
Efficacy of high intensity focused ultrasound treatment for cystic adenomyosis: a report of four cases
Introduction
Cystic adenomyosis or cystic adenomyoma is a particular type of adenomyosis that manifests cystic growth in the adenomyotic lesion (1). The maximum diameter of the cyst is larger than 5 mm. Patients usually have moderate to severe dysmenorrhea, which may be primary or secondary to other menstrual disorders. Cystic degeneration of an adenomyoma first was described in the world by Parulekar in 1990 (2). And in the year of 1996, Tamura et al. first reported the case of an adenomyotic cyst of the uterus in a 16-year-old girl (3). The pathogenesis is still unclear. There are two main points of view, (I) congenital diseases; some parts of the Mullerian duct are damaged during the development process, and the parts remain. After the menarche, the residual Mullerian duct epithelial cells under the action of estrogen periodically hemorrhages and form the cystic cavity. Thus, dysmenorrhea occurs due to increased pressure in the cyst. (II) Iatrogenic diseases; patients have different types of uterine operation history before the onset, the lesions may be related to the surgical operation site, clinical manifestations often appear after uterine operation history, and trauma may be the inducement. Severe dysmenorrhea often characterizes this population, which is difficult to control with drugs (4). It is easily misdiagnosed as female obstructive genital malformation and uterine fibroid degeneration. Due to misdiagnosis and delay treatment, the quality of life and gestation potential of adolescents and young women are affected. Currently, the treatment methods for cystic adenomyosis is to remove the lesion and protect the reproductive function. Treatment methods should be determined according to the patient’s age, fertility status, clinical symptoms, among many others; however, the most important is medication. Clinically used for conventional adenomyosis, treatment drugs are applicable, including nonsteroidal anti-inflammatory drugs (NSAIDs). It is an only temporary relief of symptoms, and it is easy to relapse after stopping the drug. The effect of treatment was not satisfactory. (III) Surgical treatment, the most important and effective method, including open surgical resection, laparoscopic resection of the lesions, and excision of the lesions under hysteroscopy (5-8). Although partial uterine resection can effectively alleviate the symptoms of dysmenorrhea, it is difficult for patients to accept it due to sizeable surgical trauma and postoperative complications.
High intensity focused ultrasound (HIFU), as a non-invasive method, can be used to kill the target tissues without damaging surrounding structures effectively. The previous studies have shown that HIFU is safe and effective in treating patients with solid tumors, superior to other methods, including no radiation damage, less pain, and rapid recovery after treatment (9-12). Recently, HIFU has also been used to treat adenomyosis (13,14). However, no study has reported on the feasibility of HIFU treatment for cystic adenomyosis. In this study, it reported four cases of patients with cystic adenomyosis treated with HIFU.
We present the following article in accordance with the AME Case Series reporting checklist (available at http://dx.doi.org/10.21037/apm-20-1599).
Methods
This study was conducted following the declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the Second Hospital of Hebei Medical University (No. 2019-R024). Written informed consent was obtained from all participants.
Clinical data
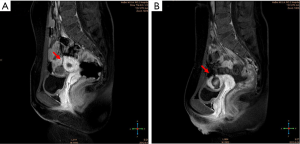
Four cystic adenomyosis patients with severe dysmenorrhea, aged from 20 to 34 years old, underwent HIFU treatment between January 2012 and January 2015 in the Second Hospital of Hebei Medical University. A thorough history, including age (at the time of treatment), age at onset of symptoms, marital status, fertility, delivery method, the severity of dysmenorrhea, and other menstrual disorders, was obtained. All patients underwent ultrasonography and magnetic resonance imaging (MRI) to determine the exact location and size of the lesion and its relation to the uterine cavity. The preoperative and postoperative symptoms and patient satisfaction were evaluated using the Uterine Fibroid Symptom and Quality of Life (UFS-QOL) questionnaire subscales, consisting of Symptom Severity Score (SSS) and Heath Related Quality of Life (HRQL) (15). All patients were admitted to the hospital due to progressive dysmenorrhea with inadequate response to medical management, which affected their quality of life. No apparent abnormalities were found in the detection of routine blood, routine urine, biochemistry, coagulation, and cervical liquid-based smears. Baseline patient profiles and imaging findings were presented in Table 1. Figure 1 describes the MRI features of the lesions and their relation to the uterine cavity.

