
Circulating endothelial progenitor cells from septic patients are associated with different infectious organisms
Introduction
Sepsis is a serious complication in patients with severe infection, burns, wounds, or shock (1-3). Sepsis-induced endothelial barrier damage and microcirculatory disturbance, including cell separation, cell ablation, changes in vasomotor function, and apoptosis, are important pathological mechanisms that contribute to the development of multiple organ failure (4,5). Therefore, repair of endothelial damage plays a crucial role in the treatment of sepsis (6-8).
Endothelial progenitor cells (EPCs) have a high capacity for proliferation and differentiation in the pathogenesis of severe sepsis, and can repair damaged endothelial cells (9-11). Through mobilization, proliferation, adhesion, migration, and homing to damaged blood vessels, EPCs can repair the damaged vascular intima, alleviate ischemia and hypoxia in the local tissues and organs by promoting neovascularization, and decrease the inflammatory response (12-14). Increasing the percentage and function of EPCs could provide a new treatment for patients with sepsis (15,16).
In recent decades, studies on the effects of sepsis on the percentage of EPCs in the peripheral blood have reported different results. In 2007, Mayr et al. found that the percentage of EPCs in healthy volunteers decreased significantly after the infusion of 2 mg/kg lipopolysaccharide (LPS) (17). Recent studies have revealed the percentage of EPCs in patients with severe sepsis to be significantly elevated and negatively correlated with mortality (18-20). Patschan et al. reported that the numbers of colony-forming unit endothelial cells were lower in patients with sepsis than in healthy controls (21). However, the relationship between quantities and functions of EPCs in septic patients with different infectious organisms has remained unclear. In this study, septic patients were enrolled and divided into three groups: the negative blood culture group, the gram-negative bacteria group, and the gram-positive bacteria group. Differences in the percentages of EPCs in peripheral blood samples were detected and analyzed in different groups of patients.
We present the following article in accordance with the MDAR checklist (available at http://dx.doi.org/10.21037/apm-20-2458).
Methods
Study patients
From February 2017 to July 2017, 39 patients with sepsis who were admitted to the Critical Care Medicine (CCM) at Tai’an City Central Hospital were enrolled. The patient screening process is outlined in Table 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was granted approval by the hospital’s medical ethics committee (NO.: 2016-05-040), and each participating patient provided written informed consent. Sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Patients with septic shock were identified with a need for a vasopressor to maintain a mean arterial pressure of >65 mmHg and a serum lactate level >2 mmol/L (>18 mg/dL) in the absence of hypovolemia (22). Only patients aged 18–79 years old were included. The exclusion criteria were: (I) pregnant patients; (II) patients with a history of an acute ischemic event within the past 2 months; (III) patients with a history of metastatic cancer; and (IV) patients with autoimmune diseases or other conditions that inhibit the host immunity. For clinical characterization, the Acute Physiology and Chronic Health Evaluation (APACHE) II and Sepsis-Related Organ Failure Assessment (SOFA) scores were calculated on admission to the CCM. Age- and sex-matched healthy subjects (n=20) were included as a control group, which had not cardiovascular risk factors or diseases and were not taking any medications.
Full table
Blood culture
Blood culture were collected before the start of antimicrobial therapy or at the onset of fever, and were analyzed according to local standards. The results of blood culture were carefully assessed by the treating physicians; if no bacterial growth was detected within the incubation period, the blood culture was considered negative. Pathogens were grouped into gram-positive bacteria and gram-negative bacteria. Depending on the blood culture results, patients were classified into the three following groups: the negative blood culture group, the gram-negative bacteremia group, and the gram-positive bacteremia group. Blood culture of patients in the gram-negative bacteremia group detected at least one of these organisms: Escherichia coli, Klebsiella, Enterobacter cloacae, or Acinetobacter baumannii. The results of blood culture in the gram-positive bacteremia group revealed infection with the following organisms: Staphylococcus aureus or Staphylococcus hominis. The treating physician retrospectively determined the focus of the infection based on all available clinical and microbiological data. For the analysis, infections were divided into six categories as follows: pneumonia, urethritis, peritonitis, pancreatitis, skin/soft tissue infection, and others.
