Multiple combination therapy based on intrathecal pemetrexed in non-small cell lung cancer patients with refractory leptomeningeal metastasis
Introduction
Approximately 3–5% of patients with non-small cell lung cancer (NSCLC) will develop leptomeningeal metastasis (LM) at the time of initial diagnosis or during treatment, and it is more common in the adenocarcinoma (1). However, the incidence of LM is increased in a subset of patients with targeted mutations, and can be as high as 9.4% in patients with epidermal growth factor receptor (EGFR) mutations (2). The diagnosis of LM can be a positive radiological discovery with supportive clinical findings; however, positive cerebrospinal fluid (CSF) cytology remains the gold standard (3).
The LM cause damage to cerebral hemisphere, cranial nerves and spinal cord and associated roots, resulting in a progressive decline in the general state of the patient and a rapid progression to death if not treated. Even with maximal therapy, the median survival after diagnosis of LM only increases from 1–3 to 3–11 months (1,2,4), with molecularly targeted therapy being the major contributor. Targeted therapy is the first choice for LM patients with target mutations, while chemotherapy is the first choice for wild-type patients (3). The standard of care for chemotherapeutic regimens in LM and the role of newer agents such as bevacizumab and pemetrexed have not yet been established (5). Also, the survival benefit of whole-brain radiotherapy (WBRT) remains controversial (6,7). With appropriate treatment, intrathecal chemotherapy (ITC) provides a promising response rate and survival benefit, with a pooled study reporting a median survival of 7.5 months (8). However, patients’ progress after chemotherapy without targeted mutations and patients’ progress after targeted therapy with actionable mutations may deteriorate quickly. For refractory LM (rLM) patients with epidermal growth factor receptor (EGFR) mutations, the median overall survival (mOS) was 6.2 months for high-dose erlotinib (9), 7.2 months for standard-dose osimertinib, and 11.0 months for high-dose osimertinib (10,11). The current literature on rLM has focused on patients with EGFR mutations, defined as progression after classical or conventional dose targeted therapy, mainly after first generation or second generation targeted drug therapy. However, with the approval and widespread use of three generations of targeted drugs, differences in the definition of rLM lead to dissimilar patient characteristics in trials, resulting in varying survival outcomes. At present, rLM lacks a standard definition and there are no standard treatment guidelines.
We conducted a single-center retrospective study to explore the efficacy and safety of multiple therapy based on intrathecal pemetrexed (IP) in patients with rLM in NSCLC, and investigate the guiding significance of CSF next generation sequencing (NGS) testing for clinical decision-making. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/apm-20-2086).
Methods
Patients
From March 2019 to June 2020, a total of 23 patients were enrolled in this study. This study was approved by the Ethical Committee of Fujian Cancer Hospital (No. SQ2017-015-01). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. We defined rLM as: (I) for patients with actionable mutations, diagnosis of LM during systemic therapy with tyrosine-kinase inhibitors (TKIs) or progression of known LM on treatment with TKIs; (II) for patients with EGFR Thr790Met (T790M) mutation or anaplastic lymphoma kinase (ALK)-positive NSCLC, LM progression after failure of third generation EGFR-TKIs or second/third generation ALK inhibitors; for patients without EGFR T790M mutation, LM progression after failure of first/second/third generation EGFR-TKIs; (III) diagnosis of LM in wild-type patients after first-line standard treatment failure.
Eligibility criteria were as follows: (I) cytological identification of malignant cells within the CSF; (II) no severe hepatic and renal dysfunction, with a glomerular filtration rate (GFR) of >80 mL/min, white blood cell count of ≥3.5×109/L, and platelet count of ≥100×109/L. Patients with only radiological evidence of LM not confirmed by positive CSF cytology were excluded. The analysis of patients receiving salvage treatment included at least one dose of IP and/or one cycle of systemic therapy.