Full table
Devices
Philips iU22 color Doppler ultrasonic diagnostic apparatus (iU22; Philips Healthcare, Hamburg, Germany) was applied for trans-vaginal ultrasound examination with a trans-vaginal probe frequency of 9 Hz. Simultaneously, the blood flow signal distribution of the lesions was detected by color Doppler flow imaging (CDFI) apparatus, and the ultrasonic diagnosis was obtained.
A Philips Achieva 3.0T magnetic resonance imaging system (Achieva; Philips Medical Systems, Best, The Netherlands) and standard body coil were used. All patients underwent routine scanning on the transect, coronal, and sagittal sections with spin echo (SE) T1WI sequence and turbo spin echo (TSE) T2WI sequence, and parts received an additional fatty suppression sequence.
HIFU treatment is performed using JC model HIFU tumor therapeutic system (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, China), which consists of the host, motor system, console, monitoring system, power source, and water management system.
Pre-HIFU preparation
Patients are instructed to ingest easily digestible semi-fluid or fluid food according to requirements three days before the treatment, a 12-hour fasting period before treatment, and a cleansing enema on the morning of treatment. The patients received routine shaving, degreasing, and degassing of the skin of the anterior abdominal wall from the umbilicus to the upper margin of the pubic symphysis. A urinary catheter was inserted to control the size of the bladder to optimize the therapeutic acoustic pathway.
HIFU treatment
The patients were carefully positioned on the HIFU treatment table in the prone position, with the lower abdomen in contact with degassed water. A cold degassed water balloon was placed on the abdominal wall to compress and push the bowel away from the acoustic pathway. HIFU treatment was performed under conscious sedation (fentanyl at 0.8–1 µg/kg, administered at 30–40 min intervals; midazolam hydrochloride, at 0.02–0.03 mg/kg, administered at 30–40 min intervals). The patients were asked to report any discomfort during HIFU treatment, and vital signs, including heart rate, blood pressure, respiration, and oxygen saturation, were checked.
Treatment begins from the posterior aspect of the lesion to the anterior. Then, it moved from the inferior aspect to the superior. The focal point is at least 1.5 cm away from the endometrium and 1 cm from the margin of the adenomyotic lesion. During treatment, the ultrasonic energy was adjusted with patient feedback and changes in grayscale on ultrasonographic imaging. This process was repeated on a section-by-section basis until hyperechoic changes covered the entire target lesion, or the contrast-enhanced ultrasound showed no blood supply.
All patients were followed up at the first menstrual cycle after HIFU and then every three months to check for any recurrence of dysmenorrhea or any other menstrual disorder.
Statistical analysis
The UFS-QOL questionnaire was used to evaluated HRQOL and SSS. A higher HRQOL score means a better quality of life. A lower SSS stands for improvement of symptoms. Patients with a SSS <20 in combination with a total HRQOL score >80 were considered asymptomatic.
If patients did not respond to the questionnaires telephone contact was attempted, we inquired about participation, additional treatment and patient satisfaction
Results
Four patients were successfully treated with HIFU, and no skin burns, bowel injuries, or bladder injuries were observed. Post-HIFU contrast-enhanced ultrasound or MRI showed no perfusion in the lesions (16) (Figure 1).
Follow-up results showed that the symptoms were significantly relieved by the first follow-up, with no dysmenorrhea and a higher health-related quality of life score compared to pre-HIFU treatment (Table 1). During the follow-ups one month, three months, and six months after HIFU, the four patients still had no dysmenorrhea and were highly satisfied with the HIFU ablation (Table 1).