Blood sampling and flow cytometry
Peripheral blood samples were collected in heparin anticoagulant tubes via venipuncture. Peripheral blood mononuclear cells (PBMCs) were obtained by performing density gradient centrifugation with Ficoll (1.077 g/mL; Sigma). The PBMCs were incubated for 30 minutes in the dark at room temperature with appropriate concentrations of the following monoclonal antibodies: Alexa Fluor 647-conjugated KDR (kinase insert domain receptor, BD Biosciences Cat# 560495, RRID: AB_1645404), Fluorescein Isothiocyanate (FITC)-conjugated CD34 (Thermo Fisher Scientific Cat# 11-0349-42, RRID:AB_1518732), and Phycoerythrin (PE)-conjugated CD133 (Thermo Fisher Scientific Cat# 12-1339-42, RRID:AB_2016660). The corresponding isotype controls were used. Samples were assessed using a flow cytometer (BD FACSCalibur Flow Cytometry System, RRID:SCR_00040). At least 1,000,000 events were recorded in the mononuclear cell gate set on the SSC/FSC morphological plot, and the results were analyzed with FlowJo software (FlowJo, v10, RRID:SCR_008520). EPC counts were expressed as a percentage of the total number of PBMCs in each septic patient or control.
Characterization of EPCs
PBMCs obtained through density gradient centrifugation were seeded on fibronectin-coated 24-well plates (Corning, USA). After 4 days, non-adherent cells were removed, and fresh medium was added. After culture for 3 more days, the cells were incubated with 2.5 mg/L DiI-ac-LDL (Acetylated Low Density Lipoprotein, labeled with 1,1’-dioctadecyl-3,3,3’,3’-tetramethyl-indocarbocyanine perchlorate, Thermo Fisher Scientific Inc.) for 2 hours at 37 °C and then with 10 mg/L FITC-UEA (FITC labeled Ulex Europaeus Agglutinin I, Sigma-Aldrich) for 1 hour at 37 °C. Following that, the cells were fixed with 1–2% paraformaldehyde (Sigma-Aldrich) for 5 minutes. Double-positive cells and total cell numbers were counted in five random fields by using a microscope.
Statistical analysis
All data were presented as mean ± standard deviation or standard error of the mean. Intergroup comparisons were made by performing the paired Student’s t-test or Mann–Whitney U test. Statistical significance was indicated by P<0.05.
Results
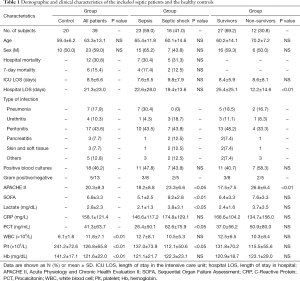
Demographics of septic patients and the healthy controls
This study enrolled 39 septic patients and 20 healthy controls who met the respective inclusion and exclusion criteria. Clinical data of the study participants are summarized in Table 1. Except for differences in counts of white blood cell, platelet, and hemoglobin (P<0.01, Table 1), there were no significant differences in the demographic data of the healthy controls and the septic patients. Compared with patients with sepsis, patients with septic shock had significantly increased APACHE II scores (23.3±6.6 vs. 18.2±8.8, P<0.05), SOFA scores (9.2±2.8 vs. 5.1±2.5, P<0.01), increased levels of lactate (3.8±3.1 vs. 2.1±1.3, P<0.05) and procalcitonin (62.6±75.9 vs. 26.4±50.1, P<0.05), as well as significantly decreased level of platelet (112.1±50.6 vs. 137.0±73.9, P<0.05). Compared with the surviving patients, patients who died had significantly increased APACHE II scores (26.6±6.4 vs. 17.5±7.5, P<0.01) and significantly decreased length of stay in hospital (12.2±14.6 vs. 25.4±25.1, P<0.01) (Table 1).
