A case report of rash induced by cefoperazone sodium and sulbactam sodium plus metronidazole sodium chloride combined with morphine hydrochloride
Introduction
Cefoperazone sodium and sulbactam sodium are mainly used for various infections caused by sensitive bacteria. The infection sites include the reproductive system, respiratory system, and urinary system. Although the drugs have good effects in clinical applications, there are also allergic reactions, including skin rash, fever, anaphylactic shock, and blood pressure drop. The allergic reactions not only affect the treatment of the patient but also affect the prognosis and recovery, and in severe cases, it may even endanger the patient’s life (1,2). Metronidazole is used in the treatment of anaerobic infections and is also widely used in clinical practice. Its adverse side effects include nausea, vomiting, lack of appetite, abdominal cramps, headache, and dizziness, and more rarely urticaria, itching, and leukopenia (3). After cesarean section, morphine hydrochloride is usually given epidurally for analgesia. Compared with other administration methods, a single epidural bolus of morphine hydrochloride can provide the same long-term analgesia (4). Among the neurological side effects of morphine, pruritus is one of the most troublesome problems. Mild pruritus is sometimes ignored, but sometimes severe pruritus can significantly affect the patient’s daily life and recovery (5). Several cases of rash or pruritus after the administration of cefoperazone sodium and sulbactam sodium or metronidazole sodium chloride or morphine hydrochloride have been reported.
This study reports a female undergoing cesarean section who had itching and skin rash after the administration of cefoperazone sodium and sulbactam sodium, metronidazole sodium chloride, and morphine hydrochloride. The occurrence of adverse side effects in special populations should arouse clinicians’ attention. This study aims to provide a reference for the clinical treatment of similar patients.
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2192).
Case presentation
The patient, female, 35 years old, was admitted to the hospital on May 18, 2020 due to “no period for 39+5 weeks, vaginal fluid for 4+ hours”. There was no special medical history, and the patient denied a history of chronic diseases such as hypertension, diabetes, and coronary heart disease; a history of chronic infectious diseases such as hepatitis and tuberculosis; a history of food or drug allergies; a history of blood transfusion; and a history of scheduled immunization. Physical examination showed that uterine height was 33 cm, the abdominal circumference was 3 cm, the fetus was in the cephalic presentation, the fetal heart rate was 144 beats/min, the fetal head was exposed, and the fetus had descended into the pelvis. The fetal membrane was broken, and the amniotic fluid was clear. The complete blood count (CBC) showed that the total number of white blood cells was 25.33×109/L, the percentage of neutrophils was 0.9130, and the C-reactive protein (CRP) was 47 mg/L. Fetal monitoring showed that the fetus had a good intrauterine response, and no abnormalities were found in the urine routine, liver function, biochemistry, or blood coagulation test. After admission, the patient tried vaginal delivery first, but the labor progressed slowly, and the patient was switched to a cesarean section.
On May 19, 2020, a lower uterine segment cesarean section was performed under combined spinal-epidural anesthesia. The patient received preoperative intravenous infusion of cefoperazone sodium and sulbactam sodium 3.0 g (batch number CH0491, Pfizer Pharmaceutical Co., Ltd.) once + metronidazole sodium chloride 0.5 g (batch number 200412402, Shijiazhuang No. 4 Pharmaceutical Co., Ltd., China) once, morphine hydrochloride injection 2 mg (batch number 190310-1, Northeast Pharmaceutical Group Shenyang First Pharmaceutical Co., Ltd., China) once in the epidural cavity at the end of the operation, then morphine hydrochloride 7 mg + NaiLePin 135 mg via an analgesic pump for 24 hours, and intramuscular injection of carboprost tromethamine 250 µg once to stop bleeding.