Discussion
Cystic adenomyosis is a rare type of adenomyosis with the manifestation of a cyst more substantial than 5 mm in diameter. Large cystic adenomyosis is easily misdiagnosed as uterine malformation and broad ligament myoma (17,18); therefore, imaging is helpful for the diagnosis of cystic adenomyosis. Trans-vaginal sonography (TVS) is a standard imaging examination method for the diagnosis of adenomyosis. If there is a large cystic lesion (the diameter is greater than 5 mm) within the muscular layer of the uterus, the boundary is not clear, and the surrounding color blood flow signal is not apparent or presents as star pointed shapes, it is diagnosed as cystic adenomyosis. MRI is a correct and non-invasive method; its sensitivity can reach 78–88%, and its specificity can reach 67–93% (19). The features of this disease on MRI include uterine volume enlargement, diffuse, or localized thickening in the uterine wall and mottling abnormal signals in the uterine wall. T1WI indicates the lesions show an uneven low signal or high signal mixed with mottling. T2WI indicates the lesions show a low signal mixing with a high mottling signal and cannot be separated from the standard muscular layer. Uniform enlargement of the uterus, smooth outlines, and diffuse thickening of the connecting belt occur in cystic adenomyosis.
The fluid in the cyst shows a high signal on T1 weighted images, and the cystic wall shows a visible low signal on T2 weighted images (20-22). The cystic adenomyosis gets worse gradually as the menstrual cycle changes, and if the treatment is not prompt, there will be a risk of rupture. The management strategy of cystic adenomyosis depends primarily on the symptoms and the patient’s fertility. The traditional surgical approaches to cystic adenomyosis include hysterectomy, laparoscopic surgery, and hysteroscopic surgery. Hysterectomy is the definitive treatment possibility for intractable symptomatic adenomyosis when medical or other conservative treatments do not control the symptoms. Patients undergoing hysterectomy for adenomyosis may have an increased risk of bladder injury and persistent pelvic pain, diminished sexual life quality, among many others. The effect of laparoscopic surgery is remarkable. Several cases have been reported in the literature that dysmenorrhea in cystic adenomyosis was significantly improved by laparoscopic resection (6,7). Laparoscopic surgery, which produces less trauma and less bleeding, is superior to traditional surgery in the aspects of postoperative analgesic time, activity time, and hospitalization time. However, it still has complications, including abdominal bleeding, abdominal adhesion, and intestinal obstruction, among many others (23). Hysteroscopy is also a method for cyst excision, which is suitable for adenomyosis with a cyst protruding into the uterine cavity, but not suitable for a larger cystic cavity (5,8).
Additionally, due to the standard muscle layer surrounding the lesion in isolated cystic adenomyosis, laparoscopic or hysteroscopic surgery will destroy the healthy tissue of the muscle layer, presenting complications including the increased risk of uterine rupture after re-pregnancy. It is also not easy to avoid the occurrence of new iatrogenic endometriosis during surgery. At present, although hysterectomy is still the complete radical treatment for adenomyosis, it is unsuitable for young women, especially unmarried and childless patients, due to the substantial surgical trauma and undesirable influence on the patients’ body and mind. Although the operation method of uterine preservation can preserve the patient’s fertility, the wound on the uterus is more extensive, which not only has a higher recurrence rate but also may lead to iatrogenic pelvic endometriosis lesions.