Analysis of the patients’ baseline characteristics also revealed that 16 patients (41.0%) developed septic shock and 12 patients (30.8%) died in the hospital. However, no significant differences were found in mortality, or in the ICU or hospital length of stay between patients with sepsis and septic shock. In all patients, the most common infection was peritonitis (43.6%,17/39), followed by pneumonia (17.9%,7/39), urethritis, peritonitis, skin/soft tissue infection, and other types of infection. Bacteremia was observed in 18 patients (46.2%), 5 and 13 of whom had gram-positive bacteria and gram-negative bacteria, respectively.
Characterization of EPCs
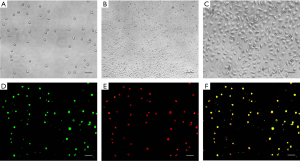
The cultured cells were round or oval in the first few days (Figure 1A), after culturing for about 10 days, cell proliferation could be observed, and the prolific EPCs were showed as a cobblestone-like morphology (Figure 1B,C). Because EPCs are able to take up LDL (Low Density Lipoprotein) and bind lectin, LDL uptake and lectin binding were used as differentiation markers of EPCs (23). In this study, the percentage of double-positive cells in septic patients showed no significant difference compared with those in healthy controls, and both were >95% (Figure 1D,E,F).
Septic patients had a higher percentage of EPCs in their peripheral blood than healthy controls
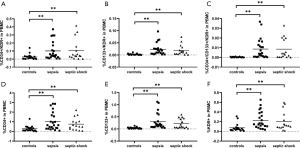
EPCs were absent of uniform specific phenotypic markers in previous studies. Asahara et al. were the first to use CD34 and KDR as markers to identify EPCs (24). CD34 is a marker of hematopoietic stem cells, and KDR is a VEGF (vascular endothelial growth factor) receptor that play an important role in the homing of EPCs. In order to differentiate mature endothelial cells (ECs) from EPCs, CD133, an undifferentiated marker, was used to identify undifferentiated EPCs (25). In this study, six subpopulations of progenitor cells: single-positive (CD34+, CD133+ and KDR+) cells, double-positive (CD34+/KDR+ and CD133+/KDR+) cells, and triple-positive (CD34+/CD133+/KDR+) cells were detected in all groups.
The percentages of CD34+/KDR+, CD133+/KDR+, CD34+/CD133+/KDR+, CD34+, CD133+, and KDR+ progenitor cells in septic patients were significantly higher than those in healthy controls [0.1027±0.0190 vs. 0.0270±0.0073, P<0.01; 0.0215±0.0036 vs. 0.0025±0.0006, P<0.01; 0.0086±0.0016 vs. 0.0003±0.0001, P<0.01; 0.9208±0.1322 vs. 0.2777±0.0646, P<0.01; 0.2636±0.0444 vs. 0.0370±0.0072, P<0.01; 0.2176±0.0292 vs. 0.0727±0.0178, P<0.01 (mean ± SEM, % of total PBMCs)] (Figure 2).

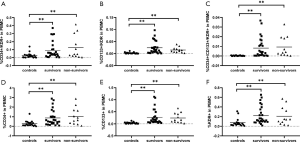
Patients were further divided into a sepsis group and a septic shock group. Analysis of the EPC markers revealed no statistically differences between the two subgroups, although the percentages of all progenitor cell markers in the septic shock group were lower than those in the septic group (Figure 3). In this paper, the data showed that the percentages of EPCs in the septic patients who survived were similar to those in the non-surviving septic patients (Figure 4).