Molecular test
All patients had the genetic testing status of the initial specimens, and some of the patients had undergone a second biopsy and CSF testing. The mutation status of the tumor tissue sample or blood sample was determined by amplification-refractory mutation system (ARMS) assay, droplet digital polymerase chain reaction (ddPCR), or NGS. All CSF specimens were taken before intrathecal injection and tested with NGS. The NGS test was performed using a panel consisting of 168 cancer-related genes (Burning Rock Biotech, Guangzhou, China) or of 1,021 cancer-related genes (Geneplus-Beijing Ltd., Beijing, China).
IP
Pemetrexed (10 mg) was administered by intrathecal injection via lumbar puncture or ommaya reservoir, once a week until: two consecutive negative CSF cytology results were achieved, the side effects were not tolerated, or the disease progressed. Dexamethasone (5 mg, 2 mL) was administered by intrathecal injection combined with pemetrexed simultaneously. Approximately 15 mL of CSF was drained from the reservoir for discarding or testing prior to the intrathecal injection. Personalized systematic therapy and supporting treatment were administrated to patients.
Evaluation of responses and adverse events (AEs)
The clinical response was determined according to the Response Assessment in Neuro-Oncology (RANO) proposal criteria based on three fundamental elements (clinical, neuroimaging, and CSF analysis) (12). CSF cytology examination was performed prior to intrathecal injection therapy every week. Two consecutive negative CSF cytology results were interpreted as negative CSF analysis. Cerebrospinal magnetic resonance imaging (MRI) examination was performed before and every 6±2 weeks after treatment using a scanner (3.0 T field strength). AEs were assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
Statistical analysis
Descriptive statistics were used to summarize patient and treatment characteristics. Progression-free survival (PFS) was defined as the time from diagnosis of rLM to intracranial or extracranial progression, or death. Overall survival (OS) was defined as the time from diagnosis of rLM to death or last follow-up. Survival analyses were performed using Kaplan-Meier estimates, and were reported along with their 95% confidence intervals (95% CIs). All analyses were performed using IBM SPSS Statistics 22.0. The date of last follow-up was July 31, 2020.
Results
Baseline characteristics
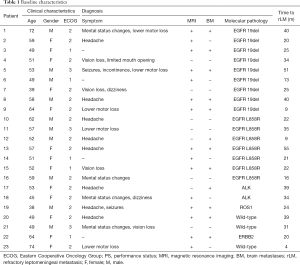
From March 2019 to June 2020, 23 patients who received IP and systemic pharmacotherapy for rLM were enrolled in Fujian Cancer Hospital. The baseline patient characteristics are listed in Table 1. All patients were diagnosed with lung adenocarcinoma and the median age of the population was 53 years (range, 38–74 years). There were 10 (43.5%) males and 13 (56.5%) females. The most common manifestations were headache (34.8%), lower motor loss (21.7%), vision loss and mental status changes (17.4%), dizziness and seizures (8.7%), and incontinence and limited mouth opening (4.3%). The median time from initial diagnosis of advanced NSCLC to development of rLM was 25 months (range, 4–55 months).
Full table
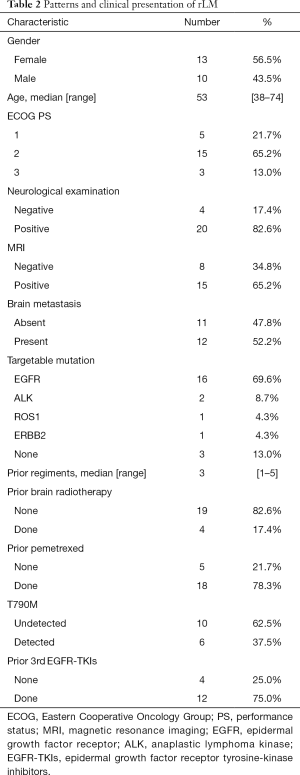
In total, 20 (87.0%) patients carried druggable mutations, most commonly EGFR mutations (16/23, 69.6%). At diagnosis of rLM, 18 (78.3%) patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score >1. Concurrent brain metastases were reported in 12 (52.2%) patients at rLM diagnosis, four of whom were treated with radiation therapy. The characteristic abnormal enhancement of MRI was reported in 15 patients (65.2%), four of whom were asymptomatic (Table 2). All patients with EGFR/ALK/ROS proto-oncogene 1 (ROS1) mutation had received first or second generation TKIs as the first-line therapy. RLM was diagnosed after systemic therapy consisting of first and third generation TKIs in 15 of 19 (78.9%) patients with EGFR/ALK/ROS1 mutation, chemotherapy in 18 of 23 (78.3%) patients, and both TKIs and chemotherapy in 14 of 23 (60.9%) patients. Acquired T790M mutations were reported in 6 of 16 (37.5%) patients before rLM diagnosis, however there were 12 of 16 (75.0%) patients who had used third generation TKIs including osimertinib and alflutinib (AST2818). Overall, patients received a median number of three systemic therapies (range, 1–5) before diagnosis of rLM. Wild-type patients received chemotherapy or immunotherapy in combination with anti-angiogenic therapy. No patients had received ITC previously, and all of the 18 chemotherapy-treated patients received pemetrexed.