HIFU is a non-invasive surgical technique for the treatment of tumors that emerged in the 1990s. Proper targeting characterizes it, real-time monitoring, non-invasive, no need for anesthesia, minor adverse reactions, repeatable treatment, and brief hospitalization time. HIFU is a non-surgical treatment for uterine fibroids that focuses on the ultrasound beams in the target lesion, causing coagulative necrosis and shrinkage of the lesion. Additionally, ultrasound-guided HIFU is less costly and offers real-time anatomic monitoring imaging, and a greyscale change during treatment is a reliable indicator in treatment response. It is effective in both focal and diffuse lesions (24,25). In a review of 2,549 patients with symptomatic adenomyosis among ten different centers, ultrasound-guided HIFU was shown to be technically successful in up to 94.6% of patients (26). Cystic adenomyoma is a category of adenomyoma, which has the same pathogenesis as adenomyosis. HIFU is also suitable for the treatment of cystic adenomyosis. Because there are more cystic adenomyotic lesions, the real-time monitoring of the lesions is accurate, and the damage to the standard muscle layer is minor for the entire treatment, which can avoid the occurrence of new iatrogenic endometriosis. In this study, the four patients were accurately diagnosed as cystic adenomyosis and successfully treated with HIFU, showing no skin burns, bowel injuries, or bladder injuries. It showed that HIFU, a non-invasive treatment method to preserve the integrity of the uterus, has gradually been accepted by the patients. Due to the non-invasive nature of its treatment, it avoids premature ovarian failure caused by traditional surgery and reduces the risk of iatrogenic implantation. During the treatment, the integrity of the uterine structure and function was preserved, the uterine environment was improved, and pregnancy was positively affected.
As no specific questionnaire exists in the literature to assess symptoms of adenomyosis, and as adenomyosis symptoms are like those of uterine myoma, we used the UFS-QOL questionnaire subscales (27). They were designed for assessing symptom severity of uterine myoma, including SSS and HRQL. The SSS was calculated used to assess the severity of a patient’s clinical symptoms. A higher score means the patient’s symptoms are more severe. In our study, the SSS results showed that the preoperative baseline score was about 36.5. Compared with preoperative, it decreased by 10, 10, and 10.25 after one month, three months, and six months. It can be seen that the score decreased significantly with time. Our results showed that the clinical symptoms of the two patients were severe, obvious dysmenorrhea, and the preoperative SSS score is notably higher than the other two patients. The postoperative symptoms improved at once, and the prognosis was stable, which was considered primary.
The other two had a history of surgery, and their clinical symptoms were alleviated after HIFU, and their preoperative SSS score was lower than the two original patients. Due to iatrogenic implantation, there was a higher possibility of recurrence of new lesions in the future, and the SSS score might be repeated. After HIFU treatment, the quality of life has been fundamentally changed. Higher scores on the HRQL scale indicate a better quality of life. During the follow-up, no dysmenorrhea was reported, and the patients were highly satisfied with the HIFU ablation. These results suggested that HIFU therapy for cystic adenomyosis with reasonable effectiveness, it reduced the size of the lesion and alleviated the symptoms of patients. It improves the patient’s quality of life significantly while preserving the uterus to achieve treatment, reducing the patient’s fear of undergoing surgery, avoiding hysterectomy, psychological, and physical trauma to the patient.
Progressive dysmenorrhea is the characteristic symptom of adenomyosis. The functional endometrial stroma causes dysmenorrhea and glands in the myometrium to occur periodic bleeding and the local inflammatory mediator release, triggered by the changes of ovarian hormone levels. It is believed the lesion more extensive, the dysmenorrhea symptoms more severe. Therefore, relieving dysmenorrhea is the primary therapeutic goal of the disease. HIFU ablation disables the ectopic endometrial by local “thermal resection” of the lesion, thus blocking the release of bleeding and inflammatory mediators caused by its periodic response to ovarian hormones. Yang et al. confirmed the postoperative histopathological changes of adenomyosis after HIFU treatment. They showed that the typical pathology was coagulative necrosis observed only with the naked eye and microscopy examination (28). In our case, the diagnosis of cystic adenomyosis was passed through trans-vaginal ultrasound and MRI. Ultrasound-guided HIFU ablation was performed under conscious sedation. HIFU, as a non-invasive treatment for adenomyosis, keeps the uterine and reproductive functions. These results made it possible to get pregnant and support. Rabinovici et al. recently reported a case of HIFU-treated adenomyoma patients (29). Symptoms significantly relieved at 16 weeks after the surgery. The tumor reduces to 50% before surgery and then naturally conceives and delivers a healthy baby through the vagina.
