Acoustic parameters for the evaluation of voice quality in patients with voice disorders
Introduction
Acoustic analysis is an objective, noninvasive modality for the evaluation of voice quality in patients with voice disorders such as laryngitis, laryngospasm, laryngeal tumors, spasmodic dysphonia, and vocal cord paralysis (1-5). The widely used parameters of acoustic analysis mainly include the standard deviation of the fundamental frequency (F0 SD), jitter, and shimmer (3,6). Jitter refers to the short-term variations in the F0 between contiguous glottal cycles, and shimmer represents the short-term variations in the amplitude of sound waves (6).
According to the existing literature, these acoustic parameters have been extensively used for the identification of voice abnormalities. In 2016, Lopes and colleagues investigated the accuracy of acoustic parameters to discriminate between patients with different laryngeal diagnoses. They found that isolated F0 SD was the optimal parameter for distinguishing between vocal nodules and unilateral vocal fold paralysis, vocal nodules and gastroesophageal reflux-induced voice impairment, and between a vocal polyp and sulcus vocalis. Shimmer exhibited high accuracy for the differential diagnosis between vocal nodules and sulcus vocalis, and a combination of F0 SD and jitter aided in the identification of unilateral vocal fold paralysis (6). Subsequently, based on a much larger cohort, they proposed that combined acoustic measurements can facilitate the discrimination of voice deviation intensity and predominant voice quality in patients with dysphonia (7). Ayoub et al. investigated the impact of smoking on voice acoustics, and demonstrated that F0 SD and jitter did not alter remarkably, although the mean F0 and speaking F0 were significantly reduced in cigarette smokers (8). Searl et al. compared the acoustic parameters before and after executing treatment tasks in patients with Parkinson’s disease, and they noted that voice intensity was significantly increased after treatment, while there was no significant alteration in F0 SD (9). Additionally, Kang et al. found that some acoustic parameters (jitter, relative average perturbation, and noise-to-harmonic ratio) were associated with aspiration risk in patients with swallowing disorders (10). Jesus et al. found that unilateral vocal fold paralysis may cause significant alterations in various acoustic parameters including mean F0, F0 SD, jitter, shimmer, and mean harmonics-to-noise ratio (11).
Although these acoustic parameters have shown promise for describing voice characteristics across a number of conditions, there is still a paucity of evidence comparing the clinical values of F0 SD and perturbation parameters (jitter and shimmer). Previous studies indicated that F0 SD may have a higher sensitivity for an objective clinical voice assessment than jitter and shimmer, as a rapidly changing or shifting F0 may not alter the perturbation parameters (12), though there remains a lack of solid evidence. Therefore, this study aimed to compare the value of F0 SD, jitter, and shimmer for the evaluation of voice quality and the description of vocal characteristics in patients with voice disorders.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2102).
Methods
Subjects
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the local Ethics Committee (No. 2020-P2-074-01). Written informed consent was obtained from each participant. All the participants were male and they were enrolled from our hospital between January 2017 and December 2018. The inclusion criteria were as follows: (I) aged 18–60 years; and (II) no history of acute respiratory tract infection in the past 2 weeks. This study included 4 groups. For the vocal cord polyps (VCP) group, including 55 consecutive patients with VCP: electronic laryngoscope or stroboscopic laryngoscope demonstrated unilateral localized polyps at the edge of the vocal cord, with the base length less than 1/3 of the length of the vocal cord membranous part. For the early-stage laryngeal carcinoma (ELC) group, including 35 consecutive patients with early-stage (stage I–II) ELC: electronic laryngoscopy, stroboscopic laryngoscope, computed tomography or magnetic resonance imaging showed a tumor confined to unilateral or bilateral vocal cords, with mobility of the vocal cords. Postoperative pathology confirmed a diagnosis of squamous cell carcinoma without invasion of the vocal cord muscle layer. For the mutational falsetto (MF) group, including 17 consecutive patients with MF: the falsetto occurred during puberty, with high or unstable pitch and low volume. Stroboscopic laryngoscope showed glottal dysraphism, and falsetto was the major voice mode. In addition to traditional F0 parameters, F0 SD of mutational falsetto was significantly higher than that of normal. In addition to high pitch, another characteristic is that the unstable state of the fundamental frequency is a very important feature. The value of F0 SD can reflect the overall unstable voice characteristics of the fundamental frequency. We used the combination of F0 and F0 SD to identify mutational falsetto.
In addition, 29 healthy medical staff with no history of dysphonia or hoarseness were recruited as normal controls.