Full table
Treatment after rLM
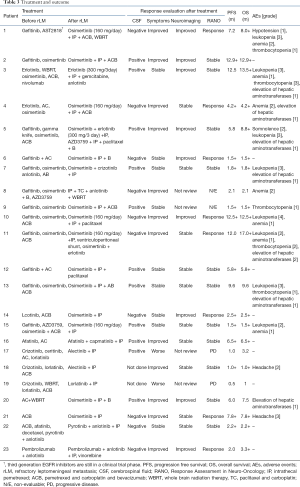
All patients received IP therapy; IP was given to all patients a total of 75 times, with a median of four times (range, 1–10). On the basis of IP, systemic therapy included targeted therapy, systemic chemotherapy, immunotherapy, and anti-vascular therapy, which were combined according to the ECOG PS status and the clinical characteristics of the patients (Table 3). Of the total cohort, 19 patients were rechallenged with TKIs, nine patients were treated with systemic chemotherapy, 10 patients were treated with anti-vascular therapy, one patient was treated with immunotherapy, and one patient was treated with WBRT due to metastatic lesions of the cerebral parenchyma requiring treatment. Three patients continued the previous systemic treatment regimen and only added IP treatment. Of the 20 patients who switched regimens, six received single-agent targeted therapy combined with IP, and the remaining 14 patients received more than two regimens of combined therapy.
Full table
Of the 20 patients who initially had active target mutations including EGFR/ALK/ROS1/ Erb-B2 receptor tyrosine kinase 2 (ERBB2) mutations, 19 chose to be rechallenged with TKIs, except for patient 8 (P8), whose rebiopsy pathology showed small cell transformation. Of the 15 patients who developed rLM after first and third generation multiline-targeted therapy, 12 had EGFR mutations, two had ALK fusions, and one had ROS1 fusion. Among the 12 patients with EGFR mutations, two received high-dose osimertinib, three received high-dose osimertinib combination chemotherapy, one received erlotinib pulse therapy combined with chemotherapy, one received osimertinib combined with erlotinib pulse therapy (C797S was detected in the CSF), three received conventional-dose osimertinib combination chemotherapy, one received osimertinib plus MET proto-oncogene (MET) inhibitor (MET amplification was detected in the CSF), and one case of small cell transformation was converted to systemic chemotherapy. Patients with ALK were switched to alectinib, which had not been used before, while the patient with ROS1 fusion continued treatment with third-generation ALK-TKIs.
Of the four patients who had received only first or second generation single-line TKIs with EGFR mutations and no detectable T790M mutations before rLM, one was treated with osimertinib alone, one was treated with osimertinib combined with anti-angiogenesis therapy, and one was treated with osimertinib combined with chemotherapy, and patient 16 (P16) with non-classical EGFR mutations was treated with maintenance afatinib in combination with a MET inhibitor. The patient with ERBB2 mutation did not adjust the original targeted drug.
Three patients with initial molecular pathology exhibited the wild type, two of whom were confirmed to have the EGFR 19-del mutation via CSF testing after rLM diagnosis, received osimertinib monotherapy or in combination with anti-angiogenesis treatment. CSF NGS was not performed in the remaining wild-type patient, who was maintained on immunotherapy combined with anti-angiogenesis treatment due to advanced age.