Nevertheless, so far, how to repair the intimal injury due to energy diffusion and improve the pregnancy rate still is to study. Using HIFU in the treatment of adenomyosis is insufficient large-scale case reports. Also, insufficient long-term efficacy observations make it a problem that needs further research.
Conclusions
In summary, our study indicated HIFU, the technology fully complied with the laws of nature and the wishes of patients, retains intact organs and showed the distinct advantages of non-invasive, safe, effective, well-tolerated, relatively comfortable, effect and lower cost, achieving the goal of “from treatment to treatment of patients.” Therefore, HIFU is a possible and safe method for the treatment of cystic adenomyosis.
Acknowledgments
Funding: Natural Science Foundation of Hebei Province (H2019206610).
Footnote
Reporting Checklist: The authors have completed the AME Case Series reporting checklist. Available at http://dx.doi.org/10.21037/apm-20-1599
Data Sharing Statement: Available at http://dx.doi.org/10.21037/apm-20-1599
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/apm-20-1599). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted following the declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the Second Hospital of Hebei Medical University (No. 2019-R024). Written informed consent was obtained from all participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Buerger PT, Petzing HE. Congenital cysts of the corpus uteri. Am J Obstet Gynecol 1954;67:143-51. [Crossref] [PubMed]
- Parulekar SV. Cystic degeneration in an adenomynoma (a case report). J Postgrad Med 1990;36:46-7. [PubMed]
- Tamura M, Fukaya T, Takaya R, et al. Juvenile adenomyotic cyst of the corpus uteri with dysmenorrhea. Tohoku J Exp Med 1996;178:339-44. [Crossref] [PubMed]
- Koga K, Osuga Y, Hiroi H, et al. Images in reproductive medicine. A case of giant cystic adenomyosis. Fertil Steril 2006;85:748-9. [Crossref] [PubMed]
- Giana M, Montella F, Surico D, et al. Large intramyometrial cystic adenomyosis: a hysteroscopic approach with bipolar resectoscope: case report. Eur J Gynaecol Oncol 2005;26:462-3. [PubMed]
- Takeda A, Sakai K, Mitsui T, et al. Laparoscopic management of juvenile cystic adenomyoma of the uterus: report of two cases and review of the literature. J Minim Invasive Gynecol 2007;14:370-4. [Crossref] [PubMed]
- Takeuchi H, Kitade M, Kikuchi I, et al. Diagnosis, laparoscopic management, and histopathologic findings of juvenile cystic adenomyoma: a review of nine cases. Fertil Steril 2010;94:862-8. [Crossref] [PubMed]
- Gordts S, Campo R, Brosens I. Hysteroscopic diagnosis and excision of myometrial cystic adenomyosis. Gynecol Surg 2014;11:273-8. [Crossref] [PubMed]
- ter Haar GR. High intensity focused ultrasound for the treatment of tumors. Echocardiography 2001;18:317-22. [Crossref] [PubMed]
- Gao HF, Wang K, Meng ZQ, et al. High intensity focused ultrasound treatment for patients with local advanced pancreatic cancer. Hepatogastroenterology 2013;60:1906-10. [PubMed]
- Knuttel FM, van den Bosch MA. Magnetic Resonance-Guided High Intensity Focused Ultrasound Ablation of Breast Cancer. Adv Exp Med Biol 2016;880:65-81. [Crossref] [PubMed]