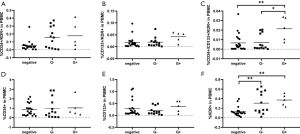
The percentages of EPCs subpopulations differed between the positive and negative blood culture groups, and the healthy controls
According to the results of blood culture, the septic patients were divided into the positive blood culture group and the negative blood culture group. Data showed that the percentage of KDR+ cells among PBMCs in the positive blood culture group was significantly higher than that in the negative blood culture group [0.3260±0.0476 vs. 0.1246±0.0206, P<0.01 (mean ± SEM, % of total PBMCs)] (Figure 5). Although the percentages of CD34+/KDR+, CD133+/KDR+, CD34+/CD133+/KDR+, CD34+ progenitor cells were higher in the positive blood culture group than in the negative blood culture group, the differences were not significant.
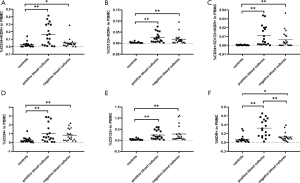
The percentages of EPC subpopulations in the gram-positive bacteremia group, gram-negative bacteremia group, and negative blood culture group differed
According to the results of blood culture, the septic patients in clinical study were usually divided into negative blood culture, gram-positive bacteremia, gram-negative bacteremia, etc. According to the results of blood culture, the septic patients in this study were divided into three groups: the gram-positive bacteremia group, the gram-negative bacteremia group and the negative blood culture group. Of the 39 septic patients, 21 patients had negative microbial results and 18 patients had positive blood culture, including 13 with gram-negative bacteremia and 5 with gram-positive bacteremia. Analysis of EPCs in the three different groups of patients, showed that the percentages of CD34+/KDR+, CD133+/KDR+v cells showed no significant differences (Figure 6A,B); the percentage of CD34+/CD133+/KDR+ cells in the gram-positive bacteremia group was significantly higher than that in the gram-negative bacteremia group and the negative blood culture group (G+ vs. G−, 0.0214±0.0055 vs. 0.0070±0.0023, P<0.05; G+ vs. negative, 0.0214±0.0055 vs. 0.0065±0.0020, P<0.01 [mean ± SEM, % of total PBMCs] (Figure 6C); the percentages of CD34+ and CD133+ cells showed no significant differences among groups (Figure 6D,6E); and the percentages of KDR+ cells in both gram-negative bacteremia group and gram-positive bacteremia group were significantly higher than the negative blood culture group (G− vs. negative, 0.3083±0.0632 vs. 0.1246±0.0206, P<0.01; G+ vs. negative, 0.3722±0.0526 vs. 0.1246±0.0206, P<0.01, [mean ± SEM, %of total PBMCs] (Figure 6F).
Discussion
Mutunga et al. observed an increase in circulating endothelial cells (ECs) during sepsis, which indicated endothelial barrier damage (26), and then microvascular dysfunction can be occurred. Due to their limited proliferation ability, terminally differentiated ECs cannot sufficiently repair severe vascular damage, which results in dysfunction of different organs. EPCs have been shown to possess the potential to repair vascular endothelial damage in sepsis, acute respiratory distress syndrome, hindlimb ischemia, and cardiovascular and cerebrovascular diseases. Fan et al. verified that the exogenous administration of EPCs is beneficial in cercal ligation and puncture-induced sepsis, and it can improve lung vascular integrity and reduce mortality in septic patients (15). We have found that LPS-induced lung injury repair may be correlated with elevated EPC percentage and functions induced by high-density lipoprotein mimetic peptide reverse-D-4F (high-density lipoprotein mimetic peptide reverse-D-4F) (27). EPCs may be a therapeutic target for sepsis-induced lung dysfunction in the future.
At present, EPCs markers are controversial, and different markers of EPCs have been used by different research groups. CD34, CD133, and KDR are commonly used surface markers of EPCs. CD133 is a marker of progenitor cells, and it can distinguish mature ECs from progenitor cells. CD34 is present in hematopoietic stem cells (HSCs), EPCs, and mature ECs, KDR is another endothelial marker that can define EPCs. In this study, CD34+/CD133+/KDR+ cells were used to identify EPC phenotypes, and the percentages of CD34+/KDR+, CD133+/KDR+, CD34+/CD133+/KDR+, CD34+, CD133+, and KDR+ cells were analyzed through flow cytometry.
