
Secondary and cumulative meta-analysis of olanzapine for antiemetic prophylaxis for chemotherapy-induced nausea and vomiting: do we still need to study its effectiveness?
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a common and burdensome adverse effect for cancer patients receiving chemotherapy treatment (1). Patients frequently report a lower quality of life and may present with clinical conditions of dehydration, malnutrition and treatment non-response (2). These debilitating side effects may ultimately lead to decreased chemotherapy dose administration and poor patient adherence, both compromising management for patients on chemotherapy.
Antiemetics have been studied and developed to target specific pathways that are postulated to be involved in CINV. Neurokinin-1 receptor antagonists such as aprepitant, rolapitant and netupitant, are prescribed to block substance P, which initiates impulses to the emesis center in the medulla (3-5). 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists such as ondansetron, granisetron and palonosetron, are administered to interrupt the pathway where serotonin from enterochromaffin cells bind to 5-HT3 receptors (6,7).
Over the past decade, olanzapine has been used in the antiemetic setting. Originally approved by the Food and Drug Administration to treat psychosis, it was speculated that olanzapine may be an effective antiemetic due to its ability to inhibit multiple serotonergic, dopaminergic, alpha-1 adrenergic and histamine receptors (8). A review of phase I and II trials by Chow et al. reported good efficacy of olanzapine (9). A meta-analysis of phase III trials by Chiu et al. in 2016 reported that olanzapine is more efficacious than other standard antiemetics in the prophylactic setting (10). Since then, international clinical guidelines by the American Society of Clinical Oncology (ASCO) (11) and National Comprehensive Cancer Network (NCCN) (12) have subsequently recommended olanzapine to be added as the fourth drug in a standard prophylactic CINV regimen, citing these aforementioned reviews.
However, since the 2016 review, many clinical trials using olanzapine for CINV have been started, completed, and published—in many cases, citing the paucity of literature at the time as support for initiating their trial and the need for further research in this setting (13-15). It remains to be seen whether there was indeed a paucity of data on the use of olanzapine for the CINV setting in 2016. Indeed, if there were already sufficient evidence for the use of olanzapine in 2016, one would expect the most recent clinical trials to have little effect on the summary estimate in a meta-analysis; that is to say, the existing body of literature would have encompassed such a large sample of patients such that the results of additional trials would only shift the effect size minimally and unremarkably (16).
The primary aim of this study is to conduct a secondary cumulative meta-analysis to provide insight into the effect of the most recent trials on the published effect estimate of olanzapine as an anti-nausea and anti-emesis agent, and to determine whether the trials published since 2016 have significantly changed the summary estimate.
Methods
Included studies and data
As specified a priori (Appendix 1), all papers and data reporting on olanzapine for the prophylaxis of CINV included by Chiu et al. (10) were included in this secondary meta-analysis. As reported previously, a literature search was conducted up until 2015, of Ovid Medline, Embase and the Cochrane Central Register of Controlled Trials; 10 randomized controlled trials (RCTs) with a total of over 1,000 patients (17-26) were included, that compared olanzapine to other antiemetics in the prophylactic setting, which reported on at least one of two endpoints—no emesis and no nausea. Two endpoints of emesis and nausea were included in this analysis, analyzed separately by time of incidence of CINV—acute (0 up to 24 hours post-chemotherapy), delayed (beyond 24 up to 120 hours post-chemotherapy) and overall phases (0–120 hours post-chemotherapy). Data was collected in duplicate by two authors (LC, RC).
Statistical analysis
The Mantel-Haenszel, random-effects analysis model was used to compute cumulative risk ratios (RR) and their accompanying 95% confidence intervals (CIs). All analyses were conducted using Comprehensive Meta-Analysis (Version 3), by BioStat (AnalystSoft Inc., Alexandria, VA, USA). Funnel plots were also generated, to visually assess for publication bias.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1462.
Results
Cumulative meta-analysis: emesis and nausea
For the endpoint of emetic control, the cumulative meta-analysis shows that the summary effect did not change noticeably with the inclusion of the most recent trials. In the acute phase, the RR shifted from 1.07 before 2011 to 1.10 after 2015, even after the inclusion of 7 trials. Similar small changes were noted in the delayed phase, with the RR shifting from 1.23 before 2011 to 1.40 after 2015 and the overall phase, with RR shifting from 1.22 before 2011 to 1.53 after 2015. As expected, the CI did narrow with the inclusion of the most recent trials in all phases (Figure 1).
For the endpoint of nausea control, the cumulative meta-analysis does show a significant visual change in summary effect, except for nausea control in the acute phase. In the delayed phase, the RR shifts from 1.58 before 2011 to 1.50 after 2015. In the overall phase, the RR shifts from 1.642 before 2011 to 1.53 after 2015. The CI in the acute phase is narrow relative to the summary estimates for the delayed and overall phases (Figure 2).