After the operation, the patient returned to the ward without complaining of special discomfort. After returning to the ward, the patient complained of mild itching on the skin of the waist, abdomen, and back. In the evening after the operation, the patient continued to receive intravenous infusion of cefoperazone sodium and sulbactam sodium 3.0 g and metronidazole sodium chloride 0.5 g as part of the anti-infective treatment, as well as intravenous infusion of oxytocin injection solution 20 IU, vitamin C injection solution 2 g, and compound amino acid injection solution (18AA-VII) 200 mL for symptomatic and supportive treatments for promotion of contraction, hemostasis, and nutritional supplementation.
On the first day after the operation, the patient still complained of mild itching on the skin of the waist, abdomen, and back and had a fever with the highest body temperature at 38 °C. No dizziness or headache was reported, and the patient had occasional pain due to uterine contractions and no passing gas through the anus. Physical examination showed that the vital signs were stable, the heart and lungs were normal, the fundal height was about one finger width above the umbilicus, the uterine contraction was good, the breasts were not swollen, the amount of lochia was moderate, and the incision was dry and clean, without redness, swelling, or exudation. The CBC showed that the total number of white blood cells was 19.14×109/L, the percentage of neutrophils was 0.8610, and the CRP was 74.1 mg/L. Due to the patient's high infection index and fever, she continued to be given cefoperazone sodium and sulbactam sodium + metronidazole sodium chloride for anti-infective treatment. The patient received oral polysaccharide iron complex capsules 0.15 g bid for iron supplementation and fresh motherwort capsules 0.4 g bid to promote lochia discharge. Symptomatic treatments such as fluid supplementation and uterine contraction stimulation were continued, and the changes in condition were observed.
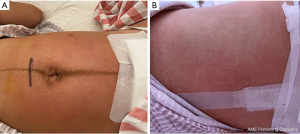
On the second day after the operation, the patient complained of more severe itchiness on the skin of the waist, abdomen, and back. The patient's body temperature was lower than before with the highest body temperature at 37.4 degree. There was no dizziness or headache, there was occasional pain due to uterine contractions, and gas had been passed through the anus. On physical examination, the vital signs were stable, and large patches of rashes were seen on the waist, abdomen, and back (Figure 1). There was no obvious abnormality in the heart or lungs, the fundal height was at the umbilicus (approximately 20 cm), the uterus was well contracted, the breasts were not swollen, the amount of lochia was small, lochia was bloody, urination was normal, and the incision was dry and clean, without redness, swelling, and exudation. It is common for cefoperazone sodium and sulbactam sodium, metronidazole sodium chloride, and morphine hydrochloride to cause skin rashes. Considered the possibility of a severe rash caused by the above drugs, the use of morphine hydrochloride was discontinued. After relevant examination results indicated a downward trend in infection indicators, the cefoperazone sodium and sulbactam sodium and the metronidazole sodium chloride were discontinued. The patient recovered well after surgery, and intravenous fluids were terminated. For skin rash and pruritus, loratadine tablets 10 mg po qd and topical calamine lotion tid were given to the patients for anti-allergic and anti-itch therapy. Oral medications promoting contractions and iron supplementation were continued. The body temperature was monitored, and changes in condition were observed.
On the third day after the operation, the patient had no complaints of fever or dizziness, and still had occasional pain due to uterine contractions, and the skin itching was better than before. The CBC showed that the total number of white blood cells was 13.52×109/L, and the percentage of neutrophils was 0.7770. On physical examination, the vital signs were stable, the rash was reduced, the heart and lungs were normal, the fundal height was one finger width below the umbilicus (approximately 18 cm), the uterus was well contracted, the breasts were slightly swollen, the amount of lochia was small, the abdominal wounds were well healed, and there was no bleeding, exudation, or redness. Oral medication was continued to promote uterine contractions, iron was supplemented, and allergic reactions were treated, and changes in her condition were observed.