In this study, the percentages of CD34+/KDR+, CD133+/KDR+, CD34+/CD133+/KDR+, CD34+, CD133+, and KDR+ progenitor cells in septic patients were significantly higher than those in healthy controls. This finding is consistent with the observations of Rafat et al., whose data showed that the percentage of EPCs (CD34+/CD133+/KDR+) was correlated with survival in septic patients (28). In a study on pediatric sepsis, the percentage of EPCs (CD34+/CD144+/CD133+) in surviving patients were found to be significantly higher than those in non-surviving patients (29). However, Patschan et al.’s study identified no differences in circulating EPCs (CD133+/KDR+) between non-surviving and surviving septic patients (21). The inconsistency in results among above studies may be attributable to differences in blood collection time, EPC phenotypes, and patient selection. In this study, the percentages of CD34+/KDR+, CD133+/KDR+, CD34+/CD133+/KDR+, CD34+, CD133+, and KDR+ cells in surviving patients and non-surviving patients were measured, and the data showed that there were no significant differences between the two groups.
Becchi et al. further classified septic patients into septic patients and severe septic/septic shock patients. Their study found that the percentage of CD133+/KDR+ cells in the CD34+ cell population in patients with severe sepsis or septic shock was significantly higher than that in septic patients (30). In the current study, to further analyze the contribution of EPCs to the development of sepsis, septic patients were divided into the septic group and septic shock group. The results showed that the percentages of CD34+/KDR+, CD133+/KDR+, CD34+/CD133+/KDR+, CD34+, CD133+, and KDR+ cells in the septic group were similar to those in the septic shock group. The discrepancy between our findings and those reported by Becchi et al. may be due to the use of different counting methods for EPCs and different inclusion criteria for the subgroups. The CD34+/KDR+, CD133+/KDR+, CD34+/CD133+/KDR+, CD34+, CD133+, and KDR+ cell counts in this study were expressed as percentage of PBMCs, while in another study performed by Becchi et al., EPC counts were expressed as percentage of CD133+/KDR+ cells in the CD34+ population. The sample sizes of our study and Becchi et al.’s study was relatively small; thus, a future study with a larger population of septic patients is needed to analyze the contribution of EPCs to septic patients.
Previous studies of the percentages of EPCs in septic patients have not conducted further analysis of the organisms of infection. Our results showed that the percentages of KDR+ cells among PBMCs in both the gram-positive bacteremia group and the gram-negative bacteremia group were significantly higher than those in the negative blood culture group. Moreover, the percentage of CD34+/CD133+/KDR+ cells among PBMCs in the gram-positive bacteremia group was significantly higher than that in the gram-negative bacteremia and negative blood culture groups. Although the number of patients was relatively small, the percentages of EPCs in the peripheral blood showed difference according to various infectious organisms. In the Menchinelli et al.’s study (31), the median time to detection (TTD) for gram-negative species was 9.3–12.0 hours, and the median TTD for gram-positive species was 13.5–15.5 hours; consequently, the diagnosis of bacteremia was often delayed in the clinical treatment of sepsis patients. In our study, the time taken to acquire the percentages of EPCs was only 2–3 hours, and the percentages of KDR+ and CD34+/CD133+/KDR+ cells were positively correlated with the organisms detected by blood culture, thus antibiotics may be applied more quickly and accurately according to the percentages of EPCs in patients with sepsis in clinical. But more large-population experiments are needed to establish the criteria for diagnostic percentages of EPCs.
