDA was low in the control group. In MRL/lpr mice, SOD and GPx were significantly reduced, along with a mass accumulation of MDA. This reduction in SOD and GPx were rescued by taurine administration in LN mice, and MDA storage was also released (Figure 3A,B,C). The generation of ROS was also detected to evaluate oxidative stress levels in each treatment group. In the LN group, ROS generation increased significantly compared to the control group (Figure 3D). Taurine addition reduced the oxidative stress seen in LN mice. The effect of taurine is close to the positive drug group. These data suggest that taurine can alleviate oxidative stress in LN mice.
Taurine treatment suppresses kidney tissue apoptosis in LN mice
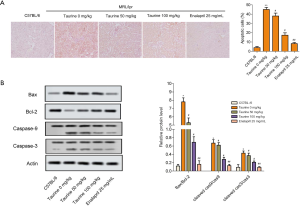
The results of TUNEL staining revealed that, compared with the control group, apoptotic percentages significantly increased in the LN group. Taurine treatment reversed this abnormal increase in apoptosis (Figure 4A). The results of western blotting showed that in the LN group, the expression of Bax increased significantly, accompanied by a downregulation in Bcl-2 (Figure 4B), though taurine administration mitigated this. Taurine treatment also significantly decreased the Bax/Bcl-2 ratio (Figure 4B). Furthermore, in the LN group, cleaved caspase-3 and cleaved caspase-9 expression were significantly upregulated (Figure 4B). These results indicate that taurine treatment suppresses kidney tissue apoptosis in LN mice.
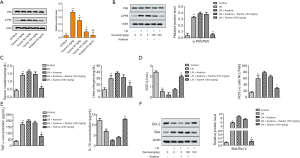
Taurine-mediated therapeutic effects in LN mice via inactivation of the NF-κB p65 signaling pathway
To search for the signaling pathways that might be affected in kidney tissues of MRL/lpr mice administered with taurine, we analyzed NF-κB activity and p65 phosphorylate. As shown in Figure 5A, taurine treatment can reduce the phosphorylation of p65 in kidney tissues. To further determine whether taurine modulated LN via the NF-κB p65 pathway, we added asatone, an activator of NF-κB p65. As shown in Figure 5B, compared with the LN group, asatone addition enhanced the phosphorylation of p65. However, taurine treatment mitigated this increased phosphorylation of p65. Correspondingly, asatone addition elevated BUN and CRE production (Figure 5C), decreased the content of SOD, and increased the generation of ROS (Figure 5D). Additionally, asatone treatment increased the levels of TNF-α and decreased levels of IL-10 (Figure 5E). As seen in Figure 5F, asatone addition also upregulated the ratio of Bcl-2/Bax. In general, asatone significantly counteracted the effects of taurine on LN mice. These data suggest that the modulatory role of taurine in LN is associated with NF-κB p65 reactivation.
Discussion
LN treatments are mainly determined by the severity of the disease. Classified from stage I to V, doses and types of immunosuppressants and hormones vary for the treatment of each stage of LN. The higher the stage of LN, the greater the difficulty patients have in responding to immunosuppressants and hormones (34,35). Several reasons contribute to the difficulties in LN treatment. Firstly, the molecular and cellular mechanisms underlying how SLE patients abnormally generate autoimmune antibodies to disrupt kidney function are not completely understood (34,36). Secondly, most of the current therapies for LN can only mitigate the immune response and stop the damage process, but are not effective for the existing impairments of the kidney, consistent with all other types of refractory nephropathies, especially impacting end-stage LN patients (37). Thirdly, not all patients have a sufficient response to treatments, and for those who react poorly to drugs, the disease will gradually progress to end-stage (35). Meanwhile, for patients who respond well to the drugs, there are still risks of relapse. Finally, though immunosuppressants and hormones have therapeutic efficacy, they also have side effects.