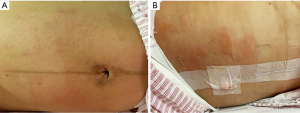
On the fourth day after the operation, the patient had no complaints of fever or dizziness but had occasional pain due to uterine contractions, and skin itching was improved. On physical examination, the vital signs were stable, the rash had improved significantly (Figure 2), there was no obvious abnormality in the heart or lungs, the bilateral breasts were swollen, the abdomen was flat, the fundal height was two finger widths below the umbilicus (approximately 16 cm), the uterus was contracted well, the abdominal incision was well aligned, the healing was good, and there was no redness, bleeding, or oozing and a small amount of bloody lochia. The patient recovered well after surgery, all drugs were terminated, and the patient was discharged from the hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Discussion
Drug-induced rash (also called measles-like eruption) is the most common drug skin reaction, accounting for approximately 90% of all drug eruptions. Most cases involve the most used prescription drugs, such as antibiotics (6). The patient in this study had mild itching on the day after the operation and the first day after operation, and a large rash was seen on the skin on the second day after operation. It is likely that the skin rash was caused by one or more of the drugs. Itching of the skin was the first symptom of the rash. The drugs used before the appearance of the rash were cefoperazone sodium and sulbactam sodium, metronidazole sodium chloride, morphine hydrochloride injection, ropivacaine hydrochloride injection, and carboprost tromethamine. Ropivacaine hydrochloride injection and carboprost tromethamine have not been reported to cause adverse rash reactions, so we can rule out any relationship between these two drugs and rash. The patient received postoperative intravenous infusion of vitamin C injection, coenzyme Q10 sodium chloride, and compound amino acid injection (18AA-VII) and oral administration of polysaccharide iron complex capsules, and fresh motherwort capsules. These drugs were only used after the patient had skin itching. Therefore, the rash could not have been caused by these drugs. According to the relationship between the patient’s physical signs and the time of drug injection along with the adverse side effects reported in the drug instructions and related literature, it is most likely that the drug rashes were caused by cefoperazone sodium and sulbactam sodium, metronidazole sodium chloride, and morphine hydrochloride.
Cesarean section is class II incision surgery. The common pathogens are gram-negative bacilli, Enterococcus, group B streptococcus, and anaerobic bacteria. The “Guidelines for Clinical Application of Antimicrobial Agents” (2015 edition) recommends the use of preventive antimicrobials such as the first- and second-generation cephalosporins ± metronidazole. The timing of preventive medication is 30 minutes after cutting the umbilical cord or before the operation. The preoperative blood examination of this patient suggested elevated white blood cells, neutrophils, and CRP, so the possibility of infection was considered. Therefore, it was reasonable to give cefoperazone sodium and sulbactam sodium + metronidazole sodium chloride as anti-infection treatment before surgery, and the same regimen was applied for postoperative anti-infection treatment. On the first and second days after operation, the patient had a fever, and infection-related indicators were elevated. It was reasonable to continue intravenous infusion of cefoperazone sodium and sulbactam sodium + metronidazole sodium chloride.
Cefoperazone sodium and sulbactam sodium belong to the class of cephalosporins + enzyme inhibitors. They achieve their bactericidal effect by inhibiting the biosynthesis of cell wall mucopeptides in sensitive bacteria during the bacterial reproduction period. They can be clinically used for multisite infections and have a good antibacterial effect (2). The common adverse side effects after the use of cephalosporin antibiotics are fever, itching, urticaria, and rash. Cephalosporin antibiotics are incomplete antigenic drugs. They undergo chemical recombination to produce antigenic determinants during in vivo metabolism, which combine with serum proteins in the body to form hapten-carrier complexes, and can cause the body to produce a variety of allergic reactions. The allergic reaction to drugs may also be related to the proteins and their polymers introduced in the production process, and the sensitization to cephalosporins of different varieties, batches, and manufacturers may be different (7,8). Metronidazole is a nitroimidazole derivative. It is mainly used to treat intestinal and parenteral amebiasis. It is widely used in the treatment of anaerobic infections. Its adverse side effects include nausea, vomiting, lack of appetite, abdominal cramps, headache, dizziness, and less often urticaria, itching, and leukopenia (3).