The chemotherapy regimen was pemetrexed or paclitaxel with or without carboplatin, and gemcitabine was also used. Ten patients were treated with anti-angiogenesis therapy, seven with bevacizumab, and three with the small-molecule vascular inhibitor, anlotinib.
Outcome
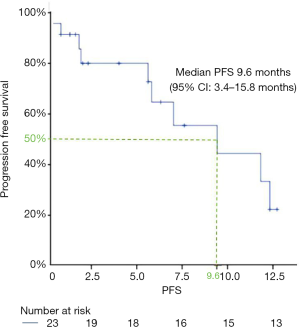
Five patients (21.7%) had died at the time of follow-up, and another five patients received posterior palliative treatment after progression. Thirteen patients (56.5%) remained at the progression-free stage, and the longest PFS was 12.9 months. The median PFS was 9.6 months (95% CI: 3.4–15.8 months) at follow-up (Figure 1). Due to insufficient follow-up time, OS data was immature. The clinical assessment was: response in eight patients (34.8%), stable in 11 patients (47.8%), worse in two patients (8.7%), and non-evaluable in two patients (8.7%) (Table 3). Of the two patients who were assessed as non-evaluable, one patient had not been reexamined neuroimaging by the end of the follow-up period, and the other patient died suddenly of unknown causes after treatment despite showing marked improvement in his symptoms. There were also two patients assessed as worse who could not be reviewed on imaging due to rapidly deteriorating status.
Genetic profiles
Molecular analysis collected the primary lesions from all 23 patients, secondary tissue or blood biopsy of 19 patients, tertiary tissue or blood biopsy of two patients, and CSF testing of 16 patients. In all 60 samples, including 31 tissue samples, 13 plasma samples, and 16 CSF samples, a total of 40 genes were detected (Figure 2). CSF and extracranial samples of gene detection were not collected at the same time, and CSF NGS detection was performed for patients who were diagnosed as rLM but did not receive IP treatment.
Of the 16 CSF samples, 14 patients had EGFR/ALK mutations in their primary tumors, and driver gene mutations were also detected in the CSF sampled (14/14, 100%). Acquired T790M mutations were reported in 6 of 16 (37.5%) patients before rLM diagnosis, and in three of these patients, CSF specimens were collected after diagnosis of rLM, but the T790M mutation was not detected. In addition, T790M was detected in the CSF in two patients with undetectable extracranial lesions.
A large number of copy number variations were detected in the CSF, which were not identified in primary tissue or plasma. Of the 16 patients who had both extracranial and CSF specimens, the latter detected a more frequent loss of heterozygosity of tumor protein 53 (TP53) (31.6% vs. 68.8%, respectively), amplification of EGFR (5.3% vs. 25%, respectively), cyclin E1 (CCNE1) (5.3% vs. 18.8%, respectively), and MET (5.3% vs. 12.5%, respectively), and loss of retinoblastoma 1 (Rb1) (10.5% vs. 12.5%, respectively). The most noteworthy detection was that in two patients whose extracranial lesions were confirmed to be wild-type by testing, EGFR 19-del was detected in the CSF. Both patients were evaluated for response after switching to osimertinib. C797S was detected in the CSF of patient 5 (P5), but T790M was not detected simultaneously. This patient, who had previously received multi-line targeted therapy and chemotherapy, received combination targeted therapy of first and third TKIs and IP, and achieved a PFS for 5.8 months and was still alive at the time of writing this report. The genetic similarities and differences between extracranial lesions and CSF are shown in Figure 3.

Safety and AEs
None of the patients died from treatment-related toxicities. The most common AEs were myelosuppression and liver enzyme increase, and these were often associated with side effects of chemotherapy and targeted therapy. AEs related to any component occurred in 14 patients (60.9%). Grade 3 or higher AEs occurred in seven (30.4%) patients, including six cases of myelosuppression and one case of headache. Four patients changed their treatment strategy due to AEs, including three patients who discontinued chemotherapy because of myelosuppression and one patient who discontinued IP because of IP-related headache.