Up to 10–20% of LN patients progress to end-stage disease (34), making it vital to discover novel drugs against this autoimmune disease. To overcome the treatment barriers discussed above, we chose taurine as a potential therapeutic agent for LN, and our results are promising. In this study, we used the MRL/lpr mouse model, which is a widely used model with a spontaneous mutation on its genome to naturally generate systemic autoimmunity to mimic SLE in humans, and its corresponding C57BL/6 control mouse strain. We found that taurine not only reduced CRE, proteinuria, and BUN concentration levels, but also reversed the cell death-stimulating conditions of the pathological kidney. During LN development, glomeruli, interstitium, and atrophic changes of tubules are generally observed (38). These pathological symptoms usually induce kidney weight gain, elevated BUN, and serum CRE levels (39). Proteinuria can predict preliminary LN renal damage. Generally, the urinary albumin excretion rate in 24 hours is used to evaluate renal damage in LN patients (40). Therefore, proteinuria can be improved by reducing glomerular hyperpermeability and kidney damage. In this study, taurine treatment increased apoptotic activity in MRL/lpr mice.
TNF-α, iNOS, IL-10, and IL-4 play important roles in inflammatory processes. Previous studies have shown that Huangqi injection exerted therapeutic effects on LN by regulating iNOS activity (41). Our research revealed that taurine treatment improved the abnormal increase in iNOS and TNF-α levels, whilst also elevating IL-10 and IL-4 levels. Inflammation and oxidative stress are intrinsically linked processes. Redox signaling and redox homeostasis are essential for the maintenance of a normal physiological steady-state (42). In our study, MDA and ROSproduction increased significantly, while SOD levels decreased in the LN group, indicative of oxidative stress. We found that taurine treatment significantly mitigated this oxidative stress in LN mice. Studies have shown that NF-κB mediates the occurrence and development of nephropathy (43). To suppress inflammatory responses in LN, taurine may interact with a variety of signaling pathways to produce its modulatory functions. Here we report that taurine treatment can deactivate the NF-κB signaling pathway by suppressing p65 phosphorylation. It has been previously reported that attenuation of NF-κB p65 alleviated renal failure in a Rho kinase inhibition mouse model (44). The deactivation of the NF-κB pathway through suppression of p65 phosphorylation observed in our study is consistent with this report. We found that p-p65/p65 significantly increased in the LN group after taurine administration, while asatone significantly weakened this therapeutic effect, suggesting that taurine inhibits the NF-κB p65 pathway. Therefore, our findings suggest that taurine can inhibit inflammation, oxidative stress, and tissue apoptosis via the NF-κB p65 pathway, thereby alleviating LN in a mouse model.
To summarize, our study provides a novel strategy to treat LN. Treatment with taurine may have less side effects compared to other traditional therapies using immunosuppressants and hormones. It may even reverse LN progression by preventing apoptosis of renal cells, mitigating oxidative stress in kidneys, and importantly, ameliorate kidney injury.
Although this study has highlighted taurine as an effective strategy to treat LN, clinical trials are still needed to verify effects in humans, and further studies exploring the cellular and molecular mechanisms of taurine in LN are needed to comprehensively understand its therapeutic effect.