Assessment for publication bias
There were no remarkable concerns for publication bias, based on visual assessment of the generated funnel plots (Figures 3,4).
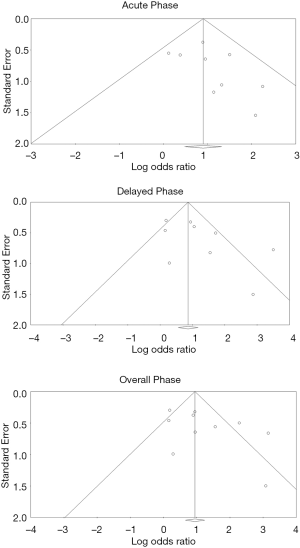
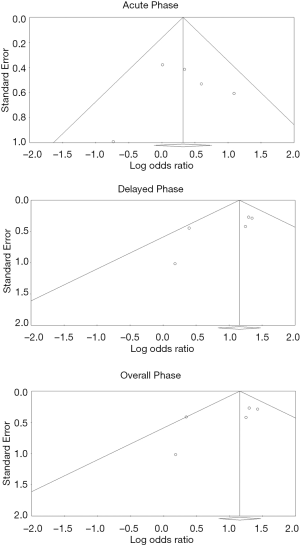
Rescue of breakthrough CINV
There were only three studies reporting on the efficacy of olanzapine in the rescue of breakthrough CINV. The summary estimate effect did change over time, and olanzapine remains significantly superior to other compared interventions (Figure S1). For this analysis, assessment for publication bias via funnel plot similarly did not lead to any concerns of bias, but the paucity of data does not lead to a definitive conclusion (Figure S2).
Discussion
The purpose of this paper was to appraise the review by Chiu et al. to see the impact of the most-recent publications on the meta-analysis. A cumulative meta-analysis is a sequence of meta-analyses performed on a subset of studies, starting with a single study and adding the other studies one at a time. In this way, we can assess the effect of the last few trials included in the Chiu et al. review and see how each study affects the meta-analysis’ point estimate and CI. A lack of significant change with the addition of the last couple trials suggests that the published literature had already generated a precise treatment effect—supporting the conclusion that further trials assessing olanzapine for CINV are unnecessary.
In this cumulative analysis, the summary effect size seems to be well-established for the endpoint of emetic control, as the summary effect did not change appreciably with the inclusion of the most recent trials. The same cannot be said for nausea control for the delayed and overall phases—the summary estimate did change noticeably. These observations are accompanied by wide CIs, supporting the view that there remains a paucity of data reported in the literature on nausea control.
The lack of publication bias seen in this analysis confirms that the existing published literature provides an accurate report and assessment of olanzapine’s efficacy. The lack of publication bias found in this study indicates that there is not an oversaturation of studies that over- or under-state olanzapine’s efficacy. When interpreted alongside the minimal change in effect size for the endpoint of emetic control, there is minimal support from this analysis for studies since 2016 that solely report on emetic control. However, given the paucity of data for nausea control, it may be appropriate to assess both endpoints in recent trials. In the interest of efficiently allocating scarce trial resources, studies solely reporting emetic control may not have been necessary.
This study is not without limitations. As it is a meta-analysis, it is subject to the same constraints and biases inherent in the design of the individual RCTs. Furthermore, the use of funnel plots to visually assess for publication bias is not as robust as a quantitative statistical test such as Egger’s test. But, the lack of obvious visual evidence of bias mitigated the need to employ Egger’s test.
In conclusion, olanzapine’s efficacy for the prophylaxis of CINV has been sufficiently documented with respect to emetic control. There is, however, a paucity of data with respect to nausea control. As an aside, there is also a paucity of data documenting the efficacy of olanzapine for the rescue of breakthrough CINV. To better allocate trial resources, further studies studying olanzapine for CINV in terms of emetic control may be unnecessary, whereas future studies should focus efforts on better documenting nausea control.