Acoustic examinations
Acoustic examinations were performed in a quiet room with a sound field less than 45 dB, and vocal data were collected using the ZOOMH6 recorder (sampling frequency, 44,000 Hz; 16 bits). The microphone was positioned 15–20 cm away from the subject’s mouth. The recorded sample was analyzed using the PRAAT software (version 3.9).
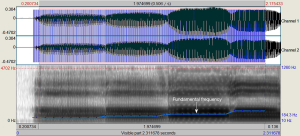
The participants were asked to emit a sustained vowel /a/, starting with a steady pronunciation for 0.5–1 seconds. Subsequently, the participants were asked to abruptly raise the pitch and keep it stable for 0.5–1 seconds, then abruptly raise the pitch again and maintain stable pronunciation until the pitch reached the upper limit of the natural vocal range. The shifting sections were defined as 4 continuous sections, starting from the comfort pitch and characterized by continuously increasing F0, in which the voice signal was stable (Figure 1). Acoustic analyses were performed in the first-section stable pronunciation (vocal sample I), first 2 shifting sections (vocal sample II), and 4 continuous shifting sections (vocal sample III), respectively. Acoustic parameters, including F0, F0 SD, jitter, and shimmer, were recorded.
Statistical analysis
SPSS 24.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analyses. Continuous variables were expressed as the mean ± standard deviation (SD) when data was normally distributed, or median when non-normally distributed. For univariate statistical analysis, Mauchly’s test of sphericity or the Mann-Whitney U-test for continuous variables were used as appropriate. Probability (P) values <0.05 were considered significant.
Results
Vocal samples in normal controls
The F0 (202.0±38.6) and F0 SD (54.15±26.65) in the voice sample III was significantly higher than those in the voice sample II (P=0.000; F0, 145.4±14.1; F0 SD, 18.28±9.97), and these parameters were also significantly higher than those in the voice sample I (P=0.000; F0, 128.2±13.9; F0 SD, 1.66±1.06). There were no significant differences in jitter between any 2 groups (all P>0.05). The shimmer in the voice sample III (2.76±1.11) was significantly lower than that in the voice sample II (3.35±1.17) (P=0.005), while there was no significant difference in shimmer between the voice sample II and the voice sample I (P>0.05). The detailed data are summarized in Table 1.

Full table
Acoustic parameters in voice disorder cases and controls
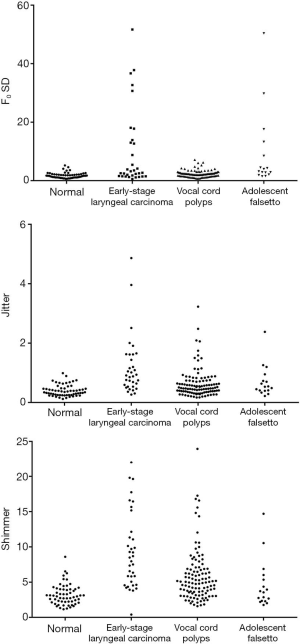
The F0 SD was highest (median, 4.00) in the MF group, and the phonogram showed abrupt alterations in F0 (Figure 2). The F0 SD in the MF group (median, 4.00) and the ELC group (median, 3.85) was significantly higher than that in the VCP group (median, 2.20) and the control group (median, 1.17) (P<0.05), and the F0 SD in the VCP group was significantly higher than that in the control group (P<0.05). However, there was no significant difference in F0 SD between the MF group and the ELC group (P>0.05).
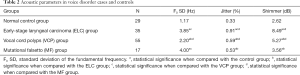
The jitter (median, 0.91) and shimmer (median, 8.49) in the ELC group were significantly higher compared to the other groups (P<0.05), and the jitter (median, 0.59) and shimmer (median, 5.27) in the VCP group were significantly higher than those in the MF group (median jitter, 0.53; median shimmer, 3.56) and the control group (median jitter, 0.33; median shimmer, 2.62) (P<0.05). There were no significant differences in jitter or shimmer between the MF group and the control group (P>0.05). The detailed results are summarized in Table 2 and Figure 3.
Full table
Discussion
MF and voice disorders all lead to pitch changes, though the clinical differentiation may be challenging. Voice disorders are usually characterized by phonatory instability, and acoustic analysis provides objective measures of phonatory characteristics (13). Perturbation parameters (jitter and shimmer), reflecting the perturbations in the frequency of the neighboring vibration cycles, are sensitive to phonatory deviations (14-16). In contrast to jitter and shimmer, which are perceived as voice roughness, F0 SD represents the F0 variation that is captured by measuring the SD in voice pitch. Therefore, F0 SD captures the amount of within-utterance variation in pitch, and low values of F0 SD are perceived as monotony (17).