Discussion
In this study, patients with rLM from NSCLC received multiple therapy based on IP treatment with a promising survival benefit and tolerable side effect profile. We observed a prolongation of PFS to 9.6 months (95% CI: 3.4–15.8 months) in treated patients, while OS is currently immature. This survival result is superior to a previous study of TKI rechallenging in rLM patients with EGFR mutations, which showed a median survival time of 6.1 months (13). This is primarily due to the fact that our study used a combination of intrathecal therapy coupled with targeted therapy and a more complex chemotherapy and/or antivascular treatment paradigm. This combination therapy model has potential clinical application prospects.
ITC is one of the treatment options for LM, which directly delivers the chemotherapeutic agent to the CSF (3). Methotrexate, cytarabine, and tiatipa are the most commonly used intrathecal chemotherapeutic agents, however they are mainly used for lymphoma and leukemia patients, and are not specifically designed for NSCLC patients (14). A pooled analysis that included patients with NSCLC treated with ITC reported a median survival time of 6.0 months (8). However, the clinical research involved in this article was performed prior to 2013, and cannot represent the current state of new target drugs and treatment modes. Similar to MTX, pemetrexed is a cell-cycle specific and antimetabolite folate inhibitor. Pemetrexed in combination with platinum is considered to be one of the first-line treatment options for NSCLC, especially for patients with adenocarcinoma histology. Although there is no standard treatment for LM patients, pemetrexed chemotherapy reduces the risk of death in patients with brain metastases or LM lung cancer (15,16). This suggests that pemetrexed has the potential to overcome CNS involvement. Moreover, an IP model of rats had been established, which showed that the IP dose in rats is 1 mg/kg, indicating that the high concentration of pemetrexed in CSF is maintained for an extended period (17). A pilot phase 1 study of IP in the treatment of rLM in NSCLC showed controlled toxicity and good efficacy of pemetrexed (10 mg) and vitamin supplements administered 1–2 times per week (18). In our study, 18 patients were treated with pemetrexed intravenous chemotherapy before rLM diagnosis, and a high disease control rate can still be achieved by IP, especially in the clinical observation, which has a significant effect on the remission of symptoms. IP is a fairly effective treatment for patients with rLM in NSCLC, and further prospective studies with well-designed pharmacokinetic measures are warranted. Although ITC is a reasonably effective treatment for patients with LM from NSCLC, the optimal drug, dose, and regimen remain to be determined (19).
Some previous studies support the hypothesis that the occurrence of LM may be related to limited central nervous system (CNS) diffusion (20,21). High-dose or pulsatile dosing has been reported as an attempt to increase TKI concentrations in the CNS. High-dose erlotinib (200 or 300 mg every 2 days, or 300 or 450 mg every 3 days, or 600 mg every 4 days) and pulsatile high-dose erlotinib (median dose 1500 mg, weekly) achieved radiographic responses of 30% and 67%, respectively, compared with standard doses of erlotinib or gefitinib in patients with EGFR mutant NSCLC with rLM (9,22). Osimertinib is a third generation EGFR-TKI that effectively penetrates the blood-brain barrier (23), and a previous study had reported that osimertinib improves OS in NSCLC patients with rLM regardless of T790M mutation status (24). The BLOOM study demonstrated a meaningful treatment effect of high-dose osimertinib (160 mg daily) in patients with EGFR-mutant NSCLC with rLM (positive CSF cytology) and a manageable safety profile (11). The median investigator-assessed PFS was 8.6 months (95% CI: 5.4–13.7 months), which was comparable to our results. Thus, current data supports the use of high-dose TKIs in EGFR-mutated rLM patients when standard-dose TKIs are ineffective. However, very few patients enrolled in these studies were diagnosed with rLM after progression to osimertinib, and as the use of osimertinib in the first line increases, more attention will be given to the management of osimertinib-resistant rLM.