Acknowledgments
Dr. Carlo DeAngelis is the senior author of this project.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1462
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1462). CBS II serves as an unpaid Editor-in-Chief of Annals of Palliative Medicine and reports honorarium from Varian Medical Systems. ML reports consulting fees from Ferring, Abbvie, Sanofi, and AstraZeneca in the past 10 years outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chow R, Valdez C, Chow N, et al. Oral cannabinoid for the prophylaxis of chemotherapy-induced nausea and vomiting – a systematic review and meta-analysis. Support Care Cancer 2020;28:2095-103. [Crossref] [PubMed]
- Osoba D, Zee B, Warr D, et al. Effect of postchemotherapy nausea and vomiting on health-related quality of life. Supportive Care in Cancer 1997;5:307-13. [Crossref] [PubMed]
- Sankhala KK, Pandya DM, Sarantopoulos J, et al. Prevention of chemotherapy-induced nausea and vomiting: a focus on aprepitant. Expert Opin Drug Metab Toxicol 2009;5:1607-14. [Crossref] [PubMed]
- Chow R, Tsao M, Chiu L, et al. Efficacy of the combination neurokinin-1 receptor antagonist, palonosetron, and dexamethasone compared to others for the prophylaxis of chemotherapy-induced nausea and vomiting: a systematic review and meta-analysis of randomized controlled trials. Ann Palliat Med 2018;7:221-33. [Crossref] [PubMed]
- Chasen MR, Rapoport BL. Rolapitant for the treatment of chemotherapy-induced nausea and vomiting: a review of the clinical evidence. Future Oncol 2016;12:763-78. [Crossref] [PubMed]
- Vrabel M. Is ondansetron more effective than granisetron for chemotherapy-induced nanusea and vomiting? A review of comparative trials. Clin J Oncol Nurs 2007;11:809-13. [Crossref] [PubMed]
- Chow R, Warr DG, Navari RM, et al. Should palonosetron be a preferred 5-HT3 receptor antagonist for chemotherapy-induced nausea and vomiting? An updated systematic review and meta-analysis. Support Care Cancer 2018;26:2519-49. [Crossref] [PubMed]
- Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003;25:578-82. [Crossref] [PubMed]
- Chow R, Chiu L, Navari R, et al. Efficacy and safety of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Support Care Cancer 2016;24:1001-8. [Crossref] [PubMed]
- Chiu L, Chow R, Popovic M, et al. Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer 2016;24:2381-92. [Crossref] [PubMed]
- Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:3240-61. [Crossref] [PubMed]
- Berger MJ, Ettinger DS, Aston J, et al. NCCN guidelines insights: antiemesis, version 2.2017. J Natl Compr Canc Netw 2017;15:883-93. [Crossref] [PubMed]
- Ithimakin S, Theeratrakul P, Laocharoenkiat A, et al. Randomized, double-blind, placebo-controlled study of aprepitant versus two dosages of olanzapine with ondansetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving high-emetogenic chemotherapy. Support Care Cancer 2020;28:5335-42. [Crossref] [PubMed]
- Vimolchalao V, Sakdejayont S, Wongchanapai P, et al. The efficacy and safety of the addition of olanzapine to ondansetron and dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Int J Clin Oncol 2020;25:396-402. [Crossref] [PubMed]
- Yeo W, Lau TKH, Li L, et al. A randomized study of olanzapine-containing versus standard antiemetic regimens for the prevention of chemotherapy-induced nausea and vomiting in Chinese breast cancer patients. Breast 2020;50:30-8. [Crossref] [PubMed]
- Chow R, Aapro M, Navari RM, et al. Do we still need to study palonosetron for chemotherapy-induced nausea and vomiting? A cumulative meta-analysis. Crit Rev Oncol Hematol 2019;142:164-86. [Crossref] [PubMed]
- Tan L, Liu J, Liu X, et al. Clinical research of olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res 2009;28:131. [Crossref] [PubMed]
- Shumway NM, Terrazzino SE, Jones CB. A randomized pilot study comparing aprepitant to olanzapine for treatment of chemotherapy-induced nausea and vomiting. J Clin Oncol 2009;27:516. [Crossref]
- Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 2011;9:188-95. [Crossref] [PubMed]
- Mao WK, Peng L. Clinical observation of olanzapine combined with granisetron and hexadecadrol prevent nausea vomit induced by chemoradiotherapy. Chinese Journal of Medicine Guide 2011;13:452-4.
- Wang X, Wang L. Effectiveness of olanzapine in prevention of chemotherapy induced nausea and vomiting. Chinese Journal of Clinical 2012;6:7406-7. (Electron Ed).
- Lu YL, Liu W, Du YJ, et al. Antiemetic effect of low dose olanzapine in solid tumor chemotherapy. Chinese Journal of Cancer Prevention and Treatment 2013;20:544-54.
- Mizukami N, Yamauchi M, Koike K, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized double-blind, placebo-controlled study. J Pain Symptom Manage 2014;47:542-50. [Crossref] [PubMed]
- Navari RM, Nagy CK. Olanzapine versus fosaprepitant for the prevention of nausea and vomiting in patients receiving concurrent chemo-radiation treatment. J Clin Oncol 2015;15:9504.
- Mukhopadhyay S, Kwartra G, Jeyaraj P, et al. Role of olanzapine in chemotherapy-induced nausea and vomiting on platinum-based chemotherapy patients: a randomized controlled study. Support Care Cancer 2017;25:145-54. [Crossref] [PubMed]
- Wang X, Wang L, Wang H, et al. Effectiveness of olanzapine combined with ondansetron in prevention of chemotherapy-induced nausea and vomiting of non-small cell lung cancer. Cell Biochem Biophys 2015;72:471-3. [Crossref] [PubMed]