Morphine hydrochloride is a potent analgesic. It is suitable for acute and sharp pains that other analgesics are ineffective, such as pains related to severe trauma, war wounds, burns, and advanced cancer, and the analgesic effect of one administration lasts 4–6 hours (9). The adverse side effects of morphine hydrochloride include nausea, vomiting, and respiratory depression, and the occasional allergic reactions include itching, urticaria, and skin edema. High-dose administration of opioids can cause rash and itching. Growing evidence shows that the stimulation caused by the direct degranulation and activation of mast cells and the activation of opioid receptors lead to the release of histamine, causing skin reactions such as flushing, rash, urticaria, and itching (10-12).
The patient was previously healthy, had no history of drug or food allergies, and had no other special proneness to allergies. She did not use any medicines or eat any allergenic foods before coming to the hospital. After the patient took cefoperazone and sulbactam sodium, metronidazole sodium chloride, and morphine hydrochloride during the perioperative period, the skin began to feel itching, making it likely an adverse side effect caused by the drugs. After the patient developed the rash, cefoperazone and sulbactam sodium, metronidazole sodium chloride, and morphine hydrochloride were discontinued promptly, and loratadine tablets were given as oral anti-allergic treatment while calamine external washing was used to relieve itching. After that, the rash did not expand, the color of the rashes gradually became darker, and the skin itching gradually disappeared. There was a correlation between the use of the above-mentioned drugs and the appearance of the rash. After stopping the drugs and taking symptomatic anti-allergic measures, the allergic reaction improved. Cefoperazone and sulbactam sodium, metronidazole sodium chloride, and morphine hydrochloride can cause rash-related adverse side effects (13-16). This patient took all three of these drugs during the perioperative period, thus increasing the probability of an adverse drug reaction. After excluding previously existing diseases and other factors, we can conclude that the patient’s allergic reaction was drug-related, although it is difficult to deduce whether a single drug was responsible, or whether two or more of these drugs worked in combination.
Sensitized drugs used during the perioperative period of cesarean section include antibacterial drugs, anesthetic and analgesic drugs, and intravenous iron drugs. Pregnant women who receive these drugs should be closely observed by medical staff. The treatment of drug eruption consists mainly of detecting the rash in time, stopping allergenic drugs, treating allergic symptoms, and promoting drug excretion, because there is no evidence-based medicine to support the efficacy of preventive use of corticosteroids or antihistamines to prevent delayed drug reactions in sensitized patients. For mild drug eruptions in pregnant women, the second-generation H1 antihistamines cetirizine and loratadine are preferred, as they are much less likely to impair central nervous system function or to result in sedation or other adverse reactions compared to the first-generation H1 antihistamines. For breastfeeding women, we need to consider whether the selected drug has an impact on infants and young children when choosing anti-allergic drugs. The first-generation antihistamines, such as chlorpheniramine, and ketotifen, have a strong inhibitory effect on the central nervous system. The blood-brain barrier of infants and young children is not yet fully developed and is highly permeable. Drugs can easily penetrate the blood-brain barrier and cause adverse neurological reactions in infants and young children. People who are breastfeeding should avoid using such drugs. Among the second-generation antihistamines, loratadine has a weak central nervous system inhibitory effect and is secreted at an acceptably low level in breast milk. The American Academy of Pediatrics recommends loratadine to breastfeeding people for anti-allergic treatment because it does not affect breastfeeding. Therefore, it was reasonable to choose loratadine for anti-allergic treatment in this patient.