- Chapelon JY, Rouviere O, Crouzet S, et al. Prostate Focused Ultrasound Therapy. Adv Exp Med Biol 2016;880:21-41. [Crossref] [PubMed]
- Shui L, Mao S, Wu Q, et al. High-intensity focused ultrasound (HIFU) for adenomyosis: Two-year follow-up results. Ultrason Sonochem 2015;27:677-81. [Crossref] [PubMed]
- Liu X, Wang W, Wang Y, et al. Clinical Predictors of Long-term Success in Ultrasound-guided High-intensity Focused Ultrasound Ablation Treatment for Adenomyosis: A Retrospective Study. Medicine (Baltimore) 2016;95:e2443. [Crossref] [PubMed]
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyomata. Obstet Gynecol 2002;99:290-300. [PubMed]
- Cheng CQ, Zhang RT, Xiong Y, et al. Contrast-enhanced ultrasound for evaluation of high-intensity focused ultrasound treatment of benign uterine diseases: retrospective analysis of contrast safety. Medicine (Baltimore) 2015;94:e729. [Crossref] [PubMed]
- Jain N, Goel S. Cystic Adenomyoma simulates uterine malformation: A diagnostic dilemma: Case report of two unusual cases. J Hum Reprod Sci 2012;5:285-8. [Crossref] [PubMed]
- Calagna G, Cucinella G, Tonni G, et al. Cystic adenomyosis spreading into subserosal-peduncolated myoma: How to explain it? Int J Surg Case Rep 2015;8C:29-31. [Crossref] [PubMed]
- Popovic M, Puchner S, Berzaczy D, et al. Uterine artery embolization for the treatment of adenomyosis: a review. J Vasc Interv Radiol 2011;22:901-9. [Crossref] [PubMed]
- Hricak H, Finck S, Honda G, et al. MR imaging in the evaluation of benign uterine masses: value of gadopentetate dimeglumine-enhanced T1-weighted images. AJR Am J Roentgenol 1992;158:1043-50. [Crossref] [PubMed]
- Troiano RN, Flynn SD, McCarthy S. Cystic adenomyosis of the uterus: MRI. J Magn Reson Imaging 1998;8:1198-202. [Crossref] [PubMed]
- Tamai K, Togashi K, Ito T, et al. MR imaging findings of adenomyosis: correlation with histopathologic features and diagnostic pitfalls. Radiographics 2005;25:21-40. [Crossref] [PubMed]
- Horváth G, Simonka Z, Lázár G. Comparison of the results of laparotomy and laparoscopic surgery in patients with Crohn's disease. Orv Hetil 2014;155:24-9. [Crossref] [PubMed]
- Dong X, Yang Z. High-intensity focused ultrasound ablation of uterine localized adenomyosis. Curr Opin Obstet Gynecol 2010;22:326-30. [Crossref] [PubMed]
- Zhou M, Chen JY, Tang LD, et al. Ultrasound-guided high-intensity focused ultrasound ablation for adenomyosis: the clinical experience of a single center. Fertil Steril 2011;95:900-5. [Crossref] [PubMed]
- Zhang L, Zhang W, Orsi F, et al. Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: A review of safety and efficacy. Int J Hyperthermia 2015;31:280-4. [Crossref] [PubMed]
- de Bruijn AM, Smink M, Hehenkamp WJK, et al. Uterine Artery Embolization for Symptomatic Adenomyosis: 7-Year Clinical Follow-up Using UFS-Qol Questionnaire. Cardiovasc Intervent Radiol 2017;40:1344-50. [Crossref] [PubMed]
- Yang Z, Cao YD, Hu LN, et al. Feasibility of laparoscopic high-intensity focused ultrasound treatment for patients with uterine localized adenomyosis. Fertil Steril 2009;91:2338-43. [Crossref] [PubMed]
- Rabinovici J, Inbar Y, Eylon SC, et al. Pregnancy and live birth after focused ultrasound surgery for symptomatic focal adenomyosis: a case report. Hum Reprod 2006;21:1255-9. [Crossref] [PubMed]
(English Language Editor: J. Chapnick)