Acknowledgments
We sincerely appreciate the technical support from The First Affiliated Hospital of Hebei North University, The Third Hospital of Xingtai, Qingyun County People’s Hospital.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2087
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2087
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2087). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal procedures and experiments in this study were approved by the Animal Ethics Committee of the First Affiliated Hospital of Hebei North University, and were conducted strictly in accordance with the guidelines of International Guiding Principles for Animal Research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kronbichler A, Brezina B, Gauckler P, et al. Refractory lupus nephritis: When, why and how to treat. Autoimmun Rev 2019;18:510-8. [Crossref] [PubMed]
- Schreiber J, Eisenberger U, de Groot K. Lupus nephritis. Internist (Berl) 2019;60:468-77. [Crossref] [PubMed]
- Chesney RW, Han X, Patters AB. Taurine and the renal system. J Biomed Sci 2010;17 Suppl 1:S4. [Crossref] [PubMed]
- Zhang L, Zhang M, Chen X, et al. Identification of the tubulointerstitial infiltrating immune cell landscape and immune marker related molecular patterns in lupus nephritis using bioinformatics analysis. Ann Transl Med 2020;8:1596. [Crossref] [PubMed]
- Parikh SV, Rovin BH. Current and Emerging Therapies for Lupus Nephritis. J Am Soc Nephrol 2016;27:2929-39. [Crossref] [PubMed]
- Ripps H, Shen W. Review: taurine: a "very essential" amino acid. Mol Vis 2012;18:2673-86. [PubMed]
- McCarty MF. Nutraceutical strategies for ameliorating the toxic effects of alcohol. Med Hypotheses 2013;80:456-62. [Crossref] [PubMed]
- Park E, Park SY, Dobkin C, et al. Development of a novel cysteine sulfinic Acid decarboxylase knockout mouse: dietary taurine reduces neonatal mortality. J Amino Acids 2014;2014:346809. [Crossref] [PubMed]
- Rath M. Energy drinks: what is all the hype? The dangers of energy drink consumption. J Am Acad Nurse Pract 2012;24:70-6. [Crossref] [PubMed]
- Schaffer S, Kim HW. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol Ther (Seoul) 2018;26:225-41. [Crossref] [PubMed]
- Ginguay A, De Bandt JP, Cynober L. Indications and contraindications for infusing specific amino acids (leucine, glutamine, arginine, citrulline, and taurine) in critical illness. Curr Opin Clin Nutr Metab Care 2016;19:161-9. [Crossref] [PubMed]
- Hamilton EJ, Berg HM, Easton CJ, et al. The effect of taurine depletion on the contractile properties and fatigue in fast-twitch skeletal muscle of the mouse. Amino Acids 2006;31:273-8. [Crossref] [PubMed]
- Cuisinier C, Gailly P, Francaux M, et al. Effects of guandinoethane sulfonate on contraction of skeletal muscle. Adv Exp Med Biol 2000;483:403-9. [Crossref] [PubMed]
- Azuma J, Sawamura A, Awata N. Usefulness of taurine in chronic congestive heart failure and its prospective application. Jpn Circ J 1992;56:95-9. [Crossref] [PubMed]
- Ito T, Yoshikawa N, Schaffer SW, et al. Tissue taurine depletion alters metabolic response to exercise and reduces running capacity in mice. J Amino Acids 2014;2014:964680. [Crossref] [PubMed]
- Hara MR, Snyder SH. Cell signaling and neuronal death. Annu Rev Pharmacol Toxicol 2007;47:117-41. [Crossref] [PubMed]
- Abramov AY, Duchen MR. Mechanisms underlying the loss of mitochondrial membrane potential in glutamate excitotoxicity. Biochim Biophys Acta 2008;1777:953-64. [Crossref] [PubMed]
- Schaffer SW, Jong CJ, Warner D, et al. Taurine deficiency and MELAS are closely related syndromes. Adv Exp Med Biol 2013;776:153-65. [Crossref] [PubMed]
- Imae M, Asano T, Murakami S. Potential role of taurine in the prevention of diabetes and metabolic syndrome. Amino Acids 2014;46:81-8. [Crossref] [PubMed]
- Ito T, Schaffer SW, Azuma J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids 2012;42:1529-39. [Crossref] [PubMed]