Furthermore, in a previous retrospective study, chemotherapy was shown to improve the survival of patients with LM, and a poor ECOG PS was reported to be a poor prognostic factor (2). Patients with LM usually have poor ECOG PS scores, and patients with poor ECOG PS scores always fail to accept chemotherapy and other treatments, which may explain the poor prognosis. In our study, we found that the rapid decline of ECOG PS scores in patients with rLM was often caused by rLM itself. After administering active systemic therapy (including IP and chemotherapy), symptoms can be significantly controlled, the ECOG PS state can be restored in a relatively short time, and AEs can be accepted.
Also, considering that only a small number of patients in this study received brain radiotherapy, the role of WBRT in rLM patients needs to be further verified. Specifically, the ability of WBRT treatment to delay the occurrence of LM in patients with previous brain parenchymal metastases needs to be confirmed. In this study, the toxicities of IP in combination with multiple therapies did not overlap, and timely and more aggressive combination therapy for rLM may be a meaningful exploration model.
Liquid biopsy of CSF is a reliable method for identifying genetic characteristics of LM. This is because circulating tumor DNA from CSF more accurately represents genomic alterations in CNS lesions than circulating tumor DNA from plasma (25). A previous study compared the different genetic profiles of CSF to peripheral plasma and found that CSF as a liquid biopsy specimen could facilitate translational research programs and help personalize follow-up care (26). Our study is consistent with the findings of this study; we observed a large number of copy number variations and high frequency of TP53 loss of heterozygosity in CSF specimens, which was different to extracranial specimens. The high frequency of RB1 deletion, and MET and EGFR amplification also further described the complex drug resistance environment of rLM. Furthermore, we found a lower frequency of T790M mutations in CSF compared with extracranial samples, which is similar to the results of other studies (27-29). However, in our study, a high proportion of patients (75.0%) used osimertinib before rLM, which may have contributed to the low detection rate of T790M in CSF. We also detected T790M in CSF samples from two patients with negative extracranial samples, neither of whom had received osimertinib prior to the diagnosis of rLM. Two wild-type patients were found to have EGFR 19-del in CSF; however it is not clear whether this is due to differences in the sensitivity of different assays or the heterogeneity of resistance mechanisms between LM itself and extracranial lesions. In this study, it seems to be feasible to analyze the mechanism of rLM resistance according to the CSF test results to guide drug use.
Nonetheless, our study had limitations that should be noted. Firstly, this was a retrospective study and included a limited number of patients. The number of patient samples in this study was too small, and a large sample study should be added for verification. Secondly, extracranial and CSF samples were not contemporaneous and could not be distinguished from temporal heterogeneity. Finally, because of the short follow-up time in this study, it is easy to miss the discovery and treatment of complications miss, and further follow-up observation is needed.
Conclusions
In conclusion, IP based multimodal therapy has significant efficacy and a controlled safety in NSCLC patients with rLM. The NGS test of CSF as a liquid biopsy offers significant value in monitoring the progress of rLM and guiding clinical decision-making.
Acknowledgments
Funding: This work was supported by the Innovation of Science and Technology, Fujian province, China (GL) [grant number 2017Y9083]; Fujian Provincial Health and Family Research Talent training program, Fujian province, China (GL) [grant number 2018- CX-12]; Science and Technology Department guided projects, Fujian province, China (GL) grant number 2018Y0016].