To minimize adverse side effects of drugs in clinical practice, medical staff should pay attention to the following points: (I) before medication, the history of allergies should be inquired in detail to determine whether the patient had a tendency for allergy. (II) The early symptoms of drug eruption, such as sudden occurrence of itching, erythema, and fever should be closely monitored; suspicious drugs should be terminated immediately; the patient should be observed closely; allergenic drugs should be identified; and symptomatic treatment should be given at the same time. (III) We should strictly follow the usage and dosage specified in the drug instructions and be cautious when combining medications. (IV) We should keep up on the latest medical evidence and understand any newly discovered adverse drug reactions.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2192
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2192). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013) . Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Funk EA, Strausbaugh LJ. Antimicrobial activity, pharmacokinetics, adverse reactions, and therapeutic indications of cefoperazone. Pharmacotherapy 1982;2:185-96. [Crossref] [PubMed]
- Beloborodova NV, Kuznetsova ST, Popov DA, et al. Clinical experience with the treatment of severe nosocomial infections by inhibitor-protected 3rd generation cephalosporin cefoperazone/sulbactam. Antibiot Khimioter 2005;50:33-40. [PubMed]
- Hermida MD, Consalvo L, Lapadula MM, et al. Bullous fixed drug eruption induced by intravaginal metronidazole ovules, with positive topical provocation test findings. Arch Dermatol 2011;147:250-1. [Crossref] [PubMed]
- Alexandre MP, Mignon A, Mazoit JX, et al. Analgesic efficacy and adverse effects of epidural morphine compared to parenteral opioids after elective caesarean section: a systematic review. Eur J Pain 2010;14:894.e1-9.
- Dominguez JE, Habib AS. Prophylaxis and treatment of the side-effects of neuraxial morphine analgesia following cesarean delivery. Curr Opin Anaesthesiol 2013;26:288-95. [Crossref] [PubMed]
- Bigby M. Rates of cutaneous reactions to drugs. Arch Dermatol 2001;137:765-70. [PubMed]
- Pichichero ME, Zagursky R. Penicillin and cephalosporin allergy. Ann Allergy Asthma Immunol 2014;112:404-12. [Crossref] [PubMed]
- Macy E. Penicillin and beta-lactam allergy: epidemiology and diagnosis. Curr Allergy Asthma Rep 2014;14:476. [Crossref] [PubMed]
- Flury U, Cahill JL, Nixon RL. Occupational contact dermatitis caused by opioids: A case series. Contact Dermatitis 2019;81:332-5. [Crossref] [PubMed]
- Kaiko RF, Grandy RP, Oshlack B, et al. The United States experience with oral controlled-release morphine (MS Contin tablets). Parts I and II. Review of nine dose titration studies and clinical pharmacology of 15-mg, 30-mg, 60-mg, and 100-mg tablet strengths in normal subjects. Cancer 1989;63:2348-54. [Crossref] [PubMed]
- Portenoy RK, Maldonaldo M, Fitzamartin R, et al. Oral controlled-release morphine sulfate: Analgesic efficacy and side effects of 100mg tablet in cancer pain patients. Cancer 1989;63:2284-8. [Crossref] [PubMed]
- McNicol E, Horowicz-Mehler N, Fisk RA, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain 2003;4:231-56. [Crossref] [PubMed]
- Takahashi K, Suzuki H, Arai T, et al. Morphine induced Anaphylaxis before Induction of Anesthesia. Masui 2016;65:363-5. [PubMed]
- Shinagawa N, Takeda S, Oohira S, et al. Efficacy and safety of sulbactam/cefoperazone for hepato-biliary infections. Jpn J Antibiot 1997;50:862-70. [PubMed]
- Knowles S, Choudhury T, Shear NH. Metronidazole hypersensitivity. Ann Pharmacother 1994;28:325-6. [Crossref] [PubMed]
- Aruanno A, Parrinello G, Buonomo A, et al. Metronidazole Hypersensitivity in a Patient With Angioedema and Widespread Rash. J Investig Allergol Clin Immunol 2020;30:371-3. [Crossref] [PubMed]