Lim et al. investigated the acoustic and electroglottographic features in patients with dysphonia before and after vocal treatment, and the authors found that pitch lowered and improved in quality after treatment (18). In another study by the same authors, they found that acoustic and electroglottographic parameters, including jitter, the harmonics-noise ratio, the mean closed quotient, and the irregularity of the frequency, facilitated the objective assessment of the severity of edema and voice quality before and after surgery in patients with Reinke’s edema (19). Additionally, as cleft palate can severely affect the structure and function of the vocal tract and thus impair voice quality, some researchers attempted to perform acoustic analysis of voice in children with cleft palate and velopharyngeal insufficiency. Villafuerte-Gonzalez et al. reported that the F0 was significantly higher in children with cleft palate when compared to normal controls, and children with velopharyngeal insufficiency had a significantly higher shimmer perturbation (20). However, in a recent study, Segura-Hernandez and colleagues also conducted acoustic analyses in children with cleft lip and palate and velopharyngeal insufficiency, and they found no significant difference in mean F0 between controls and patients. Moreover, at the onset of the treatment, jitter and shimmer were significantly increased in all patients, while at the end of the treatment, jitter and shimmer were markedly decreased (21). These findings indicate perturbation parameters are more sensitive than the mean F0. Notably, all the above studies did not investigate F0 SD. In the current study, our findings suggest F0 SD may be a more reliable index than jitter and shimmer for the evaluation of voice quality.
MF, also known as puberphonia, is a functional voice disturbance characterized by failure of the male high-pitched preadolescent voice to transition to the lower pitch of adolescence and adulthood. Previous studies have confirmed that altered personality, neuropsychological, and social factors may contribute to the occurrence of MF, though the definitive pathological mechanism underlying the development of this disorder is not fully understood. Preliminary evidence indicates that irregular conversions of real pitch and falsetto are attributed to unstable vocal control (22). There has been no systematic acoustic analysis in patients with MF. Dagli et al. evaluated the outcomes of voice treatment on MF by using perceptual and acoustic analysis, and they found the F0, jitter, and shimmer were all suppressed after treatment (23). However, the F0 SD was not included in the observational indices. In the current study, we found that there was no significant difference in jitter or shimmer between patients with MF and controls when they were asked to emit a sustained vowel/a/, while the F0 SD showed marked alterations. Further analyses demonstrated that F0 SD, jitter, and shimmer were different between the laryngeal carcinoma group and the control group, as well as between the VCP group and controls. These findings indicate that MF is distinct from organic laryngopharyngeal diseases, and the disparate pathophysiologic processes lead to different acoustic characteristics.
Hohm et al. proposed that abrupt changeovers of F0 in a sustained vowel/a/ did not alter the jitter and shimmer (12), which is consistent with the results of the present study. Although the participants were asked to abruptly raise the pitch repeatedly, there was no significant difference in jitter or shimmer between different voice samples. However, the F0 SD exhibited obvious alterations with the pitch changeovers, and the variation of F0 SD was positively correlated with the degree of pitch hopping. These findings indicate that F0 SD is more sensitive to frequency hopping than jitter and shimmer, which is consistent with the acoustic results of MF.
The significant variation of F0 SD in patients with MF suggests that voice frequency is unstable and the ability to control vocal cords is weak. Moreover, the unremarkable alterations in jitter and shimmer indicate that asymmetrical vibration of the vocal cords may be involved in MF, while further studies using laryngeal high-speed photography are still warranted. In summary, acoustic analysis has the advantages of noninvasive, low price and convenience. It provides objective data for the assessment of voice disorders and becomes an indispensable detection method for voice diseases and voice disorders. In addition, it can be used to evaluate the curative effect of vocal cord polyps before and after surgery.