- Schaffer SW, Azuma J, Mozaffari M. Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 2009;87:91-9. [Crossref] [PubMed]
- Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino Acids 2014;46:7-20. [Crossref] [PubMed]
- Han X, Chesney RW. The role of taurine in renal disorders. Amino Acids 2012;43:2249-63. [Crossref] [PubMed]
- Soeters PB, van de Poll MC, van Gemert WG, et al. Amino acid adequacy in pathophysiological states. J Nutr 2004;134:1575S-1582S. [Crossref] [PubMed]
- Michalk DV, Hoffmann B, Minor T. Taurine reduces renal ischemia/reperfusion injury in the rat. Adv Exp Med Biol 2003;526:49-56. [Crossref] [PubMed]
- Mozaffari MS, Miyata N, Schaffer SW. Effects of taurine and enalapril on kidney function of the hypertensive glucose-intolerant rat. Am J Hypertens 2003;16:673-80. [Crossref] [PubMed]
- Pasantes-Morales H, Cruz C. Taurine and hypotaurine inhibit light-induced lipid peroxidation and protect rod outer segment structure. Brain Res 1985;330:154-7. [Crossref] [PubMed]
- Young TL, Matsuda T, Cepko CL. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Curr Biol 2005;15:501-12. [Crossref] [PubMed]
- Xu Y, Deng W, Zhang W. Long non-coding RNA TUG1 protects renal tubular epithelial cells against injury induced by lipopolysaccharide via regulating microRNA-223. Biomed Pharmacother 2018;104:509-19. [Crossref] [PubMed]
- Ommati MM, Heidari R, Ghanbarinejad V, et al. Taurine Treatment Provides Neuroprotection in a Mouse Model of Manganism. Biol Trace Elem Res 2019;190:384-95. [Crossref] [PubMed]
- Mansuri A, Elmaghrabi A, Alhamoud I, et al. Transient enalapril attenuates the reduction in glomerular filtration rate in prenatally programmed rats. Physiol Rep 2017;5:e13266. [Crossref] [PubMed]
- Marshall T, Williams KM. Total protein determination in urine: elimination of a differential response between the coomassie blue and pyrogallol red protein dye-binding assays. Clin Chem 2000;46:392-8. [Crossref] [PubMed]
- Lee NK, Choi YG, Baik JY, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005;106:852-9. [Crossref] [PubMed]
- Schwartz N, Goilav B, Putterman C. The pathogenesis, diagnosis and treatment of lupus nephritis. Curr Opin Rheumatol 2014;26:502-9. [Crossref] [PubMed]
- Jadot V, Krzesinski JM, Von Frenckell C, et al. Lupus nephropathy: Insight in new treatments. Nephrol Ther 2018;14:1-12. [Crossref] [PubMed]
- Lech M, Anders HJ. The pathogenesis of lupus nephritis. J Am Soc Nephrol JASN 2013;24:1357-66. [Crossref] [PubMed]
- Jaryal A, Vikrant S. Current status of lupus nephritis. Indian J Med Res 2017;145:167-78. [PubMed]
- Zhang KF, Zhang L, Wu XF, et al. Pathogenesis of rat mesangial proliferative glomerulonephritis induced by anti-Thy1 antibody. Sichuan Da Xue Xue Bao Yi Xue Ban 2004;35:188-90. [PubMed]
- Hernández-Salinas R, Vielma AZ, Arismendi MN, et al. Boldine prevents renal alterations in diabetic rats. J Diabetes Res 2013;2013:593672. [Crossref] [PubMed]
- Al-Rasheed NM, Al-Rasheed NM, Hasan IH, et al. Simvastatin Ameliorates Diabetic Cardiomyopathy by Attenuating Oxidative Stress and Inflammation in Rats. Oxid Med Cell Longev 2017;2017:1092015. [Crossref] [PubMed]
- Zhang S, Xu H, Yu X, et al. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp Ther Med 2017;14:383-90. [Crossref] [PubMed]
- Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 2002;106:672-8. [Crossref] [PubMed]
- Liu XM, Wang Z, Li CS. Viral transactivation of transcription by NF-kappaB in steroid-responsive simple nephrotic syndrome. Sichuan Da Xue Xue Bao Yi Xue Ban 2006;37:183-6. [PubMed]
- Meyer-Schwesinger C, Dehde S, von Ruffer C, et al. Rho kinase inhibition attenuates LPS-induced renal failure in mice in part by attenuation of NF-kappaB p65 signaling. Am J Physiol Renal Physiol 2009;296:F1088-99. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)