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-2086
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-2086
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-2086). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethical Committee of Fujian Cancer Hospital (No. SQ2017-015-01). All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev 2017;53:128-37. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Leptomeningeal Metastases in Patients with NSCLC with EGFR Mutations. J Thorac Oncol 2016;11:1962-9. [Crossref] [PubMed]
- Le Rhun E, Weller M, Brandsma D, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 2017;28:iv84-99. [Crossref] [PubMed]
- Le Rhun E, Galanis E. Leptomeningeal metastases of solid cancer. Curr Opin Neurol 2016;29:797-805. [Crossref] [PubMed]
- Abe M, Osoegawa A, Karashima T, et al. Erlotinib and bevacizumab combination therapy for afatinib-refractory leptomeningeal carcinomatosis from EGFR-mutated lung cancer. Int Cancer Conf J 2019;8:81-5. [Crossref] [PubMed]
- Liao BC, Lee JH, Lin CC, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors for Non-Small-Cell Lung Cancer Patients with Leptomeningeal Carcinomatosis. J Thorac Oncol 2015;10:1754-61. [Crossref] [PubMed]
- Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol 2012;7:382-5. [Crossref] [PubMed]
- Wu YL, Zhou L, Lu Y. Intrathecal chemotherapy as a treatment for leptomeningeal metastasis of non-small cell lung cancer: A pooled analysis. Oncol Lett 2016;12:1301-14. [Crossref] [PubMed]
- Kawamura T, Hata A, Takeshita J, et al. High-dose erlotinib for refractory leptomeningeal metastases after failure of standard-dose EGFR-TKIs. Cancer Chemother Pharmacol 2015;75:1261-6. [Crossref] [PubMed]
- Nanjo S, Hata A, Okuda C, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer 2018;118:32-7. [Crossref] [PubMed]
- Yang JCH, Kim SW, Kim DW, et al. Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J Clin Oncol 2020;38:538-47. [Crossref] [PubMed]
- Chamberlain M, Junck L, Brandsma D, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol 2017;19:484-92. [PubMed]
- Flippot R, Biondani P, Auclin E, et al. Activity of EGFR Tyrosine Kinase Inhibitors in NSCLC With Refractory Leptomeningeal Metastases. J Thorac Oncol 2019;14:1400-7. [Crossref] [PubMed]
- Beauchesne P. Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol 2010;11:871-9. [Crossref] [PubMed]
- Barlesi F, Gervais R, Lena H, Hureaux J, Berard H, Paillotin D, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol 2011;22:2466-70. [Crossref] [PubMed]
- Riess JW, Nagpal S, Iv M, Zeineh M, Gubens MA, Ramchandran K, et al. Prolonged Survival of Patients With Non–Small-Cell Lung Cancer With Leptomeningeal Carcinomatosis in the Modern Treatment Era. Clin Lung Cancer 2014;15:202-6. [Crossref] [PubMed]
- Sun JM, Nam MH, Chung JY, et al. Safety and pharmacokinetics of intrathecal administration of pemetrexed in rats. Cancer Chemother Pharmacol 2011;68:531-8. [Crossref] [PubMed]
- Pan Z, Yang G, Cui J, et al. A Pilot Phase 1 Study of Intrathecal Pemetrexed for Refractory Leptomeningeal Metastases From Non-small-cell Lung Cancer. Front Oncol 2019;9:838. [Crossref] [PubMed]
- Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol 2018;19:e43-55. [Crossref] [PubMed]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 2015;10:156-63. [Crossref] [PubMed]
- Grommes C, Oxnard GR, Kris MG, et al. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 2011;13:1364-9. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Lee J, Choi Y, Han J, et al. Osimertinib Improves Overall Survival in Patients With EGFR-Mutated NSCLC With Leptomeningeal Metastases Regardless of T790M Mutational Status. J Thorac Oncol 2020. [Epub ahead of print]. [Crossref] [PubMed]
- De Mattos-Arruda L, Mayor R, Ng CKY, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 2015;6:8839. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol 2018;29:945-52. [Crossref] [PubMed]
- Yang H, Cai L, Zhang Y, et al. Sensitive detection of EGFR mutations in cerebrospinal fluid from lung adenocarcinoma patients with brain metastases. J Mol Diagn 2014;16:558-63. [Crossref] [PubMed]
- Nanjo S, Arai S, Wang W, et al. MET Copy Number Gain Is Associated with Gefitinib Resistance in Leptomeningeal Carcinomatosis of EGFR-mutant Lung Cancer. Mol Cancer Ther 2017;16:506-15. [Crossref] [PubMed]
- Hata A, Katakami N, Yoshioka H, et al. Spatiotemporal T790M Heterogeneity in Individual Patients with EGFR-Mutant Non-Small-Cell Lung Cancer after Acquired Resistance to EGFR-TKI. J Thorac Oncol 2015;10:1553-9. [Crossref] [PubMed]
(English Language Editor: A. Kassem)