Conclusions
F0 SD, jitter, and shimmer are important parameters for the evaluation of pitch variation, which is of great significance in the diagnosis of MF and voice disorders.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2102
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2102
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2102). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).This study was approved by the local Ethics Committee (No. 2020-P2-074-01). Written informed consent was obtained from each participant.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gorris C, Ricci Maccarini A, Vanoni F, et al. Acoustic Analysis of Normal Voice Patterns in Italian Adults by Using Praat. J Voice 2020;34:961.e9-961.e18. [Crossref] [PubMed]
- Hanson DG, Jiang JJ, Chen J, et al. Acoustic measurement of change in voice quality with treatment for chronic posterior laryngitis. Ann Otol Rhinol Laryngol 1997;106:279-85. [Crossref] [PubMed]
- Lee SH, Hong KH, Kim JS, et al. Perceptual and Acoustic Outcomes of Early-Stage Glottic Cancer After Laser Surgery or Radiotherapy: A Meta-Analysis. Clin Exp Otorhinolaryngol 2019;12:241-8. [Crossref] [PubMed]
- Lu D, Chen F, Yang H, et al. Changes after voice therapy in acoustic voice analysis of Chinese patients with voice disorders. J Voice 2018;32:386.e1-386.e9. [Crossref] [PubMed]
- Ramírez DAM, Jiménez VMV, López XH, et al. Acoustic analysis of voice and electroglottography in patients with laryngopharyngeal reflux. J Voice 2018;32:281-4. [Crossref] [PubMed]
- Lopes LW, Batista Simoes L, Delfino da Silva J, et al. Accuracy of Acoustic Analysis Measurements in the Evaluation of Patients With Different Laryngeal Diagnoses. J Voice 2017;31:382.e15-382.e26. [Crossref] [PubMed]
- Lopes LW, Alves JDN, Evangelista DDS, et al. Accuracy of traditional and formant acoustic measurements in the evaluation of vocal quality. Codas 2018;30:e20170282. [PubMed]
- Ayoub MR, Larrouy-Maestri P, Morsomme D. The Effect of Smoking on the Fundamental Frequency of the Speaking Voice. J Voice 2019;33:802.e11-802.e16. [Crossref] [PubMed]
- Searl J, Wilson K, Haring K, et al. Feasibility of group voice therapy for individuals with Parkinson's disease. J Commun Disord 2011;44:719-32. [Crossref] [PubMed]
- Kang YA, Kim J, Jee SJ, et al. Detection of voice changes due to aspiration via acoustic voice analysis. Auris Nasus Larynx 2018;45:801-6. [Crossref] [PubMed]
- Jesus LM, Martinez J, Hall A, et al. Acoustic Correlates of Compensatory Adjustments to the Glottic and Supraglottic Structures in Patients with Unilateral Vocal Fold Paralysis. Biomed Res Int 2015;2015:704121. [Crossref] [PubMed]
- Hohm J, Dollinger M, Bohr C, et al. Influence of F0 and Sequence Length of Audio and Electroglottographic Signals on Perturbation Measures for Voice Assessment. J Voice 2015;29:517.e11-517.e21. [Crossref] [PubMed]
- Morris AE, Norris SA, Perlmutter JS, et al. Quantitative, clinically relevant acoustic measurements of focal embouchure dystonia. Mov Disord 2018;33:449-58. [Crossref] [PubMed]
- Hosseinifar S, Torabinezhad F, Ghelichi L, et al. How Do Voice Perceptual Changes Predict Acoustic Parameters in Persian Voice Patients? J Voice 2018;32:705-9. [Crossref] [PubMed]
- Torabinenezhad F, Izadi F, Pourshahbaz A, et al. Acoustic Parameters in Persian-Speaking Patients with Dysphonia. Function and Disability Journal 2018;1:8-17. [Crossref]
- Zealouk O, Satori H, Hamidi M, et al. Vocal parameters analysis of smoker using Amazigh language. International Journal of Speech Technology 2018;21:85-91. [Crossref]
- Knowles KK, Little AC. Vocal fundamental and formant frequencies affect perceptions of speaker cooperativeness. Q J Exp Psychol (Hove) 2016;69:1657-75. [Crossref] [PubMed]
- Lim JY, Lim SE, Choi SH, et al. Clinical characteristics and voice analysis of patients with mutational dysphonia: clinical significance of diplophonia and closed quotients. J Voice 2007;21:12-9. [Crossref] [PubMed]
- Lim JY, Choi JN, Kim KM, et al. Voice analysis of patients with diverse types of Reinke's edema and clinical use of electroglottographic measurements. Acta Otolaryngol 2006;126:62-9. [Crossref] [PubMed]
- Villafuerte-Gonzalez R, Valadez-Jimenez VM, Hernandez-Lopez X, et al. Acoustic analysis of voice in children with cleft palate and velopharyngeal insufficiency. Int J Pediatr Otorhinolaryngol 2015;79:1073-6. [Crossref] [PubMed]
- Segura-Hernandez M, Valadez-Jimenez VM, Ysunza PA, et al. Acoustic analysis of voice in children with cleft lip and palate following vocal rehabilitation. Preliminary report. Int J Pediatr Otorhinolaryngol 2019;126:109618. [Crossref] [PubMed]
- Hodges-Simeon CR, Gurven M, Puts DA, et al. Vocal fundamental and formant frequencies are honest signals of threat potential in prepubertal males. Behav Ecol 2014;25:984-8. [Crossref] [PubMed]
- Dagli M, Sati I, Acar A, et al. Mutational falsetto: intervention outcomes in 45 patients. J Laryngol Otol 2008;122:277-81. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)