In conclusion, we have demonstrated for the first time that the percentages of CD34+/CD133+/KDR+ cells in septic patients with gram-positive bacteremia were significantly higher than those in septic patients with gram-negative bacteremia or negative blood culture. The percentage of KDR+ cells in the positive blood cultures patients was significantly higher than that in the negative blood cultures patients. Based on this, we suggest that EPCs may be a new early indicator for the differential diagnosis of blood culture organisms in septic patients, which can help to provide guidance for clinical treatment decisions. Further animal- and population-based experiments are needed to confirm our findings and to deduce possible therapeutic approaches to improve the outcomes of patients with sepsis.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (No. 81600360), the Province Natural Science Foundation of Shandong (No. ZR2012HL18, ZR2020KH008), the Province Science and Technology Development Foundation of Shandong (2014GSF118105), the Province Higher University Science and Technology Development Project of Shandong (No. J14LK03), and the Science and Technology Development Project of Taian (No. 2018NS0160).
Footnote
Reporting Checklist: The authors have completed the MDAR checklist. Available at http://dx.doi.org/10.21037/apm-20-2458
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2458
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2458). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the medical ethics committee of Tai’an City Central Hospital (NO.: 2016-05-040) and with the Helsinki Declaration (as revised in 2013). Each participating patient provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gill SE, Rohan M, Mehta S. Role of pulmonary microvascular endothelial cell apop-tosis in murine sepsis-induced lung injury in vivo. Respir Res 2015;16:109. [Crossref] [PubMed]
- Ammer-Herrmenau C, Kulkarni U, Andreas N, et al. Sepsis induces long-lasting im-pairments in CD4+ T-cell responses despite rapid numerical recovery of T-lymphocyte populations. PLoS One 2019;14:e0211716. [Crossref] [PubMed]
- Belba MK, Petrela EY, Belba AG. Epidemiology and outcome analysis of sepsis and organ dysfunction/failure after burns. Burns 2017;43:1335-47. [Crossref] [PubMed]
- Goldenberg NM, Steinberg BE, Slutsky AS, et al. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med 2011;3:88ps25. [Crossref] [PubMed]
- Osuchowski MF, Welch K, Siddiqui J, et al. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mor-tality. J Immunol 2006;177:1967-74. [Crossref] [PubMed]
- Mu S, Liu Y, Jiang J, et al. Unfractionated heparin ameliorates pulmonary microvas-cular endothelial barrier dysfunction via microtubule stabilization in acute lung injury. Respir Res 2018;19:220. [Crossref] [PubMed]
- Barabutis N, Khangoora V, Marik PE, et al. Hydrocortisone and Ascorbic Acid Syn-ergistically Prevent and Repair Lipopolysaccharide-Induced Pulmonary Endothelial Barrier Dysfunction. Chest 2017;152:954-62. [Crossref] [PubMed]
- Leligdowicz A, Richard-Greenblatt M, Wright J, et al. Endothelial Activation: The Ang/Tie Axis in Sepsis. Front Immunol 2018;9:838. [Crossref] [PubMed]
- Cribbs SK, Sutcliffe DJ, Taylor WR, et al. Circulating endothelial progenitor cells inversely associate with organ dysfunction in sepsis. Intensive Care Med 2012;38:429-36. [Crossref] [PubMed]
- Schlichting DE, Waxman AB, O'Brien LA, et al. Circulating endothelial and endothelial progenitor cells in patients with severe sepsis. Microvasc Res 2011;81:216-21. [Crossref] [PubMed]
- Zhou Y, Li P, Goodwin AJ, et al. Exosomes from Endothelial Progenitor Cells Improve the Outcome of a Murine Model of Sepsis. Mol Ther 2018;26:1375-84. [Crossref] [PubMed]
- Sun R, Huang J, Sun B. Mobilization of endothelial progenitor cells in sepsis. Inflamm Res 2020;69:1-9. [Crossref] [PubMed]
- Padfield GJ, Tura O, Haeck MLA, et al. Circulating endothelial progenitor cells are not affected by acute systemic inflammation. Am J Physiol Heart Circ Physiol 2010;298:H2054-61. [Crossref] [PubMed]
- Vandoorne K, Rohde D, Kim H, et al. Imaging the Vascular Bone Marrow Niche During Inflammatory Stress. Circ Res 2018;123:415-27. [Crossref] [PubMed]
- Fan H, Goodwin AJ, Chang E, et al. Endothelial progenitor cells and a stromal cell-derived factor-1α analogue synergistically improve survival in sepsis. Am J Respir Crit Care Med 2014;189:1509-19. [Crossref] [PubMed]
- Tianhang L, Bo W, Zhengmao L, et al. Autologous transplantation of endothelial progenitor cells to prevent multiple organ dysfunction syndromes in pig. J Trauma Acute Care Surg 2013;74:508-15. [Crossref] [PubMed]
- Mayr FB, Spiel AO, Leitner JM, et al. Effects of low dose endotoxemia on endothe-lial progenitor cells in humans. Atherosclerosis 2007;195:e202-6. [Crossref] [PubMed]
- Kung CT, Su C, Chen CT, et al. Circulating endothelial progenitor cells may predict outcomes in adult patients with severe sepsis in the emergency department. Clin Chim Acta 2016;455:1-6. [Crossref] [PubMed]
- Jin Y, Yang C, Sui X, et al. Endothelial progenitor cell transplantation attenuates lipopolysaccharide-induced acute lung injury via regulating miR-10a/b-5p. Lipids Health Dis 2019;18:136. [Crossref] [PubMed]
- Yeh C, Pai M, Shih Y, et al. Intravenous Arginine Administration Promotes Proan-giogenic Cells Mobilization and Attenuates Lung Injury in Mice with Polymicrobial Sepsis. Nutrients 2017;9:507. [Crossref]
- Patschan SA, Patschan D, Temme J, et al. Endothelial progenitor cells (EPC) in sepsis with acute renal dysfunction (ARD). Crit Care 2011;15:R94. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- George AL, Bangalore-Prakash P, Rajoria S, et al. Endothelial progenitor cell biology in disease and tissue regeneration. J. Hematol Oncol 2011;4:24. [Crossref] [PubMed]
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964-7. [Crossref] [PubMed]
- Shmelkov SV, St Clair R, Lyden D, et al. AC133/CD133/Prominin-1. Int J Biochem Cell Biol 2005;37:715-9. [Crossref] [PubMed]
- Mutunga M, Fulton B, Bullock R, et al. Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med 2001;163:195-200. [Crossref] [PubMed]
- Yang N, Tian H, Zhan E, et al. Reverse-D-4F improves endothelial progenitor cell function and attenuates LPS-induced acute lung injury. Respir Res 2019;20:131. [Crossref] [PubMed]
- Rafat N, Hanusch C, Brinkkoetter PT, et al. Increased circulating endothelial progen-itor cells in septic patients: correlation with survival. Crit Care Med 2007;35:1677-84. [Crossref] [PubMed]
- Zahran AM, Elsayh KI, Mohamad IL, et al. Circulating Endothelial Cells and Endo-thelial Progenitor Cells in Pediatric Sepsis. Pediatr Emerg Care 2016;32:163-7. [Crossref] [PubMed]
- Becchi C, Pillozzi S, Fabbri LP, et al. The increase of endothelial progenitor cells in the peripheral blood: a new parameter for detecting onset and severity of sepsis. Int J Immunopathol Pharmacol 2008;21:697-705. [Crossref] [PubMed]
- Menchinelli G, Liotti FM, Fiori B, et al. In vitro Evaluation of BACT/ALERT® VIRTUO®, BACT/ALERT 3D®, and BACTEC™ FX Automated Blood Culture Systems for Detection of Microbial Pathogens Using Simulated Human Blood Sam-ples. Front Microbiol 2019;10:221. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


